Abstract
Background
Obstructive sleep apnea (OSA) is associated with poorer quality of life (QoL) and increased cardiovascular risks, which may be exacerbated by hypovitaminosis D. Sodium glucose transporter-2 inhibitor (SGLT2i) provides cardiovascular benefits beyond glycemic control. As vitamin D3 and SGLT2i act through different pathways with similar mechanisms in improving cardio-metabolic health, this study aimed to investigate the synergistic effects of this combination therapy in improving these parameters and QoL in hypertensive obese OSA patients.
Methods
Patients who fulfilled the study criteria were randomized to receive: (i) Dapagliflozin, (ii) vitamin D3, (iii) Dapagliflozin plus vitamin D3, or (iv) no treatment, for 16 weeks. The parameters evaluated included anthropometric measurements, uric acid, HbA1c, lipid profile, steatotic liver disease grade, plasma aldosterone concentration, plasma renin concentration, ultrasound flow-mediated dilatation of brachial artery, hsCRP, heart rate variability (HRV), Epworth Sleepiness Scale (ESS), and QoL scores.
Results
A total of 163 patients were recruited and 153 completed the study. The combination of vitamin D3 and Dapagliflozin treatment led to significant improvements in metabolic parameters and nocturnal heart rates, and prevented deterioration of HRV, with healthier HRV at the end of study visit compared to the control group. Only the combination group exhibited improvements in both ESS and QoL scores.
Conclusions
This is the first study to demonstrate beneficial effects of combining vitamin D3 and SGLT2i in cardio-metabolic outcomes and QoL in hypertensive obese OSA patients. These findings highlight the potential of this combination therapy in addressing the cardio-metabolic challenges and QoL in this patient population.
Trials registration
NCT06690723. Registered 10 October 2024—Retrospectively registered, https://register.clinicaltrials.gov/prs/beta/studies/S000EWGF00000085/recordSummary
Supplementary Information
The online version contains supplementary material available at 10.1186/s12967-025-06312-w.
Keywords: Cardiovascular, Dapagliflozin, Endothelial function, Epworth Sleepiness Scale, Heart rate variability
Background
Obstructive sleep apnea (OSA) is one of the most common sleep-related breathing disorders, with increasing prevalence worldwide due to its close association with obesity and aging [1]. Although still unclear, the recurrent episodes of upper airway obstruction during sleep leading to intermittent hypoxia and sleep fragmentation may contribute to endothelial dysfunction, cardiac autonomic dysfunction, and inflammatory processes [2]. This may explain the elevated cardiovascular risks observed in this cohort of patients. Furthermore, the presence of metabolic syndrome commonly found in OSA, especially hypertension, which has been reported to be highly prevalent in these patients, may worsen the cardio-metabolic risks [3]. Untreated OSA patients may also exhibit excessive daytime sleepiness [4] and report poorer quality of life (QoL) when compared to the general population [5]. The current gold standard treatment for OSA is continuous positive airway pressure (CPAP) treatment, however compliance has been reported to be poor and it does not confer metabolic improvement and cardiovascular protection [6, 7].
OSA is associated with activation of the renin–angiotensin–aldosterone system (RAAS), which partly contributes to the high prevalence of hypertension and plays a critical role in the pathophysiology of various cardiovascular disease and all-cause mortality seen in these patients [8–10]. Vitamin D, a fat-soluble secosteroid, is primarily obtained through sunlight exposure. Despite Malaysia’s abundant sunshine, vitamin D deficiency (VDD) remains prevalent [11]. Increasing evidence suggests a bidirectional relationship and independent correlation between OSA and VDD [12, 13]. This association remained significant even when age and body mass index (BMI) were controlled for, supporting the independent correlation between these two entities [13]. Beyond its effect on the skeletal system, vitamin D is now believed to play a role in cardiovascular health. Vitamin D receptors (VDR) and the enzymes responsible for metabolizing vitamin D are found in the cardiovascular tissues [14]. The mechanism of which vitamin D supplementation contributes to cardiovascular benefits is hypothesized to be driven by its effect on reducing inflammatory markers thus improving endothelial function [15], mitigating insulin resistance [16], and ameliorating RAAS derangement [17]. Although these benefits remain controversial, the observed inconsistencies may be attributed to heterogenous study designs, diverse populations, varying vitamin D dosages, and treatment duration differences [18]. Nevertheless, vitamin D supplementation has shown therapeutic benefits in addressing metabolic derangements, including improving glucose homeostasis [19], enhanced lipid profiles [20], and reduced inflammation [21], which could contribute to mitigating cardiovascular risks. However, data on vitamin D supplementation in patients with OSA remains limited.
