Abstract
Background
Notch signalling plays a crucial role in many developmental, homoeostatic and pathological processes in metazoans. The pathway is activated by binding of the ligand to the Notch receptor, which changes the conformation of the receptor by exerting a pulling force. The pulling force is generated by the endocytosis of the interacting ligand into the signal-sending cell. Endocytosis of ligands requires the action of the E3 ligases Mindbomb1 (Mib1) and Neuralized (Neur) that ubiquitylate lysines (Ks) of their intracellular domains. It has been shown that human MIB1 binds JAGGED1 (JAG1) via a bipartite binding motif in its ICD. This interaction is required for the activation of JAG1. However, it is not known whether this bipartite binding mode is of general importance. It is also not rigorously tested whether it occurs in vivo. Moreover, it is not known whether Mib1 ubiquitylates specific Ks in the ICD of ligands, or is rather non-selective.
Results
We therefore investigated how Mib1 interacts with the Notch ligand Delta of Drosophila in an in vivo trans-activation assay and determined the Ks which are required for signalling. We show that the activation of Dl by Mib1 follows similar rules as has been found for mammalian MIB1 and JAG1. We present evidence that a combination of six Ks of the ICD is required for the full signalling activity of Dl by Mib1, with K742 being the most important one.
Conclusions
Altogether, our analysis further reveals the rules of Mib1-mediated DSL-ligand-dependent Notch-signalling.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12915-025-02162-6.
Keywords: Notch-pathway, Delta, DSL-ligands, Endocytosis, Mindbomb1, Ubiquitylation, Cis-inhibition
Background
Juxtacrine Notch signalling plays a critical role in many developmental, homoeostatic and pathological processes in metazoans [1]. It is initiated by transmembrane proteins of the DSL protein family as ligands and requires endocytosis of these ligands bound to the Notch receptor in trans (reviewed in [2]). This activating endocytic event depends on the ubiquitin binding endocytic adapter Epsin and creates a pulling force that forces a conformational change in Notch. The conformational change exposes a cleavage site for the metalloprotease ADAM10, encoded by kuzbanian (kuz) in Drosophila. The S2-cleavage by Kuz sheds the extracellular domain, which is endocytosed with the bound ligand into the signal-sending cell (reviewed in [3]). The result of S2-cleavage induced ecto-domain shedding is a membrane inserted truncated Notch molecule, termed Notch EXtracellular Truncation (NEXT), which undergoes rapid intramembranous cleavage by y-secretase (S3-cleavage). The released intracellular domain (NICD) travels into the nucleus, associates with the CSL transcription factor Suppressor of Hairless (Su(H)) to initiate the expression of the target genes of the pathway.
One major way to initiate endocytosis of the ligands is the ubiquitylation (ubi) of lysines (Ks) in their intracellular domains (ICDs) by E3 ligases (reviewed in [2, 4]). Two E3-ligases have been identified that ubiquitylate the Drosophila DSL-ligands Serrate (Ser) and Delta (Dl) during Notch signalling, termed Mindbomb1 (Mib1) and Neuralized (Neur) [5]. Both are necessary in complementary Notch-dependent processes for the full signalling activity of the ligands. Dl directly binds Mib1 and Neur at different sites of its ICD and is ubiquitylated by these two E3s on Ks [6, 7]. For the mammalian Ser ortholog JAGGED1 (JAG1), the binding mode of MIB1 has been determined at the atomic level. It binds to two short epitopes in the ICD of JAG1, termed N- and C-box (NB and CB, respectively) with its N-terminal MZM and REP domains, respectively [7]. This bipartite binding mode is required for the full function of JAG1 in cell culture assays and in Drosophila in vivo experiments [7].
Corresponding boxes, termed ICD2 (= NB) and ICD3 (= CB), have been identified in the ICD of Dl and it has been shown that also the NB/ICD2 of Dl binds to the MZM domain of MIB1 in vitro and is important for the in vivo function of Dl [6, 7] (Fig. 1G). The NB/ICD2 appears to constitute the major binding site of Dl, since a Dl-variant that lacks the NB could not be co-immunoprecipitated with Mib1 [6]. It appears that, in contrast to JAG1, the CB (ICD3) is not required for Mib1-dependent signalling of Dl, although it is conserved among insect species [6]. Thus, it is not clear whether the bipartite binding mode determined for the interaction between MIB1 and JAG1 applies to the interaction of Mib1 with other ligands.
Fig. 1.
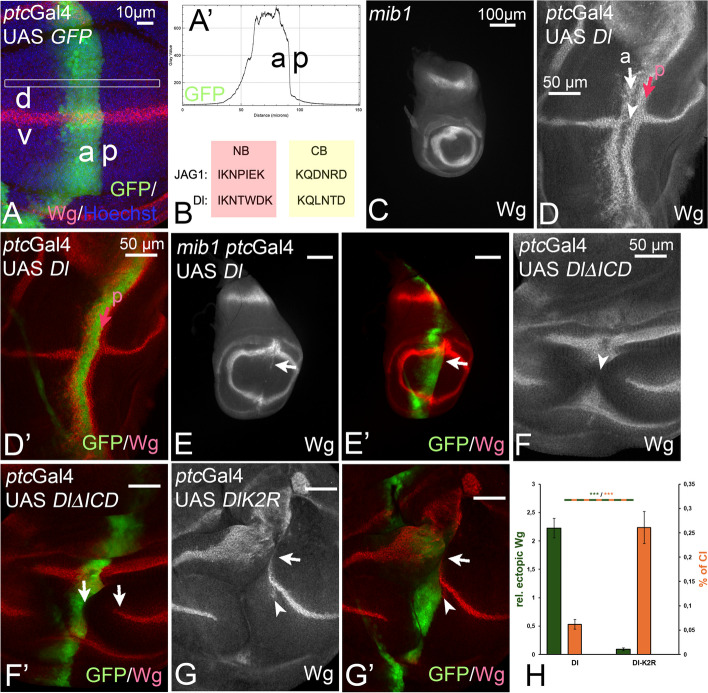
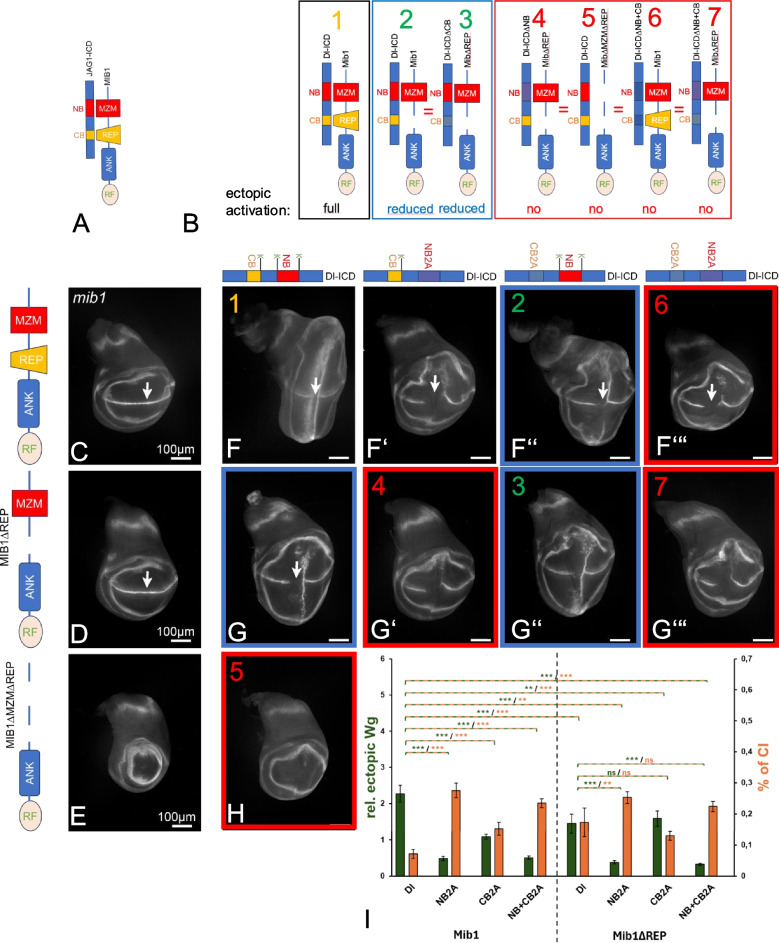
Effects of expression of DlΔICD and DlK2R during wing development. A The constructs are expressed with ptcGal4, which drives expression in a stripe along the anterior side (a) of the A/P-boundary of the wing imaginal disc. Expression in the wing disc is perpendicular to the D/V-boundary (d, v) along which the Notch target gene wg is expressed. A’ Expression is graded within the ptcGal4 domain, increasing from anterior to the A/P-boundary. The gradient is measured by the pixel intensity of the GFP-signal in the region boxed in A. B The sequence comparison of the NB and CB of JAG1 with Dl. C Expression of Wg in mib1 mutant discs. The expression along the D/V-boundary is lost and the wing area is dramatically reduced. D, D’ Ectopic expression of Dl with ptcGal4 in wildtype discs results in the induction of two stripes of ectopic Wg expression running perpendicular to the endogenous expression along the D/V-boundary. One anterior broader stripe (arrow, a) and a thinner stripe located in the posterior boundary cells (red arrow, p). At the intersection with the D/V-boundary, the endogenous expression of Wg is cell-autonomously suppressed in the domain of high expression due to CI (arrowhead). The cell-autonomy of CI is revealed by the expression of wg directly adjacent to the posterior boundary of the ptcGal4 domain (red arrow). E, E’ Expression of Dl in a mib1-mutant wing disc results in a very weak activation of Wg in the dorsal compartment of the wing area (arrow), confirming that Dl can signal weakly in the absence of Mib1 function, but requires Mib1 function for its full activity (compare with C, D, D’). F, F’ Expression of DlΔICD causes a large gap in the endogenous expression of Wg (arrowhead in F). A gap can be observed between the posterior expression boundary of the ptcGal4 domain and the endogenous expression of Wg (arrows in F’). This non-cell autonomous suppression of Wg expression indicates that DlΔICD acts in a dominant-negative manner. No ectopic expression of Wg is induced, indicating the activity of Dl is abolished if the ICD is deleted. G, G’ Expression of DlK2R results in a suppression of the endogenous expression of Wg. Note that this effect is restricted to the domain of expression and accompanied by weak ectopic activation of Wg in posterior boundary cells in the dorsal compartment (arrow and arrowhead). This indicates that DlK2R is weakly active and possess stronger cis-inhibitory abilities than Dl, indicated by the larger gap in the endogenous expression domain of Wg (arrow and arrowhead) [8]. H Quantification of the signalling activity and CI of Dl and DlK2R (see Fig. 2 and Methods for more details). It confirms that the loss of the Ks results in a dramatic reduction in signalling, accompanied by a strong increase in CI
In the wing imaginal disc of Drosophila, Mib1 is expressed ubiquitously, while Neur is restricted to single, late arising single sensory organ precursor cells (SOPs) of the peripheral nervous system. Thus, by far the most DSL-signalling (e. g. during wing development) is mediated by Mib1. The loss of function of mib1 in the wing disc results in a strong reduction of Notch activity, which causes the loss of most parts of the wing anlage and the loss of expression of wing-specific target genes, such as wingless (wg) along the dorso-ventral (D/V)-compartment boundary [9–11].
Besides the productive interaction of Notch and Dl in trans, both proteins are also engaged in cis-interactions in the same cell, which cause the cell-autonomous suppression of Notch signalling. This phenomenon is termed cis-inhibition (CI) and is exploited in several developmental processes to regulate the signalling activity of the pathway and enables directional signalling [12–14]. CI inversely correlates with the efficiency of a ligand to be endocytosed [8, 15, 16].
