Abstract
It is now clearly established that steroid hormones released from peripheral endocrine glands may, through specific receptors in the brain, directly regulate brain function. These effects may be rapid or involve long-term modifications at the genomic level. Concerning the glucocorticoids, their receptors are found in most neuronal cells, an observation which can be related to their widespread effects on neuronal metabolism. Furthermore, glucocorticoids are often related to stress. We have previously demonstrated that neonatal handling of the rat prevented excessive endocrine response to stress. In adults, this action appeared to protect the animal from potential damaging effects of glucocorticoids and from related impairment of cognitive functions. The effects of glucocorticoids are thought to involve an interaction of several central neurotransmitter systems. One such neurotransmitter is neurotensin, a neuropeptide which was reported to be closely related to central dopaminergic system regulation. This paper presents a rapid overview of the central effects of glucocorticoids and possible evidence for the interrelationship between these steroids, dopamine and neurotensin systems in the regulation of the hypothalamo-pituitary-adrenal axis. It provides a new way to approach stress responses and to develop new substances that may become potential drugs in the treatment of some psychiatric disorders.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angulo J. A., Printz D., Ledoux M., McEwen B. S. Isolation stress increases tyrosine hydroxylase mRNA in the locus coeruleus and midbrain and decreases proenkephalin mRNA in the striatum and nucleus accumbens. Brain Res Mol Brain Res. 1991 Oct;11(3-4):301–308. doi: 10.1016/0169-328x(91)90039-z. [DOI] [PubMed] [Google Scholar]
- Armanini M. P., Hutchins C., Stein B. A., Sapolsky R. M. Glucocorticoid endangerment of hippocampal neurons is NMDA-receptor dependent. Brain Res. 1990 Nov 5;532(1-2):7–12. doi: 10.1016/0006-8993(90)91734-x. [DOI] [PubMed] [Google Scholar]