The sodium glucose transporter-2 inhibitor (SGLT2i) is a class of oral anti-diabetic agent that provides cardiovascular benefits extending beyond its glucose-lowering effects. It works by inducing glycosuria and is known to reduce HbA1c and blood pressure (BP) in addition to increasing HDL and LDL while decreasing triglycerides [22]. Large cardiovascular trials such as DECLARE-TIMI and EMPA-REG have consistently shown a reduction in adverse cardiovascular outcomes with SGLT2i use [23, 24]. In addition to the metabolic improvements mentioned, the cardiovascular benefit stemming from the use of SGLT2i is also believed to be contributed by other mechanisms, such as direct cardiac beneficial effects including improvement in cardiac metabolism [25], reducing inflammatory markers [26], and natriuresis effect leading to reduction in BP [27]. Nevertheless, despite the favourable cardiovascular outcomes with the use of SGLT2i among patients with type 2 diabetes, data on the use of this drug among patients with OSA is scarce.
Since vitamin D and SGLT2i act through different pathways, with some similarities in their underlying mechanisms for improving cardiovascular and metabolic health, this study aimed to investigate the combined effects of these two treatments on (i) cardio-metabolic parameters, (ii) sleepiness symptoms, and (iii) QoL in hypertensive obese OSA patients .
Materials and methods
Study population and study criteria
This was a 4-arm parallel randomized controlled trial conducted from June 2022 till October 2024. Patients who were suspected of having OSA were referred from health clinics around Kuching and Kota Samarahan areas to Sarawak General Hospital Sleep Unit.
A lower BMI cut-off was used in this study as Asians have higher cardiovascular risks at a lower BMI than the existing World Health Organization (WHO) cut-off point for obesity [28]. Thus, the inclusion criteria were (i) aged ≥ 18 years, (ii) BMI ≥ 27.5 kg/m2, (iii) confirmed OSA on sleep study, and (iv) presence of hypertension; whereas the exclusion criteria were (i) known secondary hypertension, (ii) conditions that affect RAAS and vitamin D levels, including chronic kidney disease, congestive heart failure, recent myocardial infarction within 6 weeks of recruitment, and uncontrolled thyroid or parathyroid disorders, (iii) long-standing atrial fibrillation, (iv) CPAP treatment, (v) on vitamin D or calcium supplements, (vi) on SGLT2i treatment, (vii) on medications that could lead to weight loss, such as GLP-1 receptor analogue, (viii) malignancy, and (ix) pregnancy.
Measurements and definitions
OSA was confirmed with sleep study, and its severity was classified as mild (apnoea hypopnea index, AHI 5–15/hour), moderate (AHI > 15–30/hour), or severe (AHI > 30/hour) in accordance to the clinical practice guidelines [29]. Hypertension was defined as systolic BP ≥ 140 mmHg, and/or diastolic BP ≥ 90 mmHg on two separate occasions, or if the patient was taking anti-hypertensive treatment. Previous smokers and previous alcohol consumers were those who ceased smoking or alcohol intake for at least 1 year respectively. Vitamin D status was categorized according to the Endocrine Society guidelines [30]. Those with serum 25-hydroxyvitamin D [25(OH)D] of < 20 ng/mL were considered vitamin D deficient, 25(OH)D 20–30 ng/mL as insufficient, whereas a level of > 30 ng/mL were sufficient.