We have previously tested the requirement of ubi for the function of Dl by replacing all its Ks of its ICD by the structurally similar arginine (R, DlK2R) [8, 16]. DlK2R cannot be ubiquitylated by Mib1 and Neur [6, 8]. By analysing the activity of DlK2R, we found that Dl-signalling can be activated in three different ways: one completely independently of ubi and both E3-ligases, one dependent on Neur, but independent of ubi and one dependent on Neur- and Mib1-mediated ubi [8, 16]. During signalling events, the three ways add up to the full activity of Dl. By analysing a DlK2R knock-in allele (DlattP-DlK2R), we recently found that the two ubi-independent modes combined allow the complete development of Drosophila [16]. However, the adult DlattP-DlK2R flies displayed Notch-related defects, indicating the requirement of Mib1-mediated ubi for the full function of Dl. The previous work also indicates that, in contrast to Neur, Mib1 activates Dl solely by ubi of its ICD on Ks [8, 16].
In general, E3-ligases ubiquitylate the Ks of its substrates either non-selectively or selectively on distinct Ks [17]. In the case of mouse Delta-like1 (Dll1), a single K, K613, has been identified as the major site of ubi in cell culture experiments, suggesting that MIB1 is highly selective [18]. However, previous attempts to identify individual Ks in the ICD of Drosophila Dl relevant for its activation by Mib1 failed [6]. Moreover, the deletions of individual conserved Ks did not have a measurable impact on ubi of the ligand by Neur and Mib1 [6].
Here, we present the results of our analysis that further investigated the activation of Dl by Mib1. We show that, besides the NB, also the CB predicted in Dl is involved in signalling in vivo. We provide in vivo evidence that the MZM and REP domains of Drosophila Mib1 bind to the NB and CB of Dl, respectively. Thus, the activation of Dl by Mib1 follows similar rules as determined for the interaction between human MIB1 and JAG1. Moreover, we show that a combination of six Ks in the ICD is required for the full activation of Dl by Mib1, with K742 being the most important one. Altogether, our analysis reveals the rules of Mib1-mediated DSL/Notch signalling.
Results
For the initial analysis, we used the Gal4 expression system to ectopically express our generated Dl-variants. All constructs were inserted in the same landing site to neutralise position effects on their expression and guarantee comparable expression. patched-Gal4 (ptcGal4) induces expression of UAS constructs in a band of cells along the anterior side of the anterior–posterior compartment boundary (A/P-boundary) of the wing imaginal disc (Fig. 1A). The expression within the domain increases in a sigmoidal curve towards the posterior expression boundary, which is identical to the A/P-boundary (Fig. 1A’). Note that only Mib1 is present during wing development with the exception of single late arising expressing neural precursor cells that express Neur. Thus, the assay in the wing disc specifically addresses Mib1-mediated activation of Dl.
We used the expression of Wg as a read-out for Notch activity. It is expressed in a stripe that straddles the dorso-ventral compartment boundary (D/V-boundary) as a result of continuous Notch signalling (reviewed in [9]) (Fig. 1A). The loss of mib1 function results in the loss of Wg expression along the D/V-boundary (Fig. 1C). Ectopic expression of Dl with ptcGal4 in wildtype discs results in the induction of ectopic expression of the target gene wg in two stripes perpendicular to its normal domain along the D/V-boundary [12, 19] (Fig. 1D, D’). As previously reported, its expression with ptcGal4 interrupted the endogenous expression of Wg along the D/V-boundary not only at the point of intersection with the ptcGal4 domain, but also in adjacent non-expressing posterior cells [20] (Fig. 1D, D’, arrowhead and arrows, respectively). Expression of Dl in mib1 mutant discs induced only a very weak short stripe of Wg in a non-penetrant manner, indicating the requirement of Mib1 for signalling of Dl in wing discs (Fig. 1E, E’, arrow). We first confirmed the meaning of the ICD of Dl by generating a variant, DlΔICD-HA, which lacks most of the ICD, but is still inserted in the plasma membrane. This non-cell autonomous behaviour indicates that DlΔICD-HA is a dominant-negatively acting variant. The dominant-negative behaviour is different from the cis-inhibitory suppression of the expression of Wg by active Dl-variants, e.g. Dl and also DlK2R, which is cell-autonomous, as it is restricted to the ptcGal4 expression domain [19, 21, 22] (Fig. 1F, F’, compare with 1D–E’). The different phenotype produced by DlΔICD-HA compared to Dl and DlK2R-HA highlights the importance of the ICD for the function of Dl and also confirms that Dl can weakly signal in the absence of Ks in its ICD [8]. This ubi-independent signalling explains the finding that expression of Dl in mib1 mutants slightly induces the expression of Wg in a non-penetrant manner (Fig. 1E, E’, arrow) [8]. To quantify the effects of over-expression of the Dl-variants, we measured the length of the anterior and/or posterior ectopic Wg expression induced and divided it by the length endogenous wg expression along the D/V-boundary to account for the variation in disc size. Ten discs were measured for each genotype (Fig. 1H and subsequent figures). For the strength of CI, we measured the length of the gap within the endogenous expression of Wg along the D/V boundary and related it to the total length of the Wg domain. The quantification of the signalling properties and cis-inhibition confirmed that the signalling abilities of DlK2R were dramatically reduced, while its CI was strongly increased. This inverse correlation was observed previously and indicates that the Ks are important to adjust CI to the correct level [8, 16].
Dl has a functional CB required for Mib1-dependent activation
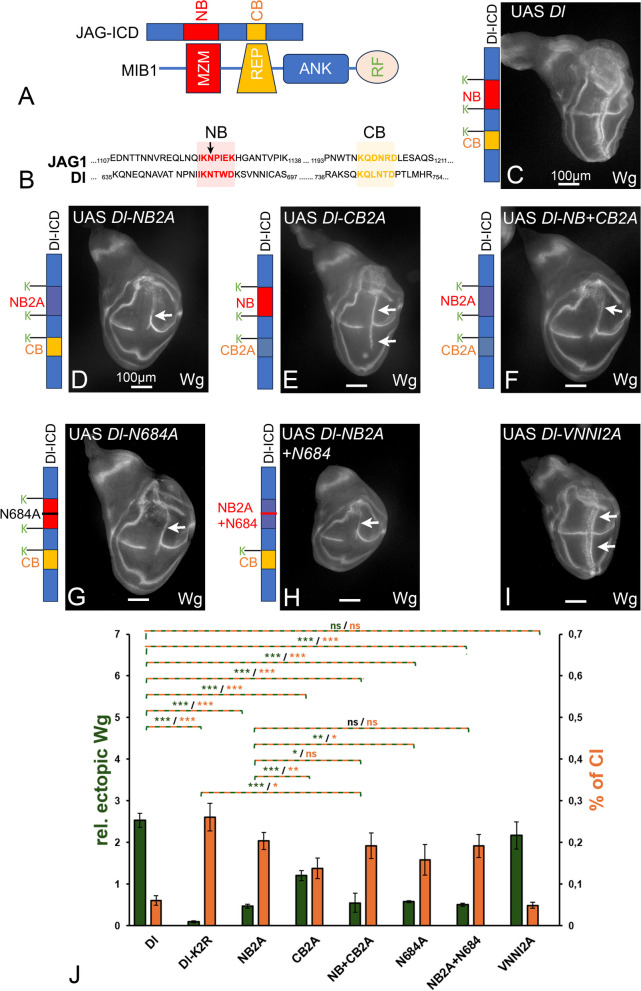
Previous work showed that bipartite binding of MIB1 (via its MZM and REP domains) to the NB and CB is required for the full activation of JAG1 [7] (Fig. 2A). However, the mutation of only the CB did not significantly affect MIB1-mediated JAG1/Notch signalling in cell culture experiments [7]. Moreover, previous experiments in Drosophila suggested that only the NB (ICD2) in Dl is required for Mib1-mediated Dl-signalling, although a sequence similar to the CB of JAG1, termed ICD3, is recognisable and conserved among insect Dl orthologs [6] (Figs. 1B, 2B and Additional file 1: Fig. S1). A caveat of the previous Drosophila work is that the conclusions were based on the analysis of random insertions of the Dl-variants in the genome. Thus, weak effects might have been missed due to the different expressivity of the constructs caused by position effects. In addition, the NB and CB were deleted in the tested variants. The deletions included strongly conserved Ks, which are important for the function of Dl because they might serve as ubiquitin adapters (see below). We therefore tested variants of Dl where the AAs of the NB, the predicted CB, or both boxes were replaced by alanine (A), but the conserved Ks were left in place. We found that the mutation of the NB (Dl-NB2A) resulted in a strong reduction of the activity of Dl expressed by ptcGal4 in wildtype discs. This is indicated by the reduced ectopic expression of Wg in comparison to Dl expression shown in Fig. 2C, D, quantification in J: only one (posterior) stripe is present, which is also dramatically reduced in length and largely restricted to the dorsal compartment (Fig. 2D, arrow). This confirms the meaning of the NB/ICD2 [6, 7]. Nevertheless, also the mutation of the predicted CB/ICD3 reduced the activity of Dl, indicated by the loss of the anterior stripe of ectopic Wg expression upon Dl-CB2A expression (Fig. 2E, arrows). However, the loss of activity of Dl-CB2A was significantly weaker than that of Dl-NB2A, indicated by the longer remaining posterior stripe reaching also into the ventral compartment (Fig. 2E, arrows, compare with C, quantification in J). This indicates that the CB is less important for the activity of Dl. This is in agreement with the cell culture experiments performed with JAG1 [7]. We also generated variants where the Ks flanking the boxes are mutated to alanine. These variants showed similar phenotypes (see Additional file 1: Fig. S2, compare with Fig. 2D, E).
Fig. 2.
The importance of the NB and CB for the activity of Dl. A Cartoon of the bipartite interaction mode between JAG1 and MIB1. The NB and CB of JAG1 interact with the MZM and REP domain of MIB1, respectively. B Sequence comparison of sections of the ICDs of JAG1 and Dl that include the NB/ICD2 (shaded in red) and the predicted CB/ICD3 (shaded in yellow). The arrow points to the conserved asparagine (N) in the NB. C Expression of Dl by ptcGal4 for comparison. D Expression of Dl-NB2A results in a weaker ectopic induction of Wg expression, largely restricted to the dorsal compartment (arrow). The anterior stripe is missing due to increased CI. E Likewise, expression of Dl-CB2A also induces weaker ectopic expression of Wg than Dl, indicating that it is required for the full activity of Dl. Note that expression of Dl-CB2A leads to a stronger Wg activation than Dl-NB2A (longer stripe of ectopic expression), indicating that the CB is less important. F The mutation of both boxes results in an ectopic induction of Wg expression comparable to Dl-NB2A (compare with D), indicating that the NB is more important for Dl function. G Dl-N684 induced ectopic expression of Wg comparable to Dl-NB2A, indicating its importance for the function of the NB. H Expression of Dl-NB2A with re-introduced N684 causes a phenotype that is similar to that caused by Dl-NB2A (compare with D). I The mutation of the NN-motif in the ICD (Dl-DNNI2A) has no effect on the activity of Dl (arrows, compare with B). J Quantification of the CI and transactivation abilities of the Dl-variants shown in C–I. CI was measured by dividing the length of the gap of endogenous Wg expression by the total length of the endogenous Wg expression along the D/V boundary. Ectopic activation of the Notch pathway was determined by measuring the length of the anterior and/or posterior ectopic Wg expression and dividing it by the endogenous wg expression along the D/V boundary. ‘relative ectopic Wg’ displays the extend of ectopic Wg expression, while ‘% of CI’ shows the % of interruption of the endogenous Wg expression domain. Ten discs were measured for each genotype. p < 0.05 = *; p < 0.01 = **; p < 0.001 = ***
The combined mutation of both boxes caused a phenotype similar to the loss of only the NB, confirming that the NB is the prevailing binding epitope for Mib1 and that the CB largely depends on the presence of the NB (Fig. 2F, compare with D, quantification in J). This is in line with the previous cell culture experiments which showed that the reduction in activity of JAG1 signalling is similar, if the NB alone, or the NB and CB are mutated together [7]. It has been shown that a peptide containing the NB of Dl can bind to the MZM domain of MIB1 and that the deletion of the NB of Dl abolishes the binding to Mib1 in a co-immunoprecipitation assay [6, 7]. Combined with our results here, it suggests a scenario in which Mib1 would initially contact the Dl-ICD via binding to the NB with its MZM domain and then strengthen the association by binding to the CB with its REP domain. In this scenario, the CB is not sufficient for efficient Mib1 binding in the absence of the CB.