- Borowsky B., Kuhn C. M. Monoamine mediation of cocaine-induced hypothalamo-pituitary-adrenal activation. J Pharmacol Exp Ther. 1991 Jan;256(1):204–210. [PubMed] [Google Scholar]
- Brindley D. N., Rolland Y. Possible connections between stress, diabetes, obesity, hypertension and altered lipoprotein metabolism that may result in atherosclerosis. Clin Sci (Lond) 1989 Nov;77(5):453–461. doi: 10.1042/cs0770453. [DOI] [PubMed] [Google Scholar]
- Casolini P., Kabbaj M., Leprat F., Piazza P. V., Rougé-Pont F., Angelucci L., Simon H., Le Moal M., Maccari S. Basal and stress-induced corticosterone secretion is decreased by lesion of mesencephalic dopaminergic neurons. Brain Res. 1993 Sep 17;622(1-2):311–314. doi: 10.1016/0006-8993(93)90836-c. [DOI] [PubMed] [Google Scholar]
- Ceccatelli S., Orazzo C. Effect of different types of stressors on peptide messenger ribonucleic acids in the hypothalamic paraventricular nucleus. Acta Endocrinol (Copenh) 1993 Jun;128(6):485–492. doi: 10.1530/acta.0.1280485. [DOI] [PubMed] [Google Scholar]
- Evans R. M., Arriza J. L. A molecular framework for the actions of glucocorticoid hormones in the nervous system. Neuron. 1989 Feb;2(2):1105–1112. doi: 10.1016/0896-6273(89)90177-3. [DOI] [PubMed] [Google Scholar]
- Gaillet S., Alonso G., Le Borgne R., Barbanel G., Malaval F., Assenmacher I., Szafarczyk A. Effects of discrete lesions in the ventral noradrenergic ascending bundle on the corticotropic stress response depend on the site of the lesion and on the plasma levels of adrenal steroids. Neuroendocrinology. 1993 Oct;58(4):408–419. doi: 10.1159/000126570. [DOI] [PubMed] [Google Scholar]
- Ganong W. F. Neurotransmitters and pituitary function: regulation of ACTH secretion. Fed Proc. 1980 Sep;39(11):2923–2930. [PubMed] [Google Scholar]
- Gould E., Woolley C. S., McEwen B. S. Short-term glucocorticoid manipulations affect neuronal morphology and survival in the adult dentate gyrus. Neuroscience. 1990;37(2):367–375. doi: 10.1016/0306-4522(90)90407-u. [DOI] [PubMed] [Google Scholar]