Metabolic parameters assessed included BP, BMI, waist circumference, neck circumference, uric acid, HbA1c, lipid profile, and presence of metabolic-associated steatotic liver disease (MASLD); whereas cardiovascular risk parameters evaluated were (i) high-sensitivity C-reactive protein (hsCRP) reflecting inflammation, (ii) cardiac autonomic function using long-term heart rate variability (HRV), i.e. standard deviation of the NN interval (SDNN), mean of the 5 min SDNN calculated over 24 h (ASDNN), and the standard deviation of the average NN interval calculated over 5 min (SDANN), and short-term HRV using the Polar H10 device, which reported SDNN, mean RR, square root of the mean of the sum of the squares of differences between adjacent NN intervals (RMSSD), parasympathetic nervous system (PNS) index, sympathetic nervous system (SNS) index, and stress index, (iii) minimum, maximum, and average heart rates evaluated over 24 h, (iv) daytime and nocturnal heart rates, (v) RAAS components, including plasma aldosterone concentration (PAC) and plasma renin concentration (PRC), and (vi) endothelial function assessed with ultrasound of flow-mediated dilatation (FMD) of the brachial artery.
MASLD was graded as normal (grade 0) or grades 1–3[31]. The hsCRP levels were classified into < 1 mg/L, 1–3 mg/L, and > 3 mg/L as low-, intermediate-, and high-risk for global cardiovascular disease respectively[32]. The normal range of each HRV parameter is displayed in Supp Tables 1 and 2. Briefly, a higher SDNN, ASDNN, SDANN, mean RR, RMSSD and PNS index, with lower SNS index, stress index, and heart rates are indicative of a healthier heart, reflecting better autonomic regulation and cardiovascular resilience. The dipping percentage was defined using the following equation:
A change of < 10% was considered as non-dipping. Daytime and nocturnal episodes were defined as occurring from 11am to 10 pm, and from 12 midnight to 4 am, respectively.
The normal ranges of PAC and PRC were 2.21–35.3 ng/dL and 4.4–46.1 μIU/mL respectively for samples taken in upright posture.
The FMD value was calculated using the following equation and expressed as a percent change in vessel caliber:
Normal endothelial function assessed by FMD was defined as ≥ 7.1% as this cut-off best discriminates those with cardiovascular risks compared to those without [33].
Sleepiness symptoms were evaluated using Epworth Sleepiness Scale (ESS), a self-administered questionnaire with scores ranging from 0 to 24, of which a score of > 10 indicates higher than normal daytime sleepiness.
QoL was evaluated using the WHOQOL-BREF, which is a shorter version of WHOQOL-100 [34]. It consists of 26 self-administered questions regarding an individual’s perception on health and well-being over the previous two weeks, with responses on a 1–5 Likert scale. The four domains covered by WHOQOL-BREF include (i) physical health, (ii) psychological health, (iii) social relationships, and (iv) environmental health, in addition to two separate questions asking specifically about the individual’s overall perception on overall quality of life and general health.
Study protocol (Supp Fig)
All anti-hypertensive treatments that affect the RAAS were discontinued for at least 6 weeks for spironolactone, 4 weeks for non-potassium sparing diuretics, and 2 weeks for angiotensin converting enzyme inhibitors, angiotensin II type 1 receptor blockers, beta blockers, and dihydropyridine calcium blockers before first visit (V1). If indicated, anti-hypertensive treatment with the least effect on RAAS, such as non-dihydropyridine calcium blockers and/or alpha blockers, were prescribed for BP control. All participants had unrestricted salt intake.
After informed consent, the study subjects were interviewed by a single investigator regarding their socio-demography and medical history. Following administration of ESS and WHOQoL questionnaires, all subjects then underwent anthropometric measurements by the same investigator. Blood was taken after an overnight fast for at least 8 h and patients being upright for at least 2 h upon waking.
Ultrasound of the hepatobiliary system and FMD of the brachial artery were then performed by a single radiologist to assess for presence of steatotic liver disease and endothelial dysfunction respectively. Subsequently, all patients underwent evaluation of the cardiac autonomic function using Polar H10 device to assess short-term HRV, followed by placement of a 24 h Holter monitoring to evaluate the long-term HRV, while maintaining their usual daily activities.
The subjects were then randomized by the primary investigator using computer-generated block randomization method to either of the 4 arms: (i) Group 1: Dapagliflozin, (ii) Group 2: vitamin D3, (iii) Group 3: Dapagliflozin plus vitamin D3, or (iv) Group 4 (control arm): no treatment, for a total duration of 16 weeks. All patients received standard education by a single investigator on lifestyle modification at V1. At the same setting, those who received Dapagliflozin were prescribed 10 mg daily and were given standard advice of precautions with its use [35]. Patients who received vitamin D3 had the doses of vitamin D prescribed according to the baseline 25(OH)D level. Those with 25(OH)D level of < 30 ng/mL received 6000 IU a day, otherwise they were prescribed with vitamin D3 dose of 4000 IU a day [30].