The quantification of the signalling activity and the extent of CI shown in Fig. 2J highlights the negative correlation between CI and signalling activity of the Dl variants, which we have previously also observed with the DlK2R variant [8, 16]. It further indicates that suppression of CI and signalling inversely depends on the presence of the NB and/or CB and therefore probably on the strength of the interaction with Mib1: strong interaction with Mib1 results in strong signalling and weak CI, while weak interaction decreases signalling and increases CI. Importantly the quantification also revealed that DlK2R is less active than Dl-NB + CB2A, since DlK2R displays less signalling activity, but tends to have more CI (Fig. 2J).
N684 of the N-box is important for the interaction of Dl with Mib1
The determined atomic structure suggests that in the NB of JAG1, a centrally located asparagine (N1124) is pivotal in the interaction with the MZM domain of MIB1 [7]. The corresponding asparagine in the NB of Dl is N684 (Fig. 2B, arrow). To determine the importance of N684 for the Mib1-induced activity of Dl in vivo, we replaced it by alanine. We found that the activity of Dl-N684A is similar to that of Dl-NB2A, indicating its importance for function of the NB (Fig. 2G, compare with D, arrows, quantification in J). However, the individual re-introduction of N684 in Dl-NB2A fails to increase the activity of Dl-NB2A, indicating that, although N684 is essential, other AAs in the N-box contribute to the binding of Dl to Mib1 (Fig. 2H, compare with D, quantification in J). This indicates the NB of Dl with the MZM domain of Mib1 in Drosophila follows similar rules than that previously found for that between JAG1 and MIB1. Altogether, the results indicate that, similar to JAG1, Dl possesses similar functional N- and C-boxes, which are both required for its full activity. They also indicate that the NB is the prevailing binding epitope for Mib1-induced Dl-signalling.
Analysis of the ICD of the Dl-ortholog DeltaD of the zebrafish (Danio rerio) identified an additional conserved di-asparagine (NN) motif which is relevant for enhancing the signalling by the NB [23]. A similar motif is present in Dl orthologs of several insect species, although located more C-terminally [6]. We mutated the corresponding VNNI sequence to A to test its significance for the function of Dl. We found that this change had no detectable effect on the signalling activity of Dl (Dl-VNNI2A, Fig. 2I, compare with C, quantification in J). Thus, the NN-motif appears to be dispensable for Mib1-mediated activation of Dl.
The importance of the NB and CB of Dl for the development of Drosophila
To further confirm the function of the NB and CB of Dl at the organismal level, we generated Dl-knock-in alleles, which encode variants with the NB, or CB, or both mutated to A. For this purpose, we used the DlattP-allele where most of the Dl coding sequence (exon 6) is replaced by an attP-landing site to generate knock-in alleles encoding Dl-variants with mutated NB, CB and both boxes [24]. As in the constructs used for Gal4 over-expression experiments, the Ks flanking the binding domains were left in place.
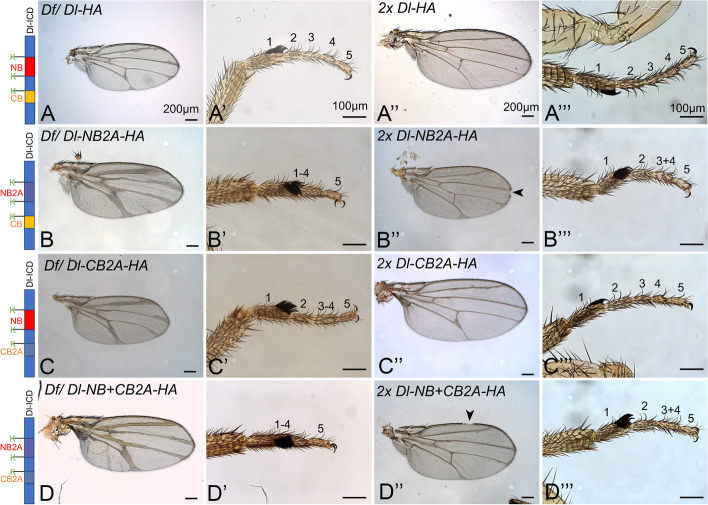
We first tested whether the addition of the HA-tag (which does not contain Ks) to the C-terminus had an effect on the activity of Dl (Additional file 1: Fig. S3). For this purpose, we compared the phenotype of DlattP-HA-Dl, a variant with the HA inserted in the extracellular domain close to the membrane, with our previously generated C-terminally tagged DlattP-Dl-HA [16]. We asked whether the presence of just one copy of the Dl-alleles in the genome can rescue Drosophila development, as has been observed for the previously characterised DlattP-Dl-HA and DlattP-DlK2R-HA [16]. For this purpose, we monitored the phenotype of the alleles over a chromosomal deficiency of Dl, Df(3R) DlBSC850 (Df). Both differently tagged Dl-variants, DlattP-HA-Dl and DlattP-Dl-HA, rescued the Dl-mutant neurogenic phenotype in a comparable manner, allowing the development to the adult stage. As expected for fully functional alleles, the flies displayed the characteristic haplo-insufficient phenotype of Dl (Additional file 1: Fig. S3A–B’, compare with Fig. 3A, A’). This indicates that the addition of the HA-tag at the C-terminus does not significantly affect the activity of Dl.
Fig. 3.
Adult phenotypes of DlattP-knock-in alleles encoding Dl-variants with NB, CB or NB + CB mutated over the deficiency Df(3R) DlBSC850 (Df) and in homozygosity. All variants contain the Ks flanking the boxes, to exclude effects caused by the absence of crucial ubi acceptors. A, A’ Dl-HA/Df results in a haplo-insufficient phenotype with strong broadening of wing veins. The tarsal region consisting of 5 segments has a wildtype appearance. A’’, A’’’ Two copies of Dl-HA restore the WT phenotype of the wing and leg. B, B’ The phenotype of one copy of Dl-NB2A/Df flies. The wing veins are broadened (B) and the tarsal segments 1–4 are fused (B’). B’’, B’’’ Phenotype of homozygous Dl-NB2A flies. The wing vein broadening and tarsal segment fusion phenotypes are less severe. The arrowhead points to a small Notch in the wing margin. C, C’ The mutation of the CB causes a milder vein and leg phenotype. C’’, C’’’ In homozygosity of Dl-CB2A, only weak wing and leg defects. D, D’ Simultaneous mutation of the NB and CB causes developmental defects similar to Dl-NB2A (compare with B–B’’’). The arrowhead points to a small notch in the wing margin
Having confirmed the functionality of the C-terminal HA-tagged DlattP-variant, we analysed the activity of DlattP-Dl-NB2A-HA, DlattP-Dl-CB2A-HA and DlattP-Dl-NB + CB2A-HA. We found that already one copy over the deficiency provided sufficient activity to prevent the embryonic neurogenic phenotype characteristic for Dl mutants and allowed the development to the adult stage in all cases (Fig. 3). However, the phenotypes of the alleles differed and were stronger than the haplo-insufficient Dl-phenotype (compare Fig. 3A, A’, B, B’, C, C’). The phenotype of DlattP-Dl-NB2A-HA/Df was stronger than that of DlattP-Dl-CB2A-HA/Df, indicated by the stronger broadening of the wing veins and fusion of more tarsal segments (Fig. 3B, B’, C, C’). Moreover, the phenotype of DlattP-Dl-NB + CB2A-HA/Df flies was comparable to that of DlattP-Dl-NB2A-HA/Df, confirming the prime importance of the NB for Mib1-dependent Dl-signalling, which was also found in the over-expression experiments (compare Fig. 3D, D’ with B, B’).
As previously reported, homozygous DlattP-Dl-HA flies have a wildtype appearance, indicating that it is a fully functional (wildtype) allele [16] (Fig. 3A’’–A’’’). In contrast, homozygosity of DlattP-DlK2R leads to a stronger phenotype due to its stronger cis-inhibitory abilities [16]. The phenotype of DlattP-Dl-NB + CB2A-HA, DlattP-Dl-NB2A-HA and DlattP-Dl-CB2A-HA weakened in homozygosity, indicating that they are not as cis-inhibitory than DlK2R-HA (Fig. 3B–D’’’). Nevertheless, the phenotype of homozygous DlattP-Dl-CB2A-HA flies was weaker than that of DlattP-Dl-NB2A-HA and DlattP-Dl-NB + CB2A-HA, while the phenotype of homozygous DlattP-Dl-NB2A-HA and DlattP-Dl-NB + CB2A-HA flies was very similar, again highlighting the importance of the NB (compare Fig. 3C’’, C’’’ with B’’, B’’’ and D’’ and D’’’).
Transport of Dl to the plasma membrane does not depend on its ICD
We were interested in the general importance of ICD of Dl at the organismal level. Therefore, we generated and analysed the knock-in allele DlattP-DlΔICD-HA, which encodes a variant that lacks most of its ICD (12 amino acids long ICD, the Ks are replaced by Rs; see Additional file 1: Fig. S4). In addition, the lysines in its residual ICD are exchanged to arginines. As expected, this allele is not functional, indicated by its lethality in homozygosity. However, anti-HA-staining of heterozygous wing discs revealed that DlattP-DlΔICD-HA was expressed in the correct pattern (Additional file 1: Fig. S4A, A’). The comparison with Dl::GFP, a genome edited fully functional allele of Dl [25], also showed that it was present at the apical membrane together with Dl::GFP (Additional file 1: Fig. S4B–C’’, white arrow in C’, C’’). In addition, it was also mis-localised in the baso-lateral membrane domain, where Dl::GFP was largely absent (Additional file 1: Fig. S4C’’, arrowhead). This finding shows that the transport of Dl to the plasma membrane (exocytosis) does not require its ICD. Importantly, DlΔICD-HA was basically absent from Dl::GFP positive intracellular punctae (Additional file 1: Fig. S4C–C’’, red arrow). These punctae were identified to be endosomes [8, 26, 27] (Fig. 4C–C’’’’’). Combined, these findings indicate that endocytosis of DlΔICD-HA is strongly impaired. The findings are in agreements with previous reports that show that a similar Dl-variant without ICD, DlΔC, accumulates in the membrane as a result of impaired endocytosis [20, 26]. Thus, the ICD of Dl is important for efficient endocytosis, but not exocytosis.
Fig. 4.
