Abstract
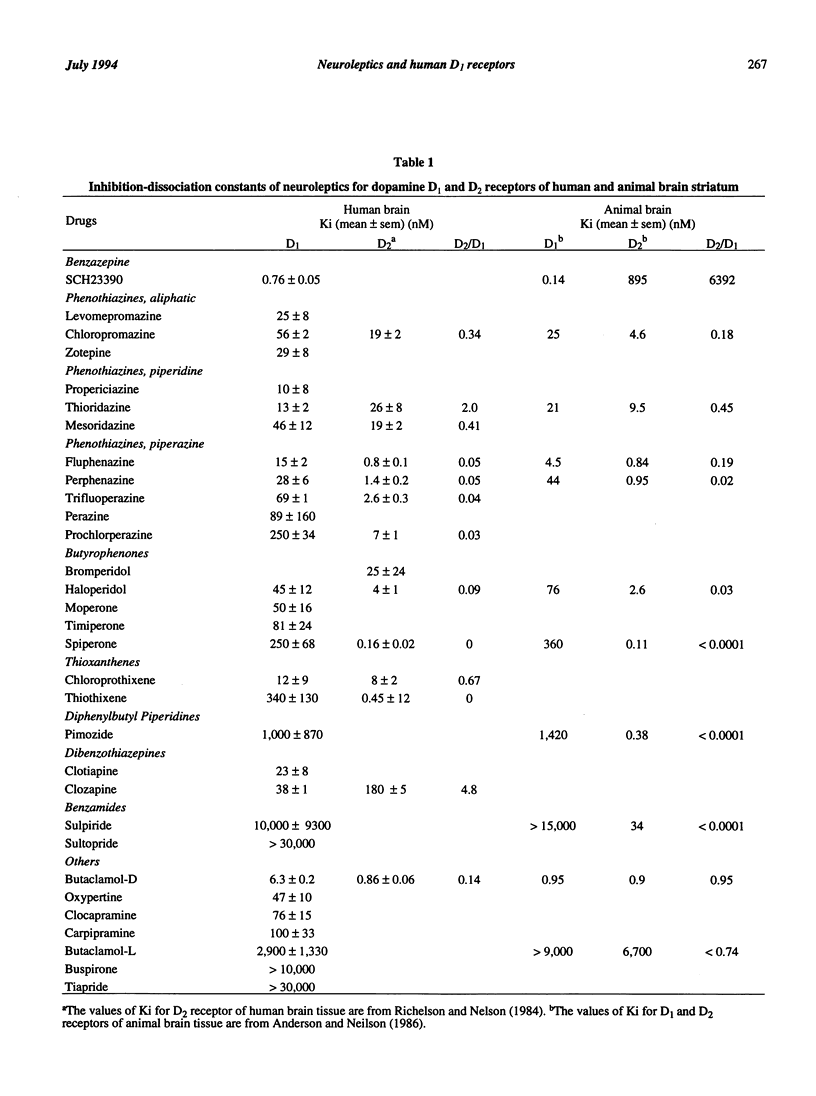
We determined the inhibition-dissociation constant (Ki) of a number of neuroleptics for D1 receptors of normal human brain tissue using [3H]SCH23390 [R-(+)-8-chloro-2,3,4,5-tetrahydro-3-methyl-5-phenyl-1H-3[benzazepine-7- ol]. SCH23390 had the highest affinity with a Ki of 0.76 nM. Among clinically used drugs, propericiazine showed the highest affinity with a Ki of 10 nM. When neuroleptics were classified according to chemical structures, the Ki values were as follows. Phenothiazines ranged from 10 nM to 250 nM. Butyrophenones ranged from 45 nM to 250 nM. Thioxanthenes ranged from 12 nM to 340 nM. Orthopramines were more than 10,000 nM. The Ki values for the binding site of this study were significantly correlated with those reported in studies using animal brain. The possible relationship between D1 receptors and negative symptoms is discussed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen P. H., Nielsen E. B. The dopamine D1 receptor: biochemical and behavioral aspects. Adv Exp Med Biol. 1986;204:73–91. doi: 10.1007/978-1-4684-5191-7_5. [DOI] [PubMed] [Google Scholar]
- Billard W., Ruperto V., Crosby G., Iorio L. C., Barnett A. Characterization of the binding of 3H-SCH 23390, a selective D-1 receptor antagonist ligand, in rat striatum. Life Sci. 1984 Oct 29;35(18):1885–1893. doi: 10.1016/0024-3205(84)90540-x. [DOI] [PubMed] [Google Scholar]
- Chipkin R. E., Iorio L. C., Coffin V. L., McQuade R. D., Berger J. G., Barnett A. Pharmacological profile of SCH39166: a dopamine D1 selective benzonaphthazepine with potential antipsychotic activity. J Pharmacol Exp Ther. 1988 Dec;247(3):1093–1102. [PubMed] [Google Scholar]
- Creese I., Burt D. R., Snyder S. H. Dopamine receptor binding predicts clinical and pharmacological potencies of antischizophrenic drugs. Science. 1976 Apr 30;192(4238):481–483. doi: 10.1126/science.3854. [DOI] [PubMed] [Google Scholar]
- Davis J. M. Comparative doses and costs of antipsychotic medication. Arch Gen Psychiatry. 1976 Jul;33(7):858–861. doi: 10.1001/archpsyc.1976.01770070088010. [DOI] [PubMed] [Google Scholar]
- Goldberg S. C. Negative and deficit symptoms in schizophrenia do respond to neuroleptics. Schizophr Bull. 1985;11(3):453–456. doi: 10.1093/schbul/11.3.453. [DOI] [PubMed] [Google Scholar]
- Hess E. J., Bracha H. S., Kleinman J. E., Creese I. Dopamine receptor subtype imbalance in schizophrenia. Life Sci. 1987 Apr 13;40(15):1487–1497. doi: 10.1016/0024-3205(87)90381-x. [DOI] [PubMed] [Google Scholar]