A follow-up phone call was made at 1-week and 4-week after V1 to inquire if the patients developed any side effects with the medications. All patients were seen 8 weeks later at visit 2 (V2) to document changes in metabolic parameters, endothelial function, and short-term HRV. The vitamin D3 dose was titrated according to the 25(OH)D level at V2, if indicated. All patients underwent the same evaluations as V1 at the end of the study (V3). Any side effects encountered during the study period were documented at each clinic visit. Compliance of the patients to treatment was assessed using pill-counting method. Those who had a compliance rate of < 80% were removed from the final analysis. All except the primary investigator were unaware of the patients’ study group.
Subgroup analysis was also performed to evaluate the effect of treatment on cardio-metabolic parameters among those who were vitamin D insufficient or deficient at baseline in all study arms, and those who achieved vitamin D sufficiency among those who received vitamin D treatment. Besides, gender-based analysis was performed to compare the effect of treatment combination on cardiovascular and metabolic parameters.
Statistical analysis
Statistical analysis was performed using SPSS software (version 29, SPSS Inc., Chicago IL). Continuous variables were presented as mean ± SD or median (IQR), whereas categorical variables were presented as absolute counts with their percentages. Variables among the 4 groups were compared at baseline and end of study visit using ANOVA if they were normally distributed, and comparison between groups were performed using Tukey–Kramer post hoc test if the differences on ANOVA analysis were significant. Otherwise, the differences were compared using Kruskal–Wallis test. For categorical variables, chi-squared test was applied to test the differences among the 4 groups. For normally distributed variables, the changes with treatment were compared using T test for baseline and end of study visits, or repeated measures ANOVA for all three study visits. For non-normally distributed variables, the changes were evaluated using Wilcoxon signed rank test for comparison between 2 study visits, or Friedman test for 3 study visits. A p value of < 0.05 was taken as statistically significant.
Results
A total of 797 patients suspected of having OSA were referred for sleep study over the period of 29 months. After screening of eligibility, 163 who fulfilled study criteria were recruited. A total of 10 patients were removed from analysis as 6 were found to be non-compliant to treatment, whereas 4 defaulted follow-up visits due to logistic reasons. Hence, 153 patients were included in the final analysis. There was no significant difference in the baseline demography, ESS, QoL scores, and cardio-metabolic parameters, as shown in Tables 1 and 2.
Table 1.
Baseline demography
| Variables | Group 1 n = 36 | Group 2 n = 41 | Group 3 n = 39 | Group 4 n = 37 | p |
|---|---|---|---|---|---|
| Demography | |||||
| Age, years | 44.7 ± 12.3 | 48.1 ± 11.6 | 46.8 ± 11.6 | 45.1 ± 12.2 | 0.574 |