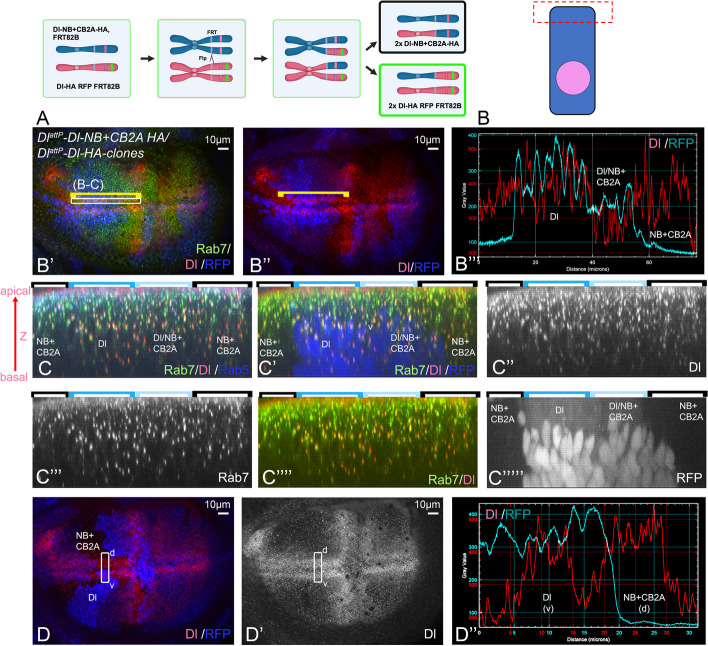
Comparison of the endocytic behaviour of Dl-NB + CB2A-HA with DlK2R-HA. A Cartoon describing the generation of the adjacently located homozygous clones by Flp/FRT-mediated clonal analysis (mitotic recombination). The mitotic recombination in G2 results in the free segregation of the replicated mutant alleles because of their localisation on different centromeres. The result is homozygous founder cells for each genotype in 25% of the cases. B–B’’ Comparison of the apical membrane localisation of the Dl-variants in cells of clones homozygous for DlattP-Dl-NB + CB2A-HA (absence of RFP) and DlattP-Dl-HA (homozygous for RFP) detected with a Dl antibody directed against the extracellular domain. B’’’ Pixel intensity plot of the RFP channel which identifies the clones and the Dl channel. The yellow bracket highlight the region of measurement adjacent to the D/V-boundary where Dl is expressed in a continuous stripe from anterior to posterior at the same level in wildtype discs. It includes regions homozygous for both Dl alleles. No significant difference is observed in the apical levels of Dl in DlattP-Dl-NB + CB2A-HA and DlattP-Dl-HA homozygous cells, indicating that both Dl variant are present in the apical membrane at comparable levels. C–C’’’’ Z section of the region of Dl expression highlight in B’ with the yellow bracket. It reveals that intracellular Dl and Dl-NB + CB2A are located in Rab7- and Rab5-positive endosomes. D–D’’ Another example of the correct location of and levels of Dl-NB + CB2A in the apical membrane. Here the Dl-homozygous clone is located in the ventral stripe of Dl and the Dl-NB + CB2A-homozygous clone in the dorsal stripe. The pixel density plot shown in D’’ confirms the similarity of the levels of the two Dl-variants in the apical membrane domain of the cells
The previous work also indicated that DlΔC acts in a dominant-negative fashion upon over-expression [20, 26]. Therefore, we were surprised to obtain the DlattP-DlΔICD-HA allele, since we expected that its expression, albeit at the endogenous level, would lead to lethality in heterozygosity and therefore of the initial transformants. Indeed, our clonal analysis revealed that DlattP-DlΔICD-HA is cell lethal, indicated by the presence of only wildtype orphan clones (Additional file 1: Fig. S4D, D’, arrows). Moreover, we found that DlΔICD-HA/Dl-A cells accumulated higher HA-levels in the apical plasma membrane than homozygous Dl-HA cells (Additional file 1: Fig. S4 D’’–F). In order to further investigate the activity of DlattP-DlΔICD-HA, we ectopically expressed the glycosyltransferase Fringe by ptcGal4. In the wildtype, this expression results in the ectopic activation of the Notch pathway at its posterior expression boundary, indicated by the ectopic expression of Wg (Additional file 1: Fig. S4G). The induction of the ectopic activity of the Notch-pathway requires the signalling of both ligands, Dl and Ser [12, 28, 29]. However, it is initiated by Dl, also indicated by the finding that the length of the ectopic stripe of Wg is reduced in Dl-heterozygous compared to wildtype discs (Additional file 1: Fig. S4G, G’, arrow, quantification in H) [29]. We found that this stripe is further shortened in discs with one wildtype copy and one copy of DlattP-DlΔICD-HA (Additional file 1: Fig. S41G’’, arrow, quantification in H). This result suggests that the presence of DlattP-DlΔICD-HA has a negative effect on the activation of the Notch pathway by the remaining copy of wildtype Dl, as it is expected from a dominant-negative acting variant. It appears that the presence of the wildtype copy in DlattP-DlΔICD-HA/ + flies can buffer the dominant-negative effect of DlattP-DlΔICD-HA. This buffering effect allows the heterozygous DlattP-DlΔICD-HA/ + flies to survive.
Endocytosis of Dl-NB + CB2A is not significantly affected
We here and previously found that the ability of Dl to cis-inhibit is inversely correlated with its endocytosis efficiency [16]. To investigate the efficiency of endocytosis of DlattP-Dl-NB + CB2A-HA relative to Dl and DlK2R, we used clonal analysis to generate cell clones that only express DlattP-Dl-NB + CB2A-HA adjacent to clones that express either only DlattP-Dl-HA (Fig. 4A). This enabled us to directly compare the subcellular localisation of the variants in wing disc cells. We previously reported that clones homozygous for DlattP-Dl-HA and clones homozygous for endogenous wildtype Dl displayed comparable levels of Dl at their surface, indicating that DlattP-Dl-HA behaves as a wildtype allele [16].
Dl is located in the apical membrane in the imaginal disc cells. In the twin clone assay, we observed no significant increase in the apical membrane levels of DlattP-Dl-NB + CB2A + K-HA compared to DlattP-Dl-HA (Fig. 4A–B’’’). Moreover, DlattP-Dl-NB + CB2A + K-HA was present in most of the Rab7-positive endosomes in a similar frequency as Dl-HA (Fig. 4C–C’’’’). Combined with the observation that the ICD of Dl is not required for exocytosis, this result suggests that Dl-NB + CB2A is endocytosed with a similar efficiency than DlattP-Dl-HA. The previous comparison of homozygous DlattP-Dl-HA with homozygous DlattP-DlK2R-HA clones revealed that DlattP-DlK2R-HA accumulated to a much higher level in the plasma membrane than Dl [16]. Thus, it appears that, just like Dl, Dl-NB + CB2A-HA is more efficiently endocytosed than DlK2R-HA. These results are in agreements with the finding that loss of mib1 function hardly affects the endocytosis of Dl [11, 27]. The difference in endocytosis efficiency between Dl-NB + CB2A-HA, which is deficient for Mib1-binding, and DlK2R-HA, which cannot be ubiquitylated, supports the previously drawn conclusion that ubi of Dl is not solely mediated by Mib1, but also by one or more unidentified E3-ligase(s) [16].
We have previously shown that the efficiency in endocytosis is crucial for the proper activity of Dl. Its decrease results in an increase in CI and a decrease in signalling [8, 16]. Thus, we conclude that the higher efficiency in endocytosis of Dl-NB + CB2A is the likely cause of its slightly weaker CI and stronger signalling activity compared to DlK2R (see quantification in Figs. 1H and 2J).
The importance of the MZM and REP domains for the function of Mib1
To further investigate the interaction of Dl with Mib1, we generated Mib1-variants lacking either the REP domain alone or the MZM and REP domains together (Additional file 1: Fig. S5). We expressed the generated variants under control of the tubulin promoter (tubP) to achieve ubiquitous expression at natural abundance, as observed for endogenous Mib1 in the wing disc [11]. All variants are inserted into the same landing site to achieve comparable expression. We tried to generate transgenic flies bearing a tub.P-mib1 insertion with the MZM domain deleted (tubP-mib1ΔMZM), but failed to obtain insertions that could be maintained as a stock, although we tried to obtain transformants several times. Although buffered by the presence of two endogenous copies of wildtype mib1, the initially transformed flies, which carried just one copy of tubP-mib1ΔMZM in their genome, displayed many Notch-related defects, were nearly sterile and survived only for a short time, which made their maintenance as a stock and analysis impossible. Thus, it appears that tubP-mib1ΔMZM acts in a dominant-negative manner. This dominant-negative activity depends on the presence of the REP domain, since we obtained transformants for a variant that lack both domains (tubP-mib1ΔMZM + REP), or only the REP domain (tubP-mib1ΔREP) without problems. It appears that the MZM domain somehow suppresses a deleterious activity of the REP domain.
Already the presence of one copy of the full-length variant tubP-mib1 resulted in a complete rescue of mib1 null-mutants (Fig. 5A, C, Additional file 1: Fig. S5A–D’). As expected from previous work [7, 10, 30, 31], the presence of tubP-mib1ΔMZM + REP, which lacks both ligand interaction domains, failed to rescue mib1 mutants, indicating that Mib1ΔMZM + REP has no activity and confirming the importance of the MZM and REP domains for Mib1 function and ligand activity (Fig. 5E, Additional file 1: Fig. S5C, D, C’’’, D’’’). Interestingly, also the presence of one copy of tubP-mib1ΔREP in the genome caused a nearly complete rescue of mib1 mutant discs, indicated by the grossly normal expression of the Notch target gene Wg along the dorso-ventral boundary of wing discs and the grossly normal wings and legs of the imago (Fig. 5D, arrow, Fig. 5S1C’’, D’’). The tubP-mib1ΔREP rescued adults had no defects with the exception of a small patch of extra vein at the tip of wing vein 2 (Additional file 1: Fig. S5C’’, arrow). Thus, the MZM domain mediates most of the activity of Mib1.
Fig. 5.
The MZM and REP domains of Mib1 interact with the NB and CB of Dl, respectively. A Reported interaction between JAG1 and MIB1. The NB and CB of JAG1 bind to the MZM and REP domains of MIB1, respectively. B Prediction for the ectopic activation of Notch by combinations of Dl- and Mib1-variants in mib1 mutant discs. Note that the combination of Dl-NB2A with Mib1ΔREP is predicted not to function, since the interaction of both the NB with the MZM and the CB with the REP should be compromised. It should therefore cause a similar phenotype as the other combinations in the red frame. However, the combination of Mib1ΔREP with Dl-CB2A should lead to the reduction of ectopic activation in a similar manner like the combination of Dl with Mib1ΔREP (combinations in the blue frame. C–E Rescue of mib1 mutant flies with Mib1-variants expressed under control of tubP. Rescue is performed with one copy of the constructs. C tubP-mib1 completely rescued mib1 mutants and the expression of Wg along the D/V boundary is re-installed (arrow, compare with Fig. 1E). D Likewise, tubP-mib1ΔREP resulted in a re-instalment of Wg expression along the D/V-boundary (arrow). E In contrast, tubP-mib1ΔMZM + REP failed to rescue mib1 mutants. The disc resembled that of mib1 mutant discs (compare with Fig. 1E). F–H’’’ Combinations of Mib1- and Dl-variants expressed in mib1 mutant discs. The Mib1 variants are expressed under control of tubP; the Dl-variants are expressed with ptcGal4. The coloured frames highlight the results, which confirmed the predictions outlined in B. I Quantification of the signalling activities and CI of the combinations
Interaction of the NB and CB of Dl with Mib1
MIB1 binds via its MZM and REP domains to the NB and CB of JAG1 (Fig. 5A). In addition, the MZM domain of MIB1 can also bind to a peptide containing the NB of Dl [7]. Having demonstrated the functionality of the predicted CB of Dl, we tested whether Dl also uses the bipartite interaction mode to bind to Mib1. To do so, we co-expressed combinations of the generated Dl-NB and -CB variants (with flanking Ks mutated) with the tub.mib1-deletion-variants in mib1 mutants and assayed the activity of the Notch pathway. As expected, the activity of the Dl-variants was dramatically suppressed in mib1 mutants (Additional file 1: Fig. S5E–G). The sole expression of Dl, Dl-NB2A and Dl-CB2A in mib1 discs rescued by tub.mib1 resulted in the activation of the Notch pathway similar to expression of the Dl-variants in wildtype discs, confirming that one copy of full-length Mib1 can provide the full function (Fig. 5F–F’’’, compare with Fig. 2C–F).
If Dl interacts with Mib1 in the bipartite binding mode, clear predictions should be fulfilled which are outlined in Fig. 5B. The main predictions are 1. The combination of Dl-NB2A with Mib1ΔREP should not lead to ectopic activation of Notch, since both of the bipartite interactions are compromised. Hence, it should produce the same phenotypes as the combinations of Dl with Mib1ΔMZMΔREP and Dl-NB + CB2A with Mib1. These predictions were fulfilled by our experiments (see Fig. 5G’, H, F’’’, respectively, framed in red, quantification in J). The second main prediction is that the combination of Dl-CB2A should produce the same reduced ectopic activation of the Notch-pathway if combined with Mib1 or Mib1ΔREP than the lack of the CB in Dl makes the presence of the REP domain in Mib1 unnecessary. Also, this prediction is fulfilled as seen in Fig. 5G and G’’, respectively, framed in blue, quantification in J. In addition, the predictions of the model for the activity produced by the other tested combinations are fulfilled by the experiments (Fig. 5B, F–H, quantification in J). Altogether, the results strongly suggest that Dl binds to Mib1 via the interaction of its NB with the MZM domain and its CB with the REP domain in vivo. Thus, it appears that the interaction between Dl and Mib1 follows similar rules as previously found for the binding of JAG1 to MIB1 and therefore are of general importance.