- Gudelsky G. A., Berry S. A., Meltzer H. Y. Neurotensin activates tuberoinfundibular dopamine neurons and increases serum corticosterone concentrations in the rat. Neuroendocrinology. 1989 Jun;49(6):604–609. doi: 10.1159/000125176. [DOI] [PubMed] [Google Scholar]
- Gully D., Canton M., Boigegrain R., Jeanjean F., Molimard J. C., Poncelet M., Gueudet C., Heaulme M., Leyris R., Brouard A. Biochemical and pharmacological profile of a potent and selective nonpeptide antagonist of the neurotensin receptor. Proc Natl Acad Sci U S A. 1993 Jan 1;90(1):65–69. doi: 10.1073/pnas.90.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horner H. C., Packan D. R., Sapolsky R. M. Glucocorticoids inhibit glucose transport in cultured hippocampal neurons and glia. Neuroendocrinology. 1990 Jul;52(1):57–64. doi: 10.1159/000125539. [DOI] [PubMed] [Google Scholar]
- Joëls M., de Kloet E. R. Corticosteroid actions on amino acid-mediated transmission in rat CA1 hippocampal cells. J Neurosci. 1993 Sep;13(9):4082–4090. doi: 10.1523/JNEUROSCI.13-09-04082.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joëls M., de Kloet E. R. Mineralocorticoid and glucocorticoid receptors in the brain. Implications for ion permeability and transmitter systems. Prog Neurobiol. 1994 May;43(1):1–36. doi: 10.1016/0301-0082(94)90014-0. [DOI] [PubMed] [Google Scholar]
- Kilts C. D., Coco M. L., Ely T. D., Bissette G., Nemeroff C. B. Differential effects of conditioned and unconditioned stress on the neurotensin content of dopamine cell body groups of the ventral mesencephalon. Ann N Y Acad Sci. 1992;668:266–276. doi: 10.1111/j.1749-6632.1992.tb27355.x. [DOI] [PubMed] [Google Scholar]
- Knych E. T., Eisenberg R. M. Effect of amphetamine on plasma corticosterone in the conscious rat. Neuroendocrinology. 1979;29(2):110–118. doi: 10.1159/000122912. [DOI] [PubMed] [Google Scholar]
- Landfield P. W., Baskin R. K., Pitler T. A. Brain aging correlates: retardation by hormonal-pharmacological treatments. Science. 1981 Oct 30;214(4520):581–584. doi: 10.1126/science.6270791. [DOI] [PubMed] [Google Scholar]