| Male, n (%) | 20 (55.6) | 19 (46.3) | 24 (61.5) | 21 (56.8) | 0.580 |
| Ethnicity, n (%) | 0.890 | ||||
| Malay | 15 (41.7) | 20 (48.8) | 17 (43.6) | 15 (40.5) | |
| Chinese | 7 (19.4) | 6 (14.6) | 8 (20.5) | 9 (24.3) | |
| Iban | 7 (19.4) | 5 (12.2) | 7 (17.9) | 4 (10.8) | |
| Bidayuh | 6 (16.7) | 10 (24.4) | 5 (12.8) | 7 (18.9) | |
| Others | 1 (2.8) | 0 (0) | 2 (5.1) | 2 (5.4) | |
| Education, n (%) | 0.112 | ||||
| No Formal Education | 1 (2.8) | 1 (2.4) | 1 (2.6) | 1 (2.7) | |
| Primary | 3 (8.3) | 11 (26.8) | 3 (7.7) | 1 (2.7) | |
| Secondary | 22 (61.1) | 16 (39.0) | 21 (53.8) | 23 (62.2) | |
| Tertiary | 10 (27.8) | 13 (31.7) | 14 (35.9) | 12 (32.4) | |
| Co-morbidities, n (%) | |||||
| Type 2 diabetes | 19 (52.8) | 27 (65.9) | 28 (71.8) | 18 (48.6) | 0.132 |
| Dyslipidemia | 24 (64.9) | 24 (58.5) | 25 (62.5) | 23 (62.2) | 0.952 |
| Hyperuricemia | 20 (55.6) | 29 (70.7) | 24 (61.5) | 23 (62.2) | 0.584 |
| Smoking, n (%) | 0.693 | ||||
| Yes | 4 (11.1) | 6 (15.0) | 8 (21.6) | 4 (10.8) | |
| No | 22 (61.1) | 21 (52.5) | 19 (51.4) | 25 (67.6) | |
| Previous | 10 (27.8) | 13 (32.5) | 10 (27.0) | 8 (21.6) | |
| Alcohol consumer, n (%) | 0.461 | ||||
| Yes | 7 (19.4) | 6 (15.0) | 8 (21.6) | 11 (29.7) | |
| No | 22 (61.1) | 27 (67.5) | 24 (64.9) | 24 (64.9) | |
| Previous | 7 (19.4) | 7 (17.5) | 5 (13.5) | 2 (5.4) | |
| OSA severity, n (%) | 0.977 | ||||
| Mild | 3 (8.3) | 4 (9.8) | 2 (5.1) | 2 (5.4) | |
| Moderate | 10 (27.8) | 12 (29.3) | 10 (25.6) | 11 (29.7) | |
| Severe | 23 (63.9) | 25 (61.0) | 27 (69.2) | 24 (64.9) | |
| AHI, /hour | 45.3 ± 27.0 | 41.7 ± 24.0 | 50.2 ± 30.6 | 46.3 ± 28.2 | 0.593 |
| Anthropometry | |||||
| Systolic BP, mmHg | 153.3 ± 16.5 | 150.6 ± 17.1 | 151.2 ± 13.8 | 153.8 ± 21.2 | 0.811 |
| Diastolic BP, mmHg | 97.4 ± 11.7 | 94.1 ± 11.6 | 97.2 ± 13.0 | 98.2 ± 15.5 | 0.524 |
| Pulse rate, bpm | 76.5 ± 13.9 | 75.4 ± 13.3 | 76.4 ± 12.2 | 80.7 ± 13.8 | 0.319 |
| BMI, kg/m2 | 42.1 ± 7.4 | 39.8 ± 7.3 | 41.6 ± 7.0 | 39.4 ± 7.8 | 0.328 |
| Neck circumference, cm | 43.5 ± 4.7 | 42.8 ± 3.9 | 44.4 ± 4.0 | 43.1 ± 4.3 | 0.357 |
| Waist circumference, cm | 122.5 ± 14.6 | 117.4 ± 12.0 | 122.3 ± 12.6 | 117.0 ± 15.9 | 0.147 |
| Patient-reported outcomes | |||||
| ESS | 8.8 ± 5.0 | 8.9 ± 5.8 | 10.4 ± 4.7 | 9.1 ± 5.7 | 0.585 |
| WHOQoL-BREF | |||||
| Quality of Life | 3.49 ± 0.77 | 3.39 ± 0.70 | 3.40 ± 0.81 | 3.61 ± 0.72 | 0.772 |
| General Health | 2.97 ± 0.87 | 3.02 ± 0.69 | 2.95 ± 0.64 | 3.11 ± 0.97 | 0.726 |
| Domain 1 | 12.2 ± 1.7 | 12.5 ± 1.7 | 12.7 ± 1.6 | 12.8 ± 1.7 | 0.653 |
| Domain 2 | 12.8 ± 1.9 | 12.5 ± 1.6 | 13.1 ± 2.1 | 13.1 ± 1.7 | 0.844 |
| Domain 3 | 14.4 ± 2.5 | 13.9 ± 2.8 | 13.9 ± 2.9 | 14.5 ± 3.4 | 0.733 |
| Domain 4 | 14.1 ± 2.2 | 13.7 ± 2.1 | 14.1 ± 1.9 | 14.6 ± 1.8 | 0.452 |
Numerical variables are presented as the mean ± standard deviation, categorical variables are defined as absolute count and percentage
OSA: obstructive sleep apnea; AHI: apnea hypopnea index; BP: blood pressure; BMI: body mass index; ESS: Epworth Sleepiness Scale; WHOQoL-BREF: World Health Organization Quality of Life—brief version
Table 2.