Note that CI of Dl is enhanced in tubP-mib1ΔREP rescued mib1 mutant discs, as previously found for the loss of the Ks in the ICD of Dl (Fig. 5D, E, arrow, quantification in J). Moreover, in discs rescued by full-length Mib1, the Dl-variants that lack one of the binding boxes are more cis-inhibitory than Dl (compare Fig. 5F with F’–F’’’, arrows, I). This observation further supports the notion that the strength of binding of Mib1 to Dl and the presence of the Ks are required to suppresses/adjust CI and signalling.
Identification of the Ks in the ICD of Dl important for signalling
Despite the ability of DlK2R to provide sufficient activity for complete development of Drosophila, the previous analysis also clearly showed that at least some of the 12 Ks in the ICD are important for the full activity of Dl in Mib1-dependent signalling events and also for adjustment of CI [8, 16]. We wondered which and how many of the Ks are important. To determine the importance of the conserved Ks, we performed two complementary sets of experiments: (A) we re-introduced individual Ks or combinations of them into DlK2R-HA and (B) replaced individual or combinations of Ks in Dl-HA by the similar R in Dl-HA (Fig. 6). The highlights of the analysis are summarised in Figs. 6 and 7; the complete results comprising all tested constructs are shown in Additional file 1: Fig. S6.
Fig. 6.
Identification of Ks in the ICD of Dl relevant for signalling. See also Fig. 6S1 for a summary of all variants tested. A Location of the 12 Ks in the ICD of Ser of Drosophila. B, C Consequences of expression of Dl and DlK2R for comparison. D Quantification of the expression of Dl and DlK2R. E–E’’’’ Consequences of the re-introduction of individual core Ks into DlK2R. Quantification in F. G, G’ Consequences of the exchange of individual core Ks to R into Dl. Quantification in I. H, H’ Exchange of all 4 core Ks (H) or the 4 core Ks and K665 and K762 to R. Quantification in I. For further information, see text
Fig. 7.
Identification of combinations of Ks in the ICD of Dl relevant for signalling. See also Fig. 6S1 for a summary of all variants tested. A Location of the 12 Ks in the ICD of Ser of Drosophila. B, C Consequences of expression of Dl and DlK2R for comparison. B–B’’’’’ The phenotype of expression of DlK2R-variants where combinations of two core Ks were re-introduced. C Quantification of the activity of the Dl-variants shown in B–B’’’’’. D–D’’’’’ Identification of the combination of Ks required for dl signalling. E Quantification of the activity of the Dl-variants shown in D–D’’’’’. It reveals the re-introduction of a combination of six Ks, including the core Ks, re-establishes the full activity of Dl, but is still insufficient to completely normalise CI. For further information, see text
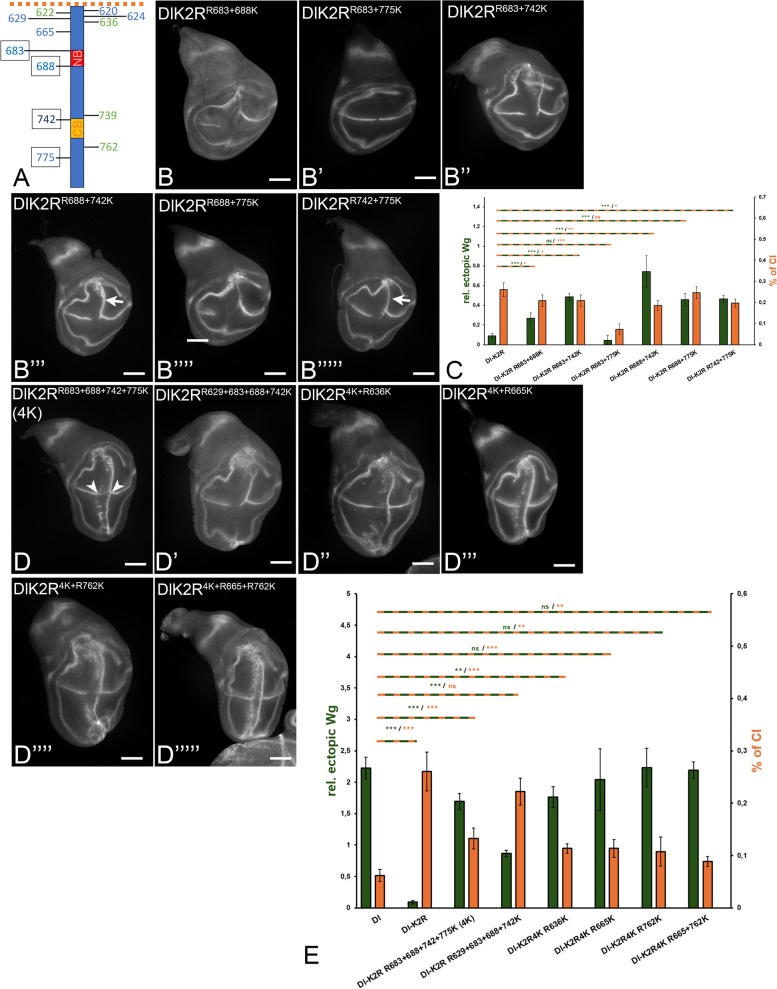
A comparison of the sequences of Dl-orthologs of insects revealed that 8 of the 12 Ks of Dl are conserved in other species to different degrees [6] (Additional file 1: Fig. S1, arrows, Fig. 6A). Three of the 8 conserved Ks are close to the transmembrane domain and together with adjacent Rs are probably required to stabilise the membrane integration of Dl by providing a positive charge that anchors the transmembrane domain in the plasma membrane. Indeed, these three Ks had no importance for the signalling activity of Dl, as their combined exchange to R in Dl, or re-introduction into DlK2R had no effect (DlK2RR620+622+624 K and DlK620+622+624R, Additional file 1: Fig. S7, also labelled with TM in Additional file 1: Fig. S6). Thus, they are not required for signalling. Of the remaining five, K683, K688, K742 and K775 are most conserved and we concentrated on these four Ks (core Ks). K683 and K688 are located at the edges of the NB and K742 at the N-terminal edge of the CB (Fig. 6A).
Re-introduction of the core Ks into DlK2R
The individual re-introduction of two Ks, K742 and K775 (DlK2RR742K and DlK2RR775K), had a stronger positive effect on the activity of DlK2R. They induced one posterior stripe of ectopic Wg expression largely restricted to the dorsal compartment (Fig. 6E’’’, E’’’’, arrow, compare with C). Introduction of K742 induced a significantly longer stripe of ectopic Wg expression than K775, suggesting that it is more important for function (compare Fig. 6E’’’ with E’’’’, arrow, quantification in F). Nevertheless, DlK2RK742 and DlK2RR775K displayed only weak activity compared to Dl (compare Fig. 6B with E’’’ and E’’’’). The individual re-introduction of the other two core Ks into DlK2R had only marginal effects on signalling (Fig. 6E’, E’’, compare with C, quantification in F). These findings suggest that K742 is the most important K in the ICD of Dl, followed by K775, K683 and K688.
The results confirmed that more than one K is required for the full function of Dl. We therefore tested combinations of two, three and four core Ks introduced into DlK2R (Fig. 7 and Additional file 1: Fig. S6). This analysis showed that 1. The re-introduction of K683 or K688 had a weak enhancing effect, only if combined with K742, indicated by the stronger stripe of ectopic Wg expression (Fig. 7B–B’’’, quantification in C). The enhancing effect of K683 and K688 on K742 was similar to the combination of K742 with K775 (Fig. 7B’’, B’’’ arrow, compare with B’’’’, quantification in C). Thus, the two Ks contribute to the activity of Dl, but only in combination with K742 and/or K775. In the case of the re-introduction of combinations of three Ks, the ones with K742 were the most active ones, indicated by the longer stripes of ectopic Wg expression (Fig. 6S1). 2. Although the re-introduction of all four core Ks into DlK2R (DlK2R4K) resulted in strong activity, it did not completely restore the activity of Dl, since the anterior stripe of ectopic Wg expression was not continuous as observed in Dl (Fig. 7D, compare with Fig. 6B, quantification in 7E). This result indicates that less conserved Ks in addition to the core Ks are required for the full activity of Dl. Note that the re-introduction of the core Ks into the ICD of DlK2R reduces the gap in the expression of endogenous Wg along the D/V boundary compared to DlK2R, indicating a reduction in CI (Fig. 7D, compare with Fig. 6C, quantification in E). Thus, the core Ks, which are important for signalling, are also important for the suppression/adjustment of CI of Dl. The finding highlights the tight inverse correlation between the ability Dl to signal and to cis-inhibit in Mib1-dependent processes. The replacement of K775 of the core by K629 (DlK2RR629+683+688+742 K) resulted in a reduction of the activity (Fig. 7D’, quantification in E). This suggests that the presence of all core Ks is required for the full activity of Dl.
Exchange of the conserved Ks by Rs in Dl
The replacement of K742 by R reduced, but not abolished the activity of Dl. In this case, the anterior ectopic stripe of Wg expression was lost (Fig. 6G’’’, compare with Fig. 6B, quantification in I). A very mild reduction was also observed in the case of the individual replacement of K688 (Fig. 6G’’). In this case, the anterior stripe of expression of Wg was slightly reduced in the dorsal compartment in comparison to Dl-HA expression (arrowheads in Fig. 6G’’, compare with Fig. 6B, quantification in I). The exchange of K683 and surprisingly K755 did not lead to a recognisable reduction (Fig. 6G’, G’’’’, quantification in I). Even the replacement of all 4 core Ks by R did not completely abolish the activity of Dl (Fig. 6S1).
Altogether, the combined analysis revealed that the 4 core Ks are important for Mib1-mediated signalling of Dl, but differ in their importance. K742 is the most important one, followed by K775 and then by K688 and K683. The results also revealed that a combination of more than the 4 core Ks is required for the full function of Dl. The additionally required Ks are less conserved among the Dl orthologs of insects. Note that the replacement of the core Ks by Rs increases the gap in the endogenous expression of Wg along the D/V-boundary increases, indicating an increase in CI (Fig. 6S1). Combined with the observation that the addition of Ks into DlK2R reduces CI, the findings confirm that the Ks are involved in adjusting the degree of CI and signalling by Mib1.
A combination of more than six Ks in the ICD restores the signalling activity of Dl
To identify additional Ks important for the activity of Dl, we tested less conserved Ks in concert with the core Ks (Fig. 6S1). Eventually, we identified K665 and K762 as additionally required Ks. Their re-introduction into DlK2R in addition to the four core Ks led to a signalling activity comparable to Dl (Fig. 7D’’’’’, compare with 6B, quantification in E). Nevertheless, DlK2R4K R665 + 762 K was still slightly more cis-inhibitory, indicating that additional Ks might be required for the re-establishment of the normal level of CI of Dl. Likewise, the replacement of these 6 Ks by Rs reduced the activity of Dl to a level close to DlK2R (Fig. 6H’, compare with C, quantification in I). Note that the addition of the Ks located at the transmembrane region to the 4 core Ks in DlK2R did not improve the activity of Dl. The same is true if K636 or 739 was added to the core Ks (see Fig. 6S1). This indicates that there is some specificity for K665 and K762 and that ubi by Mib1 is selective.