- Le Moal M., Simon H. Mesocorticolimbic dopaminergic network: functional and regulatory roles. Physiol Rev. 1991 Jan;71(1):155–234. doi: 10.1152/physrev.1991.71.1.155. [DOI] [PubMed] [Google Scholar]
- McEwen B. S., De Kloet E. R., Rostene W. Adrenal steroid receptors and actions in the nervous system. Physiol Rev. 1986 Oct;66(4):1121–1188. doi: 10.1152/physrev.1986.66.4.1121. [DOI] [PubMed] [Google Scholar]
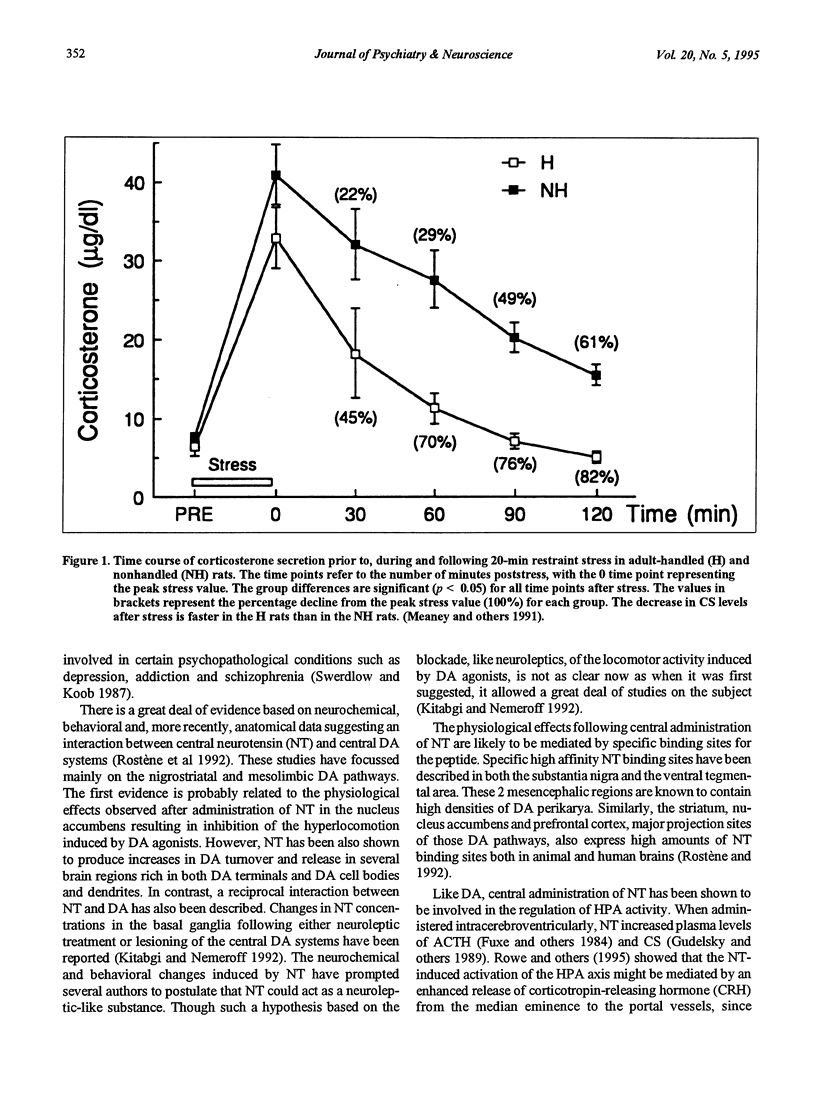
- Meaney M. J., Diorio J., Francis D., LaRocque S., O'Donnell D., Smythe J. W., Sharma S., Tannenbaum B. Environmental regulation of the development of glucocorticoid receptor systems in the rat forebrain. The role of serotonin. Ann N Y Acad Sci. 1994 Nov 30;746:260-73; discussion 274, 289-93. doi: 10.1111/j.1749-6632.1994.tb39243.x. [DOI] [PubMed] [Google Scholar]
- Meaney M. J., Mitchell J. B., Aitken D. H., Bhatnagar S., Bodnoff S. R., Iny L. J., Sarrieau A. The effects of neonatal handling on the development of the adrenocortical response to stress: implications for neuropathology and cognitive deficits in later life. Psychoneuroendocrinology. 1991;16(1-3):85–103. doi: 10.1016/0306-4530(91)90072-2. [DOI] [PubMed] [Google Scholar]
- Meaney M. J., Viau V., Aitken D. H., Bhatnagar S. Stress-induced occupancy and translocation of hippocampal glucocorticoid receptors. Brain Res. 1988 Mar 29;445(1):198–203. doi: 10.1016/0006-8993(88)91093-1. [DOI] [PubMed] [Google Scholar]
- Mitchell J. B., Rowe W., Boksa P., Meaney M. J. Serotonin regulates type II corticosteroid receptor binding in hippocampal cell cultures. J Neurosci. 1990 Jun;10(6):1745–1752. doi: 10.1523/JNEUROSCI.10-06-01745.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moga M. M., Saper C. B. Neuropeptide-immunoreactive neurons projecting to the paraventricular hypothalamic nucleus in the rat. J Comp Neurol. 1994 Aug 1;346(1):137–150. doi: 10.1002/cne.903460110. [DOI] [PubMed] [Google Scholar]
- Munck A., Guyre P. M., Holbrook N. J. Physiological functions of glucocorticoids in stress and their relation to pharmacological actions. Endocr Rev. 1984 Winter;5(1):25–44. doi: 10.1210/edrv-5-1-25. [DOI] [PubMed] [Google Scholar]