- Kanba K. S., Kanba S., Nelson A., Okazaki H., Richelson E. [3H]neurotensin(8-13) binds in human brain to the same sites as does [3H]neurotensin but with higher affinity. J Neurochem. 1988 Jan;50(1):131–137. doi: 10.1111/j.1471-4159.1988.tb13239.x. [DOI] [PubMed] [Google Scholar]
- Kanba S., Richelson E. Histamine H1 receptors in human brain labelled with [3H]doxepin. Brain Res. 1984 Jun 18;304(1):1–7. doi: 10.1016/0006-8993(84)90856-4. [DOI] [PubMed] [Google Scholar]
- Kane J., Honigfeld G., Singer J., Meltzer H. Clozapine for the treatment-resistant schizophrenic. A double-blind comparison with chlorpromazine. Arch Gen Psychiatry. 1988 Sep;45(9):789–796. doi: 10.1001/archpsyc.1988.01800330013001. [DOI] [PubMed] [Google Scholar]
- Lynch M. R. Schizophrenia and the D1 receptor: focus on negative symptoms. Prog Neuropsychopharmacol Biol Psychiatry. 1992;16(6):797–832. doi: 10.1016/0278-5846(92)90102-k. [DOI] [PubMed] [Google Scholar]
- Munson P. J., Rodbard D. Ligand: a versatile computerized approach for characterization of ligand-binding systems. Anal Biochem. 1980 Sep 1;107(1):220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- Richelson E., Nelson A. Antagonism by neuroleptics of neurotransmitter receptors of normal human brain in vitro. Eur J Pharmacol. 1984 Aug 17;103(3-4):197–204. doi: 10.1016/0014-2999(84)90478-3. [DOI] [PubMed] [Google Scholar]
- Seeman P., Lee T., Chau-Wong M., Wong K. Antipsychotic drug doses and neuroleptic/dopamine receptors. Nature. 1976 Jun 24;261(5562):717–719. doi: 10.1038/261717a0. [DOI] [PubMed] [Google Scholar]
- Seeman P., Niznik H. B., Guan H. C., Booth G., Ulpian C. Link between D1 and D2 dopamine receptors is reduced in schizophrenia and Huntington diseased brain. Proc Natl Acad Sci U S A. 1989 Dec;86(24):10156–10160. doi: 10.1073/pnas.86.24.10156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunahara R. K., Guan H. C., O'Dowd B. F., Seeman P., Laurier L. G., Ng G., George S. R., Torchia J., Van Tol H. H., Niznik H. B. Cloning of the gene for a human dopamine D5 receptor with higher affinity for dopamine than D1. Nature. 1991 Apr 18;350(6319):614–619. doi: 10.1038/350614a0. [DOI] [PubMed] [Google Scholar]
- Suzuki E., Kanba S., Nibuya M., Koshikawa H., Nakaki T., Yagi G. Plasma homovanillic acid, plasma anti-D1 and -D2 dopamine-receptor activity, and negative symptoms in chronically mediated schizophrenia. Biol Psychiatry. 1992 Feb 15;31(4):357–364. doi: 10.1016/0006-3223(92)90229-s. [DOI] [PubMed] [Google Scholar]
- Waddington J. L. Therapeutic potential of selective D-1 dopamine receptor agonists and antagonists in psychiatry and neurology. Gen Pharmacol. 1988;19(1):55–60. doi: 10.1016/0306-3623(88)90005-5. [DOI] [PubMed] [Google Scholar]