The importance of the core Ks of the ICD of Dl for development
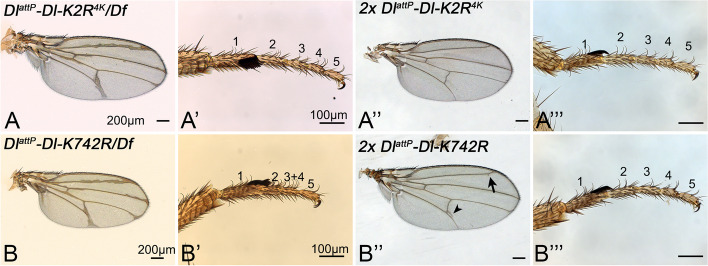
Although more than 6 Ks are required for the full function of Dl, the four core Ks are the most conserved among the Dl orthologs of insect species. Therefore, we wondered whether the presence of only the core Ks in Dl provides sufficient Dl-activity for normal development. We generated DlattP-DlK2R4K-HA and compared its phenotype to that of DlattP-Dl-HA. We found that the phenotype of DlattP-DlK2R4K over the deficiency DlBSC850 was only slightly stronger to that of DlattP-Dl-HA or endogenous Dl (Fig. 8A–A’’’, compare with Fig. 3A, A’). However, similar to DlattP-Dl-HA, homozygosity of DlattP-DlK2R4K resulted in a wildtype phenotype (Fig. 8A’’, A’’’). Thus, DlattP-DlK2R4K is a functional allele, although it has not the full activity in our over-expression analysis. It appears that a window of Dl activity exists that allows correct development. The slightly reduced signalling activity of DlattP-DlK2R4K detected in our over-expression experiments is not relevant for development as long as it provides a level of activity that lies in the window.
Fig. 8.
The importance of the four core Ks of the ICD off Dl. A–A’’’ DlattP-DlK2R4K is a functional Dl allele. The phenotype of DlattP-DlK2R4K over the deficiency resembles that of DlattP-Dl (compare with Fig. 3A, A’). A, A’ Heterozygous DlattP-DlK2R4K flies display the phenotype typical for Dl heterozygosity (compare with Fig. 3A). A’’, A’’’ Homozygosity of DlattP-DlK2R4K results in a wildtype phenotype. B, B’ Phenotype of DlattP-Dl-K742R is stronger than that of Dl-heterozygous flies, indicated by the fusion of the tarsal segments 3 + 4. B’’, B’’’ This phenotype is abolished by the addition of a second copy of DlattP-Dl-K742R. Nevertheless, a small amount of extra vein material is still observed, indicating that even two copies cannot restore the wildtype phenotype completely (arrow and arrowhead). For further information, see text
Our analysis revealed that K742 is the most important K of the ICD. To evaluate its importance for the whole of development of Drosophila, we generated the knock-in allele DlattP-Dl-K742R-HA. We found that it is not able to provide the full function of Dl, even if present in two copies (Fig. 8B–B’’’). In heterozygosity (DlattP-Dl-K742R-HA/Df), the phenotype of DlattP-Dl-K742R-HA was slightly stronger than that of heterozygosity of Dl (Fig. 7B, compare with Fig. 3A). Moreover, even in homozygosity, small amounts of extra-vein material were observed (arrow and arrowhead in Fig. 7B’’). This finding confirms the importance of K742, also for the in vivo function of Dl.
Discussion
Endocytosis of the ligands plays a critical role in the ligand-dependent activation of the Notch pathway. One important way to induce endocytosis is ubi of their ICDs by Mib1 [10, 11, 27]. We have previously shown that human MIB1 binds to JAG1 via a bipartite recognition mode that requires the NB and CB of the ICD of JAG1 and the MZM and REP domains of MIB1 [7]. However, several questions remained unanswered: 1. it was not clear whether this bipartite binding mode is of general importance and also operates in other species, e.g. Drosophila, 2. the individual importance of the MZM and REP domains were not investigated, 3. it was not sure that a functional CB exists in Dl, and 4. it was not known, which and how many Ks in the ICD of a DSL ligand are required for its full function. Here, we provided answers to these questions. In addition, we provide further evidence that ubi of Dl by Mib1 is important for signalling and also adjusting/suppressing CI. Moreover, we here show that this ability of Mib1 depends on the binding of its MZM and REP domains to the NB and CB in Dl.
We found that also Dl has a functional NB and CB. So far only the NB was identified [6]. The individual importance of the NB and CB of JAG1 was not determined. We here found that the binding boxes have different importance for function, with the NB being the dominant site. The results suggest that the strength of binding of Mib1 to Dl determines the strength of signalling, since the inactivation of both boxes reduces the activity of Dl. The large dependence of the CB on the presence of the NB and its relatively weak phenotype upon mutation is probably the reason why its importance has not been recognised in a previous study, which used randomly inserted constructs where the position effects are not neutralised [6].
We provide further evidence that, besides the MZM domain, also the REP domain is important for the function of Mib1 in Drosophila. Their combined loss resulted in a complete loss of Mib1 function and the ability to activate Dl. Moreover, our results suggest that the CB of Dl interacts with the REP domain in vivo, as it has previously been found for JAG1 in vitro [7]. We also showed that a Dl-variant with mutated NB cannot be activated by Mib1ΔREP, supporting the notion that the NB interacts with the MZM domain of Mib1. A direct interaction of a peptide containing the NB of Dl with the MZM domain of MIB1 has been demonstrated in vitro and our results provide evidence that it occurs in vivo in the context of the full-length proteins, especially as we found that the N in the NB is as important for Mib1-mediated signalling as predicted from the atomic structure. In agreement with these results is also the observation that the MZM domain of Mib1, which interacts with the NB, is more important for function than the REP domain. Our results suggest that the activation of Dl by Mib1 appears to use the same bipartite binding mode originally discovered for interaction of JAG1 with MIB1. Hence, they provide evidence that the bipartite binding mode is the general rule for binding of Mib1 proteins to DSL ligands. Combined with the previous work by McMillan et al. [7] and Daskalaki et al. [6], our findings support a model in which Mib1 contacts the ICD of Dl mainly via its MZM domain binding to the NB and subsequently contacting the CB with its REP domain to consolidate the binding. This bipartite binding results in efficient and selective ubi of Ks in the ICD of Dl. It appears that the importance of the NB and CB of JAG1 for the interaction with MIB1 is more balanced, as it has been shown that the deletion of either the NB or CB results in the abolishment of binding to MIB1 [7].
We identified 6 out of the 12 existing Ks in the ICD of Dl important for Mib1-dependent signalling. Four of these Ks, the core Ks, are highly conserved, with K742 being the most important for the activity of Dl. The individual replacement of the other core Ks by R did not (K688 and K683) or weakly (K755) affect the activity of Dl. Their importance was revealed by the combined replacement/introduction with the other core Ks, especially K742. The previous study by Daskalaki et al. [6] did not identify K742 as important for the Mib1-dependent activity of Dl. In contrast to this study, the previous one used randomly inserted insertions of Dl variants, which was the only available method at that time. The analysis allowed only the detection of large differences in the activity of Dl-variants, because of the varying position effects. DlK742R still has significant Mib1-dependent activity, which probably obscured the importance of K742 in the previous analysis because of the technical limitations.
One interesting observation is that two core Ks, K688 and K683, are effective upon the re-introduction into DlK2R only in combination with K742 and to a lesser extent with K775. An explanation for this puzzling finding might be that the initial ubi of K742 (and possible K775) is a prerequisite for ubi of the other Ks. One possible scenario would be that the ubi of K742 induces a change in the conformation of the ICD that promotes ubi of the other Ks.
In principle, K742 could also contribute to the binding of Mib1 to the ICD of Dl, as it is located at the N-terminal edge of the CB. However, previous work showed that the deletion of K742 did not have an obvious effect on the ubi of the ICD of Dl, suggesting that Mib1 can still effectively bind to the ICD of Dl in the absence of K742 [6]. Interestingly, previous work that analysed Dl alleles with trafficking defects found that one allele, DlCE16, had a K742R mutation [32]. However, this allele also bears a mutation in its ECD and the authors showed that also the ECD is somehow involved in endocytosis. Thus, it was not clear which mutation was responsible for the observed trafficking defect. Our work clarifies this issue.
The Ks required for the full activity of Dl in addition to the core Ks, K665 and K762, are less well conserved among the insect orthologs analysed, indicating a certain flexibility in the use of Ks. It appears that already the four core Ks provide the activity of Dl necessary for the Mib1-dependent developmental processes and the additional activity achieved with the ubi of K665 and K762 is not absolutely required, but might provide a certain robustness for signalling. Our finding that DlattP-DlK2R4K provided sufficient Dl-activity for normal development supports this notion. It appears there is a threshold of activity required for normal development that can be crossed already with the presence of only the core Ks. Of note here is that we have recently found that already the presence of one copy of the knock-in allele DlattP-DlK2R-HA provides sufficient activity to complete development, giving rise to adult flies with an only slightly enhanced phenotype compared to Dl heterozygous flies [16]. This work also revealed that Mib1 activates Dl solely via ubi, whereas Neur can activate Dl also in an ubi-independent manner sufficiently high to allow the correct course of Neur-dependent processes, such as neurogenesis. These findings explain the relatively weak effect of the replacement of all Ks of the ICD of Dl on Drosophila development. It also explains why the re-introduction of the core Ks in DlattP-DlK2R4K-HA results in a fully functional allele that completely resembles Dl. In fact, we think that the over-expression of Dl with the Gal4-system exaggerated the effects of loss of Ks and therefore was a more sensitive assay than a knock-in approach to reveal the meaning of the Ks and detect the contribution of the less well conserved Ks to Dl-signalling.
Conclusions
The work reveals how the ligands of the Notch pathway are activated through the interaction with Mindbomb1, the major E3-ligase devoted to Notch signalling. It reveals that, in Drosophila, Mindbomb1 interacts with the ligand Delta in a bipartite mode in vivo, as previously found for the human orthologs JAGGED1 and MINDBOMB1. This finding suggests that the bipartite interaction mode is of general importance for interaction of Mib1 with the Notch ligands. In addition, our work identifies the target sites for the enzymatic activity of Mindbomb1 in the Delta-ligand. Overall, the results contribute to a deeper understanding of how the ligands of the evolutionary Notch-pathway are activated during Notch-signalling in metazoans.
Methods
Fly strains
DlattP-Dl-NB2A + K-HA FRT82B, DlattP-Dl-NB2A-HA FRT82B, DlattP-Dl-CB2A + K-HA FRT82B, DlattP-Dl-NB2A-HA FRT82B, DlattP [24], DlattP-Dl-HA FRT82B, DlattP-Dl-K2R-HA FRT82B [16], mib1EY09870 [10, 11], Gbe + Su(H)-lacZ [33], ptcGAL4 [34], FRT82B (Bloomington stock centre (BSC), BSC2035), FRT82B ubiRFPnls (BSC30555), FRT82B ubiGFPnls (BSC32655), Dlrev10 e FRT82B [22], Df(3R) BSC850 (BSC27922) [35], lqfARI [36], Dlrev10 e SerRX82 FRT82B [22].
Stocks generated for this study: DlattP-Dl-NB2A-HA FRT82B, DlattP-Dl-NB2A-HA FRT82B, DlattP-Dl-CB2A-HA FRT82B, DlattP-Dl-NB2A-HA FRT82B, DlattP-Dl-NB + CB2A-HA FRT82B, DlattP-Dl-NB + CB2A-HA FRT82B, variants where K were re-introduced into DlattP-DlK2R-HA FRT82B and variants where Ks were replaced by Rs in DlattP-Dl-HA FRT82B, DlattP-Dl-NB2A-HA FRT82B, DlattP-Dl-CB2A-HA FRT82B, DlattP-Dl-NB + CB2A-HA FRT82B, DlattP-Dl-ΔICD-HA FRT82B DlattP-HA-Dl FRT82B, DlattP-Dl-DlK2R4K-HA FRT82B, DlattP-Dl-K742R-HA FRT82B. tubP-mib1ΔMZM + REP, tubP-mib1REP, tubP-mib1.
Quantification of ectopic Wg activation and cis-inhibition (CI)
Quantification was performed using the software ImageJ. The length of CI was measured and divided by the length of endogenous Wg expression (CI/endogenous Wg). The result indicates the percentage of CI that is acting on the endogenous Wg.
Ectopic activation of Wg was measured by assessing the length of the anterior and/or posterior Wg expression and addition of the lengths. The added lengths were divided against the endogenous Wg. The result indicates the fold-change of ectopic Wg expression relative to the endogenous Wg expression. The significance was determined by using an unpaired, two-samples t-test with H0 = the means of the two samples do not differ; with the following significance-levels: p > 0.05 = ns; p < 0.05 = *; p < 0.01 = **; p < 0.001 = ***.
Antibody staining and imaging
Antibody staining was performed according to standard protocols [37] and [38].