- Murphy B. E. Steroids and depression. J Steroid Biochem Mol Biol. 1991 May;38(5):537–559. doi: 10.1016/0960-0760(91)90312-s. [DOI] [PubMed] [Google Scholar]
- Reul J. M., de Kloet E. R. Two receptor systems for corticosterone in rat brain: microdistribution and differential occupation. Endocrinology. 1985 Dec;117(6):2505–2511. doi: 10.1210/endo-117-6-2505. [DOI] [PubMed] [Google Scholar]
- Rostène W., Brouard A., Dana C., Masuo Y., Agid F., Vial M., Lhiaubet A. M., Pelaprat D. Interaction between neurotensin and dopamine in the brain. Morphofunctional and clinical evidence. Ann N Y Acad Sci. 1992;668:217–231. doi: 10.1111/j.1749-6632.1992.tb27352.x. [DOI] [PubMed] [Google Scholar]
- Rothschild A. J., Langlais P. J., Schatzberg A. F., Miller M. M., Saloman M. S., Lerbinger J. E., Cole J. O., Bird E. D. The effects of a single acute dose of dexamethasone on monoamine and metabolite levels in rat brain. Life Sci. 1985 Jul 1;36(26):2491–2501. doi: 10.1016/0024-3205(85)90145-6. [DOI] [PubMed] [Google Scholar]
- Rotsztejn W. H., Normand M., Lalonde J., Fortier C. Relationship between ACTH release and corticosterone binding by the receptor sites of the adenohypophysis and dorsal hippocampus following infusion of corticosterone at a constant rate in the adrenalectomized rat. Endocrinology. 1975 Jul;97(1):223–230. doi: 10.1210/endo-97-1-223. [DOI] [PubMed] [Google Scholar]
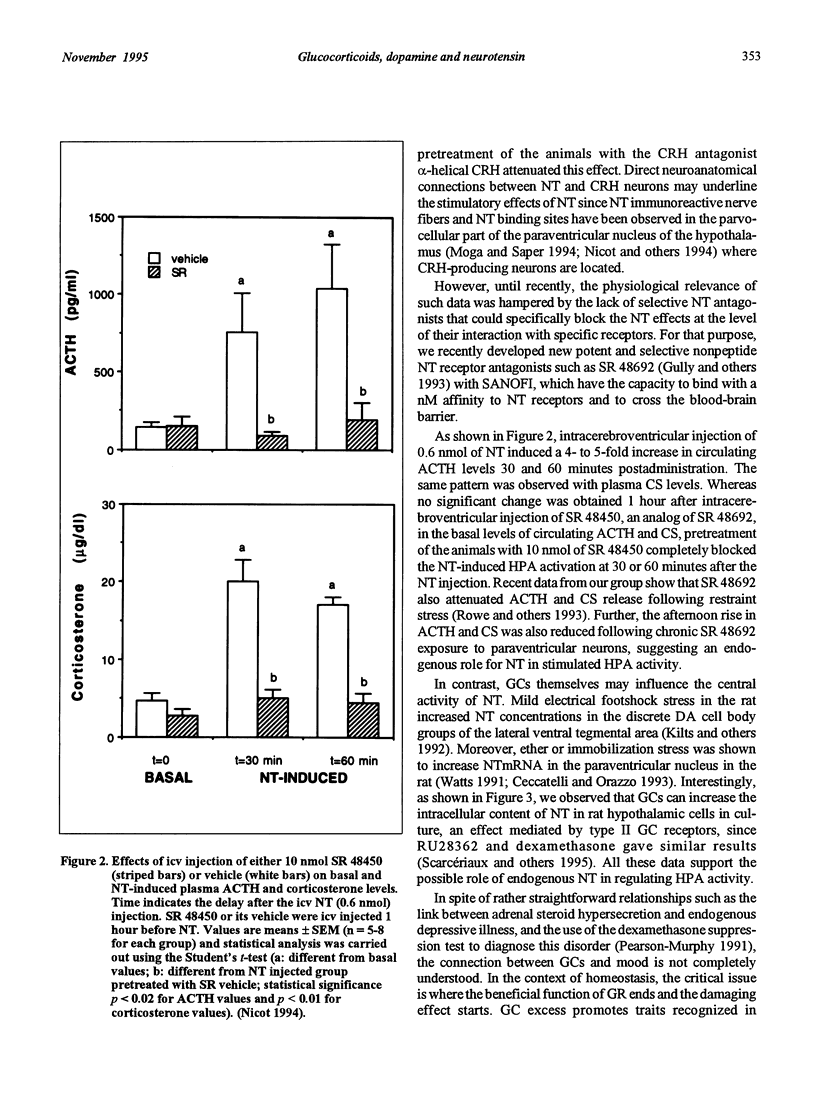
- Rowe W., Viau V., Meaney M. J., Quirion R. Stimulation of CRH-mediated ACTH secretion by central administration of neurotensin: evidence for the participation of the paraventricular nucleus. J Neuroendocrinol. 1995 Feb;7(2):109–117. doi: 10.1111/j.1365-2826.1995.tb00673.x. [DOI] [PubMed] [Google Scholar]
- Sapolsky R. M. Glucocorticoids, hippocampal damage and the glutamatergic synapse. Prog Brain Res. 1990;86:13–23. doi: 10.1016/s0079-6123(08)63163-5. [DOI] [PubMed] [Google Scholar]