Cardio-metabolic parameters of study participants at baseline
| Variables | Group 1 n = 36 | Group 2 n = 41 | Group 3 n = 39 | Group 4 n = 37 | p |
|---|---|---|---|---|---|
| Biochemistry | |||||
| Uric acid, mmol/L | 420.4 ± 84.9 | 427.2 ± 103.8 | 433.9 ± 87.3 | 422.7 ± 85.0 | 0.923 |
| HbA1c, % | 6.4 (5.9, 6.8) | 6.3 (5.9, 6.9) | 6.7 (5.9, 7.7) | 6.0 (5.8, 6.8) | 0.202 |
| Lipid, mmol/L | |||||
| Total | 4.61 ± 1.32 | 4.95 ± 0.87 | 4.52 ± 1.03 | 4.59 ± 0.94 | 0.339 |
| LDL-C | 2.65 ± 1.08 | 2.86 ± 0.79 | 2.67 ± 0.78 | 2.61 ± 0.81 | 0.593 |
| HDL-C | 1.27 ± 0.23 | 1.33 ± 0.26 | 1.29 ± 0.42 | 1.29 ± 0.26 | 0.872 |
| Triglyceride | 1.35 (1.08, 2.11) | 1.65 (1.13, 2.33) | 1.54 (1.12, 1.96) | 1.65 (1.01, 2.16) | 0.611 |
| Creatinine, umol/L | 72.4 ± 19.3 | 74.3 ± 25.7 | 74.4 ± 17.1 | 74.8 ± 22.2 | 0.966 |
| 25(OH)D, ng/mL | 18.3 ± 7.6 | 19.6 ± 9.1 | 20.3 ± 5.7 | 21.0 ± 8.1 | 0.493 |
| Vitamin D status, n (%) | 0.274 | ||||
| Deficient | 22 (59.5) | 25 (61.0) | 19 (48.7) | 20 (55.6) | |
| Insufficient | 12 (32.4) | 10 (24.4) | 19 (48.7) | 13 (36.1) | |
| Sufficient | 3 (8.1) | 6 (14.6) | 1 (2.6) | 3 (8.3) | |
| Phosphate, mmol/L | 1.14 ± 0.19 | 1.19 ± 0.24 | 1.18 ± 0.22 | 1.20 ± 0.15 | 0.664 |
| iPTH, pg/mL | 68.2 (43.8, 91.8) | 65.7 (40.7, 88.8) | 65.3 (50.5, 87.2) | 56.1 (40.3, 72.8) | 0.421 |
| hsCRP | 6.8 ± 9.9 | 6.3 ± 5.6 | 7.2 ± 5.6 | 8.4 ± 9.6 | 0.679 |
| Ultrasound of the hepatobiliary system | |||||
| MASLD, n (%) | 0.707 | ||||
| Normal | 7 (19.4) | 3 (7.3) | 2 (5.1) | 3 (8.1) | |
| Grade 1 | 6 (16.7) | 10 (24.4) | 8 (20.5) | 6 (16.2) | |
| Grade 2 | 21 (58.3) | 25 (61.0) | 23 (59.0) | 23 (62.2) | |
| Grade 3 | 2 (5.6) | 3 (7.3) | 5 (12.8) | 4 (10.8) | |
| Heart rate variability | |||||
| Long-term, ms | |||||
| ASDNN | 49.7 ± 21.4 | 48.7 ± 15.6 | 47.5 ± 14.8 | 44.4 ± 17.1 | 0.579 |
| SDANN | 105.5 ± 45.6 | 104.0 ± 39.2 | 96.5 ± 34.0 | 100.7 ± 27.6 | 0.725 |
| SDNN | 118.9 ± 48.4 | 116.9 ± 37.8 | 111.9 ± 37.6 | 111.9 ± 30.9 | 0.815 |
| Short-term, ms | |||||
| SDNN, ms | 17.9 (11.1, 25.0) | 20.2 (13.4, 30.2) | 16.8 (11.4, 28.0) | 17.0 (11.8, 21.8) | 0.647 |
| Mean RR, ms | 787.8 ± 150.0 | 794.4 ± 158.3 | 761.4 ± 148.0 | 753.7 ± 127.5 | 0.571 |
| RMSSD, ms | 16.5 (8.0, 25.3) | 17.5 (10.5, 29.8) | 15.0 (8.0, 22.0) | 15.5 (10.0, 22.8) | 0.657 |
| PNS index | − 1.1 ± 1.2 | − 1.1 ± 1.3 | − 1.3 ± 1.1 | − 1.5 ± 0.9 | 0.404 |
| SNS index | 3.0 ± 2.8 | 2.7 ± 3.1 | 3.3 ± 3.2 | 3.4 ± 3.0 | 0.694 |
| Stress index | 23.3 ± 12.1 | 21.4 ± 13.6 | 24.4 ± 15.3 | 23.4 ± 12.0 | 0.762 |
| 24 h heart rate, bpm | |||||
| Minimum | 50.3 ± 8.9 | 49.1 ± 9.5 | 49.9 ± 9.5 | 52.8 ± 9.0 | 0.341 |