Antibodies used
| Antibody list | ||
|---|---|---|
| Antibody (from species) | Source or reference | Dilution |
| Anti HA (rat) | Roche Clone 3F10 | 1:500 |
| Anti HA (rabbit) | Cell Signaling C29F4 | 1:1600 |
| Anti Dl (mouse) | DSHB C594.9B | 1:500 |
| Anti Dl (mouse) | DSHB C594.9B | 1:100 (surface staining) |
| Anti N(extra) (mouse) | DSHB C458.2H | 1:100 |
| Anti Wg (mouse) | DSHB 4D4 | 1:250 |
| Anti ß-Gal (rabbit) | Cappel | 1:1500 |
| Anti ß-Gal (rabbit) | Cell Signaling A257 | 1:1500 |
| Anti ß-Gal (mouse) | DSHB 40-1a | 1:250 |
| Anti Rab11 (rabbit) | Tanaka and Nakamura 2008 | 1:8000 |
| Anti Hnt (mouse) | DSHB 1G9 | 1:80 |
| Anti Rabbit Alexa 488 (goat) | Invitrogen/Molecular Probes | 1:500 |
| Anti Rabbit Alexa 568 (goat) | Invitrogen/Molecular Probes | 1:500 |
| Anti Rabbit Alexa 647 (goat) | Invitrogen/Molecular Probes | 1:500 |
| Anti Mouse Alexa 488 (goat) | Invitrogen/Molecular Probes | 1:500 |
| Anti Mouse Alexa 568 (goat) | Invitrogen/Molecular Probes | 1:500 |
| Anti Mouse Alexa 647 (goat) | Invitrogen/Molecular Probes | 1:500 |
| Anti Rat Alexa 488 (goat) | Invitrogen/Molecular Probes | 1:500 |
| Anti Rat Alexa 568 (goat) | Invitrogen/Molecular Probes | 1:500 |
| Anti Rat Alexa 647 (goat) | Invitrogen/Molecular Probes | 1:500 |
Generation of constructs
Generation of UAS Dl-variants
Dl-HA was generated by introduction of a synthesised fragment from the NdeI restriction site of the ICD onwards. The DlK2R-HA was generated by replacing the ICD of Dl-HA by a synthesised ICD in which all Ks are replaced by Rs. Gene synthesis was performed by GenScript. Single point mutations of each K were introduced by site directed mutagenesis (SDM) with the Pfu Polymerase by Promega and complementary primers. Each K was replaced by an R. All constructs were cloned into pUAST attB by BstEII and NdeI.
Mib1-binding mutants (NB2A, CB2A and NB + CB2A) were also generated via SDM. The Dl-constructs were injected into the landing site 51C. Transgenesis was partly performed by BestGene Inc.
Generation of tubP-(V5-)Mib1 variants
pBluescript (pBS) mib1 was generated by introduction of the amplified cDNA of mib1 (gift from C. Delidakis) into pBluescript by EcoRI and XhoI. mib1 was amplified with an N-terminal primer inserting a NotI restriction site and the V5 tag and cloned into pattB tub by NotI and XhoI. MZM or REP domains were deleted by site directed mutagenesis in pBS mib1. The mutated areas of Mib1 variants were cloned into pattB tub V5 mib1 by BbvCI and StuI. The tub.Mib1 variants were inserted into the 22A landing site.
Generation of the D|attP-variants: D|attP-variants were generated by cloning the corresponding variant from the pUAST attB-vector into the pGE-vector that was generated in our previous study by employing the restriction sites BstEll and Xbal [16]. This replaces the trans-membrane domain and the whole ICD. The vectors were injected into DlattP [24]. D|attP-ΔICD-HA was synthesised by IDT technology, bearing 12aa from the N-terminal ICD to ensure proper incorporation into the plasma membrane. Ks within these 12aa were replaced by Rs.
Alleles generated: D|attP-NB2A-HA, D|attP-CB2A-HA, D|attP-NB + CB2A-HA, D|attP-Dl-K2R4K (K683, K688, K742, K775), D|attP-K742R-HA, D|attP-ΔICD-HA. All constructs were sequenced before injection into D|attP.
The following primers were used to generate the Dl- and Mib1-constructs:
Dl-variants
| Mutation | Name | Sequence (5′ à 3′) |
|---|---|---|
| K620, 622, 624R | Dl_sdm_KTMR_fwd | C TTC TGC ATG AGG CGC AGG CGT AGG CGT GCT CAG |
| Dl_sdm_KTMR_rev | CTG AGC ACG CCT ACG CCT GCG CCT CAT GCA GAA G | |
| K629R | Dl_K629R_Fwd | CGT GCT CAG GAA AGG GAC GAC GCG GAG |
| Dl_K629R_Rev | CTC CGC GTC GTC CCT TTC CTG AGC ACG | |
| K636R | Dl_K636R_Fwd | GAC GCG GAG GCC AGG AGG CAG AAC GAA CAG |
| Dl_K636R_Rev | CTG TTC GTT CTG CCT CCT GGC CTC CGC GTC | |
| K665R | Dl_K665R_Fwd | C TCT ATG GGC GGC AGA ACT GGC AGC AAC AG |
| Dl_K665R_Rev | CT GTT GCT GCC AGT TCT GCC GCC CAT AGA G | |
| K739R | Dl_K739R_Fwd | GT GTG GCT CCG CTA CAA AGA GCC AGG TCG C |
| Dl_K739R_Rev | G CGA CCT GGC TCT TTG TAG CGG AGC CAC AC | |
| K762R | Dl_K762R_Fwd | CA GGC AGC TCA GCC AGG GGA GCG TCT GGC G |
| Dl_K762R_Rev | C GCC AGA CGC TCC CCT GGC TGA GCT GCC TG | |
| K775R | Dl_K775R_Fwd | CG GCG GAG GGC AGG AGG ATC TCT GTT TTA G |
| Dl_K775R_Rev | C TAA AAC AGA GAT CCT CCT GCC CTC CGC CG |
K2R-variants
| R620, 622, 624 K | DlK2R_sdm_RTMK_fwd | C TTC TGC ATG AAA CGC AAG CGT AAG CGT GCT CAG |
| DlK2R_sdm_RTMK_rev | CTG AGC ACG CTT ACG CTT GCG TTT CAT GCA GAA G | |
| R629K | DlK2R_R629K_Fwd | CGT GCT CAG GAA AAA GAC GAC GCG GAG GCC |
| DlK2R_R629K_Rev | GGC CTC CGC GTC GTC TTT TTC CTG AGC ACG | |
| R636K | DlK2R_R636K_for | GAC GAC GCG GAG GCC AGG AAG CAG AAC GAA CAG |
| DlK2R_R636K_rev | CTG TTC GTT CTG CTT CCT GGC CTC CGC GTC GTC | |
| R665K | DlK2R_R665K_for | C TCT CTG GGC GGC AAG ACT GGC AGC AAC AG |
| DlK2R_R665K_rev | CT GTT GCT GCC AGT CTT GCC GCC CAG AGA G | |
| R739K | DlK2R_R739K_for | GT GTG GCT CCG CTA CAA AGA GCC AAG TCG C |
| DlK2R_R739K_rev | G CGA CTT GGC TCT TTG TAG CGG AGC CAC AC | |
| R762K | DlK2R_R762K_for | CA GGC AGC TCA GCC AAA GGA GCG TCT GGC G |
| DlK2R_R762K_rev | C GCC AGA CGC TCC TTT GGC TGA GCT GCC TG | |
| R775K | DlK2R_K775R_Fwd | CG GCG GAG GGC AAG AGG ATC TCT GTT TTA GG |
| DlK2R_K775R_Rev | CC TAA AAC AGA GAT CCT CTT GCC CTC CGC CG |
Boxes Dl
| Mutation | Name | Sequence (5′ à 3′) |
|---|---|---|
| Deletion of ICD (AS 618–833) | Dl_sdm_ΔICD_for | G TGC GTG GTC TTC GCC TAC CCA TAC |
| Dl_sdm_ΔICD_rev | GTA TGG GTA GGC GAA GAC CAC GCA C | |
| N684A | Dl_sdm_N684A_fwd | C CCG AAT ATC ATC AAA GCC ACC TGG GAC AAG TCG GTC |
| Dl_sdm_N684A_rev | GAC CGA CTT GTC CCA GGT GGC TTT GAT GAT ATT CGG G | |
| IKNTWDK to AAAAAAA | Dl_sdm_NBox2A_fwd | GGC GGC AAC CCG AAT ATC GCC GCA GCC GCC GCG GCC GCG TCG GTC AAC AAC ATT TGT GCC |
| Dl_sdm_NBox2A_rev | GGC ACA AAT GTT GTT GAC CGA CGC GGC CGC GGC GGC TGC GGC GAT ATT CGG GTT GCC GCC | |
| IKNTWDK to AKAAAAK | Dl_sdm_NBox2A_K_fwd | GC GGC AAC CCG AAT ATC GCC AAA GCC GCC GCG GCC AAG TCG GTC AAC AAC |
| Dl_sdm_NBox2A_K_rev | GTT GTT GAC CGA CTT GGC CGC GGC GGC TTT GGC GAT ATT CGG GTT GCC GC | |
| IKNTWDK to AANAAAA | Dl_sdm_N-Box2A-N684_for | GGC GGC AAC CCG AAT ATC GCC GCA AAC GCC GCG GCC GCG TCG GTC AAC AAC ATT TGT G |
| Dl_sdm_N-Box2A-N684_rev | C ACA AAT GTT GTT GAC CGA CGC GGC CGC GGC GTT TGC GGC GAT ATT CGG GTT GCC GCC | |
| KQLNTD to AAAAAA | Dl_sdm_CBox2A_fwd | CAA AGA GCC AAG TCG CAA GCC GCA GCC GCC GCC GCT CCC ACG CTC ATG CAC CG |
| Dl_sdm_CBox2A_rev | CG GTG CAT GAG CGT GGG AGC GGC GGC GGC TGC GGC TTG CGA CTT GGC TCT TTG | |
| KQLNTD to KAAAAA | Dl_sdm_CBox2A_K_fwd | GCC AAG TCG CAA AAG GCA GCC GCC GCC GCT CCC ACG CTC ATG CAC C |
| Dl_sdm_CBox2A_K_rev | G GTG CAT GAG CGT GGG AGC GGC GGC GGC TGC CTT TTG CGA CTT GGC | |
| IKNTWDKSVNNI to IKNTWAKSVAAA | Dl_sdm_DNNI2A_for | C AAA AAC ACC TGG GCC AAG TCG GTC GCC GCC GCT TGT GCC TCA GCA G |
| Dl_sdm_DNNI2A_rev | C TGC TGA GGC ACA AGC GGC GGC GAC CGA CTT GGC CCA GGT GTT TTT G |
Mib1
| Name | Sequence (5′ à 3′) |
|---|---|
| mib1_NotI_V5_forward |
G TGA CCA GGC GGC CGC ATG ATC CCT AAC CCT CTC CTC TCT TGT GCG GCC ACC C NotI-restriction site, start-codon, V5-tag, binds mib1 |
| XhoI-Dmib1_backward (E. Seib) |
CAC TAG CTC GAG TCA GAA GAG CAG GAT GCG XhoI-restriction site, binds mib1 |
| Mutation | Name | Sequence (5′ à 3′) |
|---|---|---|
| Deletion of the MZM-domain (AS 100–315) | dMib1_sdm_dMZM_for | CG GCA GCG GCC CTC AAG GGT AGT AAT GTT TAC |
| dMib1_sdm_dMZM_rev | GTA AAC ATT ACT ACC CTT GAG GGC CGC TGC CG | |
| Deletion of the REP-domain (AS 333–487) | dMib1_sdm_dREP_for | CTG GGC GAA AAC GGA TCA ACT GCC TCC G |
| dMib1_sdm_dREP_rev | C GGA GGC AGT TGA TCC GTT TTC GCC CAG |
Primers were purchased from Sigma-Aldrich.