- Sarrieau A., Dussaillant M., Agid F., Philibert D., Agid Y., Rostene W. Autoradiographic localization of glucocorticosteroid and progesterone binding sites in the human post-mortem brain. J Steroid Biochem. 1986 Nov;25(5B):717–721. doi: 10.1016/0022-4731(86)90300-6. [DOI] [PubMed] [Google Scholar]
- Sarrieau A., Dussaillant M., Moguilewsky M., Coutable D., Philibert D., Rostène W. Autoradiographic localization of glucocorticosteroid binding sites in rat brain after in vivo injection of [3H]RU 28362. Neurosci Lett. 1988 Sep 23;92(1):14–20. doi: 10.1016/0304-3940(88)90734-3. [DOI] [PubMed] [Google Scholar]
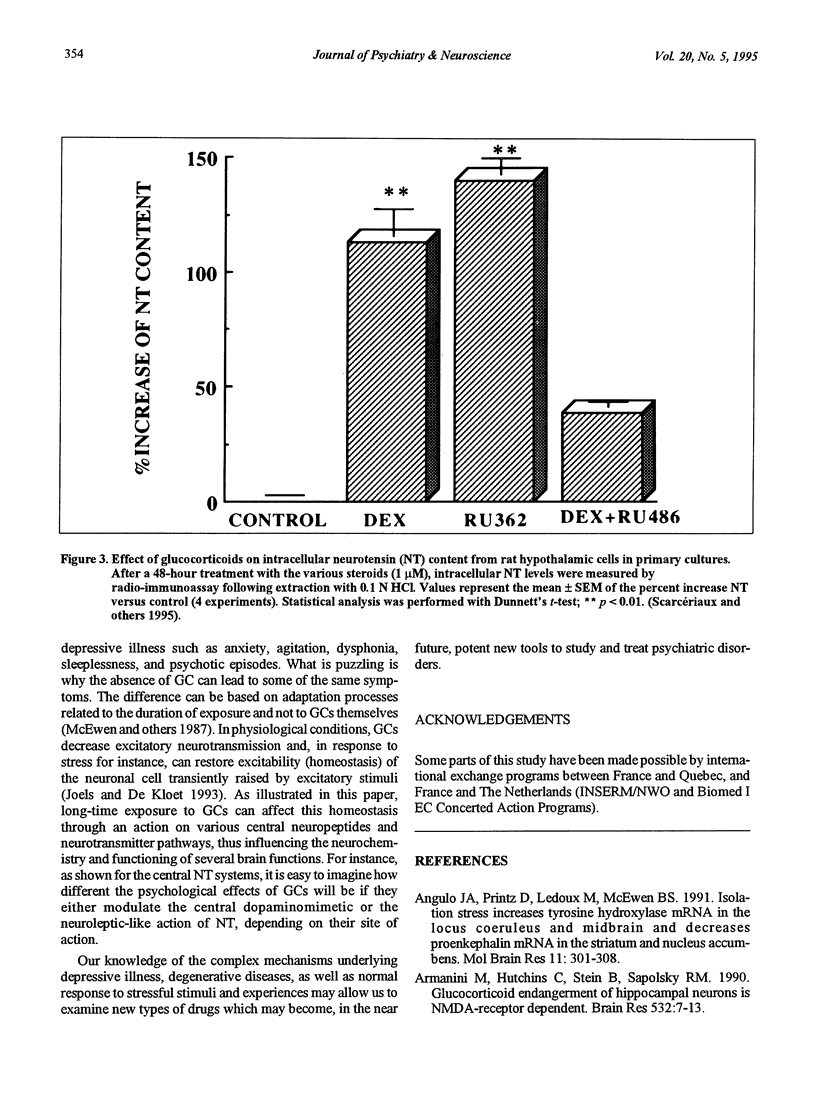
- Scarcériaux V., Pélaprat D., Forgez P., Lhiaubet A. M., Rostène W. Effects of dexamethasone and forskolin on neurotensin production in rat hypothalamic cultures. Endocrinology. 1995 Jun;136(6):2554–2560. doi: 10.1210/endo.136.6.7750477. [DOI] [PubMed] [Google Scholar]
- Versteeg D. H., Van Zoest I., De Kloet E. R. Acute changes in dopamine metabolism in the medio-basal hypothalamus following adrenalectomy. Experientia. 1984 Jan 15;40(1):112–114. doi: 10.1007/BF01959132. [DOI] [PubMed] [Google Scholar]
- Virgin C. E., Jr, Ha T. P., Packan D. R., Tombaugh G. C., Yang S. H., Horner H. C., Sapolsky R. M. Glucocorticoids inhibit glucose transport and glutamate uptake in hippocampal astrocytes: implications for glucocorticoid neurotoxicity. J Neurochem. 1991 Oct;57(4):1422–1428. doi: 10.1111/j.1471-4159.1991.tb08309.x. [DOI] [PubMed] [Google Scholar]
- Watts A. G. Ether anesthesia differentially affects the content of prepro-corticotropin-releasing hormone, prepro-neurotensin/neuromedin N and prepro-enkephalin mRNAs in the hypothalamic paraventricular nucleus of the rat. Brain Res. 1991 Mar 29;544(2):353–357. doi: 10.1016/0006-8993(91)90080-f. [DOI] [PubMed] [Google Scholar]
- Wolkowitz O. M. Prospective controlled studies of the behavioral and biological effects of exogenous corticosteroids. Psychoneuroendocrinology. 1994;19(3):233–255. doi: 10.1016/0306-4530(94)90064-7. [DOI] [PubMed] [Google Scholar]