| Maximum | 123.3 ± 16.3 | 124.8 ± 13.7 | 122.7 ± 14.3 | 127.6 ± 13.7 | 0.470 |
| Average | 80.6 ± 11.1 | 80.8 ± 9.8 | 81.9 ± 9.3 | 84.1 ± 9.4 | 0.395 |
| Daytime | 84.2 ± 12.3 | 85.1 ± 9.9 | 83.1 ± 11.3 | 88.1 ± 10.7 | 0.426 |
| Nocturnal | 71.2 ± 13.0 | 71.9 ± 11.9 | 72.9 ± 10.3 | 74.4 ± 10.1 | 0.686 |
| Renin–angiotensin–aldosterone parameters | |||||
| PAC, ng/dL | 8.98 (5.48, 12.10) | 9.36 (6.66, 11.4) | 9.41 (6.59, 13.30) | 8.82 (6.54, 11.30) | 0.820 |
| PRC, μIU/mL | 13.03 (7.10, 23.67) | 13.50 (6.88, 20.91) | 13.68 (5.05, 23.94) | 14.76 (7.45, 24.72) | 0.949 |
| Endothelial function | |||||
| FMD, % | 6.17 ± 1.31 | 5.92 ± 1.80 | 6.12 ± 1.54 | 6.06 ± 1.21 | 0.890 |
| FMD < 7%, n (%) | 30 (83.3) | 35 (85.4) | 33 (84.6) | 34 (91.9) | 0.713 |
Numerical variables are presented as the mean ± standard deviation or median (IQR), categorical variables are defined as absolute count and percentage
25(OH)D: 25-hydroxyvitamin D; iPTH: intact parathyroid hormone; hsCRP: high-sensitivity C-reactive protein; MASLD: metabolic-associated steatotic liver disease; ASDNN: the mean of the 5-min SDNN calculated over 24 h; SDANN: the standard deviation of the average NN interval calculated over 5 min; SDNN: standard deviation of the NN interval; RMSSD: the square root of the mean of the sum of the squares of differences between adjacent NN intervals; PNS: parasympathetic nervous system; SNS: sympathetic nervous system; PAC: plasma aldosterone concentration; PRC: plasma renin concentration; FMD: flow-mediated dilatation
Effect of treatment on metabolic parameters
The effect of treatment on metabolic parameters is presented in Table 3. All patients except those in the control arm demonstrated significant improvement in metabolic parameters. Specifically, patients in groups 1 and 3 demonstrated significant improvement in BP, BMI, neck and waist circumferences, uric acid, and HbA1c. A reduction in HDL-C level was noted in both groups. Patients in group 2 demonstrated significant improvement in systolic BP, uric acid, and total cholesterol levels. Two patients in group 1 had improvement of MASLD – one from grade 1 to normal, and another from grade 2 to normal; whereas 2 patients from group 3 showed improvement of MASLD from grade 1 to normal, although these changes did not achieve statistical significance. These findings were similar in the subgroup analysis (Supp Table 3). When comparing the improvements observed in group 3 by gender, females demonstrated more substantial enhancements in metabolic parameters than males (Supp Table 4).