Supplementary Information
Additional file 1: Figures S1–S7. Fig. S1 Comparison of the aa sequence of the ICD of Dl orthologs among insect species. Fig. S2 The phenotype of expression of Dl-NB2A and Dl-CB2A with the flanking Ks also exchanged to A. Fig. S3 The phenotype of a Dl knock-in allele with the HA tag inserted into the extracellular domain, close to the transmembrane domain. Fig. S4 The role of the ICD of Dl revealed by the analysis of DlattP-DlΔICD-HA. Fig. S5 The adult phenotype of mib1 mutant flies rescued with one copy of the described Mib1 variants expressed under control of tub.P. Fig. S6 Presentation of the complete analysis of all Dl variants generated and tested for this study. Fig. S7 The role of the Ks in the ICD of Dl close to the transmembrane domain.
Acknowledgements
We thank Denise Kuska, Parisa Naeli, Kritika Sharma, Patricia Blum, Jessica Nicolai for the help in some experiments, Siylvia Tannebaum and Stefan Kölzer for excellent technical assistance. The DGRC, the Bloomington stock centre and DSHB and Stefano De Renzis provided stock and reagents.
Abbreviations
- Dl
Delta
- Mib1
Mindbomb1
- NB
N-box
- CB
C-box
- MZM
Mib-Herc2-ZZ zinkfinger-Mib1-Herc2 domain
- REP
Mib repeat-Mib repeat domain
- Neur
Neuralized
- Ks
Lysines
- JAG1
JAGGED1
- ubi
Ubiquitylation
- kuz
Kuzbanian
- NEXT
Notch EXtracellular Truncation
- NICD
Notch intracellular domain
- Su(H)
Suppressor of Hairless
- ICDs
Intracellular domains
- wg
Wingless
- R
Arginine
- Dll1
Delta-like1
- ptcGal4
patched-Gal4
- A/P-boundary
Anterior-posterior compartment boundary
- D/V-boundary
Dorso-ventral compartment boundary
- N
Asparagine
Authors’ contributions
T. K: conceived the project. TK, NV, AA., T. T. and ES designed and conducted the experiments. TK wrote the manuscript. N.V. All authors reviewed and edited the manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL. The work was supported by the DFG via Sachbeihilfe KL 1028/3–4 and KL 1028/13–1.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All the authors of this manuscript consent to its publication as a research article in BMC Biology.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Nicole Vüllings and Alina Airich contributed equally to this work.
References
- 1.Siebel C, Lendahl U. Notch signaling in development, tissue homeostasis, and disease. Physiol Rev. 2017;97:1235–94. [DOI] [PubMed] [Google Scholar]
- 2.Seib E, Klein T. The role of ligand endocytosis in notch signalling. Biol Cell. 2021. 10.1111/boc.202100009. [DOI] [PubMed] [Google Scholar]
- 3.Gordon WR, Arnett KL, Blacklow SC. The molecular logic of Notch signaling–a structural and biochemical perspective. J Cell Sci. 2008;121:3109–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schnute B, Troost T, Klein T. Endocytic trafficking of the Notch receptor. Adv Exp Med Biol. 2018;1066:99–122. 10.1007/978-3-319-89512-3_6. [DOI] [PubMed] [Google Scholar]
- 5.Le Borgne R, Bardin A, Schweisguth F. The roles of receptor and ligand endocytosis in regulating Notch signaling. Development. 2005;132:1751–62. [DOI] [PubMed] [Google Scholar]
- 6.Daskalaki A, Shalaby NA, Kux K, Tsoumpekos G, Tsibidis GD, Muskavitch MA, et al. Distinct intracellular motifs of Delta mediate its ubiquitylation and activation by Mindbomb1 and Neuralized. J Cell Biol. 2011;195:1017–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McMillan BJ, Schnute B, Ohlenhard N, Zimmerman B, Miles L, Beglova N, et al. A tail of two sites: structural basis for recognition of Notch ligands by Mind bomb E3 ligases. Mol Cell. 2015;57:912–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berndt N, Seib E, Kim S, Troost T, Lyga M, Langenbach J, et al. Ubiquitylation-independent activation of Notch signalling by Delta. eLife. 2017. 10.7554/eLife.27346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klein T. Wing disc development in the fly: the early stages. Curr Op Gen Dev. 2001;11:470–5. [DOI] [PubMed] [Google Scholar]
- 10.Lai EC, Roegiers F, Qin X, Jan YN, Rubin GM. The ubiquitin ligase Drosophila Mind bomb promotes Notch signaling by regulating the localization and activity of Serrate and Delta. Development. 2005;132(10):2319–32 PubMed PMID: 15829515. [DOI] [PubMed] [Google Scholar]
- 11.Le Borgne R, Remaud S, Hamel S, Schweisguth F. Two distinct E3 ubiquitin ligases have complementary functions in the regulation of delta and serrate signaling in Drosophila. PLoS Biol. 2005;3(4): e96 PubMed PMID: 15760269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim J, Irvine KD, Carroll SB. Cell recognition, signal induction, and symmetrical gene activation at the dorsal-ventral boundary of the developing Drosophila wing. Cell. 1995;82(5):795–802 PubMed PMID: 95401269. [DOI] [PubMed] [Google Scholar]
- 13.Klein T, Brennan K, Martinez-Arias AM. An intrinsic dominant negative activity of serrate that is modulated during wing development in Drosophila. Dev Biol. 1997;189(1):123–34 PubMed PMID: 97428265. [DOI] [PubMed] [Google Scholar]
- 14.Sprinzak D, Lakhanpal A, LeBon L, Santat LA, Fontes ME, Anderson GA, et al. Cis-interactions between Notch and Delta generate mutually exclusive signalling states. Nature. 2010;465:86–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glittenberg M, Pitsouli C, Garvey C, Delidakis C, Bray S. Role of conserved intracellular motifs in serrate signalling, cis-inhibition and endocytosis. Embo J. 2006;25:4697–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Troost T, Seib E, Airich A, Vüllings N, Necakov A, De Renzis S, et al. The meaning of ubiquitylation of the DSL ligand Delta for the development of Drosophila. BMC Biol. 2023. 10.1186/s12915-023-01759-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mattiroli F, Sixma T. Lysine-targeting specificity in ubiquitin and ubiquitin-like modification pathways. Nat Struct Mol Biol. 2014;21:308–16. [DOI] [PubMed] [Google Scholar]
- 18.Zhang L, Widau RC, Herring BP, Gallagher PJ. Delta-like 1-Lysine613 regulates notch signaling. Biochim Biophys Acta. 2011;1813:2036–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Doherty D, Feger G, Younger-Shepherd S, Jan LY, Jan YN. Delta is a ventral to dorsal signal complementary to Serrate, another Notch ligand, in Drosophila wing formation. Genes Dev. 1996;10(4):421–34 PubMed PMID: 96178768. [DOI] [PubMed] [Google Scholar]
- 20.Sun X, Artavanis-Tsakonas S. The intracellular deletions of Delta and Serrate define dominant negative forms of the Drosophila Notch ligands. Development. 1996;122(8):2465–74 PubMed PMID: 96312925. [DOI] [PubMed] [Google Scholar]
- 21.de Celis JF, Bray S. Feed-back mechanisms affecting Notch activation at the dorsoventral boundary in the Drosophila wing. Development. 1997;124(17):3241–51 PubMed PMID: 97454244. [DOI] [PubMed] [Google Scholar]
- 22.Miccheli CA, Blair SS. Dorsoventral restriction in wing imaginal discs requires Notch. Nature. 1999;401:473–6. [DOI] [PubMed] [Google Scholar]
- 23.Palardy G, Chitnis AB. Identification of the Mind bomb1 interaction domain in zebrafish DeltaD. PLoS ONE. 2015. 10.1371/journal.pone.0127864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Viswanathan R, Necakov A, Trylinski M, Harish RK, Krueger D, Esposito E, et al. Optogenetic inhibition of Delta reveals digital Notch signalling output during tissue differentiation. EMBO Rep. 2019;20. 10.15252/embr.201947999. [DOI] [PMC free article] [PubMed]
- 25.Corson F, Couturier L, Rouault H, Mazouni K, Schweisguth F. Self-organized Notch dynamics generate stereotyped sensory organ patterns in Drosophila. Science. 2017;356:356. 10.1126/science.aai7407. [DOI] [PubMed] [Google Scholar]
- 26.Wang W, Struhl G. Drosophila Epsin mediates a select endocytic pathway that DSL ligands must enter to activate Notch. Development. 2004;131(21):5367–80 PubMed PMID: 15469974. [DOI] [PubMed] [Google Scholar]
- 27.Wang W, Struhl G. Distinct roles for Mind bomb, Neuralized and Epsin in mediating DSL endocytosis and signaling in Drosophila. Development. 2005;132(12):2883–94 PubMed PMID: 15930117. [DOI] [PubMed] [Google Scholar]
- 28.Panin VM, Papayannopoulos V, Wilson R, Irvine KD. Fringe modulates Notch-ligand interactions. Nature. 1997;387(6636):908–12 PubMed PMID: 97345631. [DOI] [PubMed] [Google Scholar]
- 29.Troost T, Klein T. Sequential Notch signalling at the boundary of fringe expressing and non-expressing cells. PLoS One. 2012;7:e49007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Itoh M, Kim C-H, Palardy G, Oda T, Jiang Y-J, Maust D, et al. Mind bomb is a ubiquitin ligase that is essential foe efficient activation of Notch signalling by Delta. Dev Cell. 2003;4:67–82. [DOI] [PubMed] [Google Scholar]
- 31.Chen W, Corliss C. Three modules of zebrafish Mind bomb work cooperatively to promote Delta ubiquitination and endocytosis. Dev Biol. 2003;267:361–73. [DOI] [PubMed] [Google Scholar]
- 32.Parks AL, Stout JR, Shepard SB, Klueg KM, Dos Santos AA, Parody TR, et al. Structure-function analysis of delta trafficking, receptor binding and signaling in Drosophila. Genetics. 2006;174:1947–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Furriols M, Bray S. A model response element detects Suppressor of Hairless-dependent molecular switch. Curr Biol. 2001;11:60–4. [DOI] [PubMed] [Google Scholar]
- 34.Speicher SA, Thomas U, Hinz U, Knust E. The Serrate locus of Drosophila and its role in morphogenesis of the wing imaginal discs: control of cell proliferation. Development. 1994;120(3):535–44 PubMed PMID: 94215494. [DOI] [PubMed] [Google Scholar]
- 35.Castro B, Barolo S, Bailey A, Posakony JW. Lateral inhibition in proneural clusters: cis-regulatory logic and default repression by Suppressor of Hairlesss. Development. 2005;132:3333–44. [DOI] [PubMed] [Google Scholar]
- 36.Overstreet E, Fitch E, Fischer JA. Fat facets and Liquid facets promote Delta endocytosis and Delta signaling in the signaling cells. Development. 2004;131(21):5355–66 PubMed PMID: 15469967. [DOI] [PubMed] [Google Scholar]
- 37.Klein T. Immunolabelling of imaginal discs. In: Methods in molecular biology. 2006; Volume: Drosophila, ed. Christian Dahmann Humana Press. 10.1007/978-1-59745-583-1_15.
- 38.Zipper L, Jassmann D, Burgmer S, Görlich S, Reiff T. Ecdysone steroid hormone remote controls intestinal stem cell fate decisions via the PPARγ- homolog Eip75B in Drosophila. 2020. 10.7554/eLife.55795. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figures S1–S7. Fig. S1 Comparison of the aa sequence of the ICD of Dl orthologs among insect species. Fig. S2 The phenotype of expression of Dl-NB2A and Dl-CB2A with the flanking Ks also exchanged to A. Fig. S3 The phenotype of a Dl knock-in allele with the HA tag inserted into the extracellular domain, close to the transmembrane domain. Fig. S4 The role of the ICD of Dl revealed by the analysis of DlattP-DlΔICD-HA. Fig. S5 The adult phenotype of mib1 mutant flies rescued with one copy of the described Mib1 variants expressed under control of tub.P. Fig. S6 Presentation of the complete analysis of all Dl variants generated and tested for this study. Fig. S7 The role of the Ks in the ICD of Dl close to the transmembrane domain.
Data Availability Statement
No datasets were generated or analysed during the current study.