Table 3.
Metabolic parameter changes before and after treatment (all cohort)
| Variables | Group 1 n = 36 | Group 2 n = 41 | Group 3 n = 39 | Group 4 n = 37 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before | After | p | Before | After | p | Before | After | p | Before | After | p | |
| Anthropometric | ||||||||||||
| Systolic BP, mmHg | 153.3 ± 16.5 | 146.2 ± 15.3 | 0.030 | 150.6 ± 17.1 | 142.9 ± 16.0 | 0.003 | 151.2 ± 13.8 | 143.7 ± 16.5 | 0.003 | 153.8 ± 21.2 | 149.3 ± 20.6 | 0.098 |
| Diastolic BP, mmHg | 97.4 ± 11.7 | 93.7 ± 12.4 | 0.070 | 94.1 ± 11.6 | 91.0 ± 11.0 | 0.050 | 97.2 ± 13.0 | 92.8 ± 11.2 | 0.017 | 98.2 ± 15.5 | 96.4 ± 13.9 | 0.351 |
| Pulse rate, bpm | 76.5 ± 13.9 | 74.8 ± 14.1 | 0.197 | 75.4 ± 13.3 | 72.2 ± 13.4 | 0.096 | 76.4 ± 12.2 | 75.2 ± 11.1 | 0.527 | 80.7 ± 13.8 | 77.0 ± 14.1 | 0.057 |
| BMI, kg/m2 | 42.1 ± 7.4 | 40.8 ± 6.5 | < 0.001 | 39.8 ± 7.3 | 39.9 ± 7.5 | 0.646 | 41.6 ± 7.0 | 40.3 ± 6.5 | < 0.001 | 39.4 ± 7.8 | 39.4 ± 8.0 | 0.649 |
| Neck circ., cm | 43.5 ± 4.7 | 42.5 ± 5.0 | < 0.001 | 42.8 ± 3.9 | 42.6 ± 3.9 | 0.275 | 44.4 ± 4.0 | 43.4 ± 4.1 | < 0.001 | 43.1 ± 4.3 | 42.9 ± 4.4 | 0.352 |
| Waist circ., cm | 122.5 ± 14.6 | 118.5 ± 13.2 | < 0.001 | 117.7 ± 12.1 | 116.9 ± 12.6 | 0.190 | 122.3 ± 12.6 | 118.2 ± 12.2 | < 0.001 | 117.0 ± 15.9 | 116.4 ± 16.2 | 0.316 |
| Biochemistry | ||||||||||||
| Uric acid, mmol/L | 420.4 ± 84.9 | 346.7 ± 89.5 | < 0.001 | 427.2 ± 103.8 | 402.1 ± 92.7 | 0.036 | 433.9 ± 87.3 | 362.5 ± 86.5 | < 0.001 | 422.7 ± 85.0 | 419.6 ± 83.7 | 0.744 |
| HbA1c, % | 6.4 (5.9, 6.8) | 6.1 (5.7, 6.6) | 0.005 | 6.3 (5.9, 7.0) | 6.2 (5.8, 7.1) | 0.773 | 6.7 (5.9, 7.7) | 6.0 (5.7, 6.9) | < 0.001 | 6.0 (5.8, 6.8) | 6.1 (5.7, 6.7) | 0.387 |
| Lipid, mmol/L | ||||||||||||
| Total | 4.61 ± 1.32 | 4.32 ± 1.04 | 0.029 | 4.95 ± 0.87 | 4.68 ± 1.13 | 0.023 | 4.62 ± 1.03 | 4.33 ± 1.01 | 0.056 | 4.59 ± 0.94 | 4.59 ± 0.94 | 0.974 |
| LDL-C | 2.65 ± 1.08 | 2.52 ± 0.92 | 0.235 | 2.86 ± 0.79 | 2.74 ± 0.94 | 0.262 | 2.67 ± 0.78 | 2.51 ± 0.76 | 0.149 | 2.61 ± 0.81 | 2.69 ± 0.85 | 0.547 |
| HDL-C | 1.27 ± 0.23 | 1.17 ± 0.21 | < 0.001 | 1.33 ± 0.26 | 1.27 ± 0.29 | 0.109 | 1.29 ± 0.42 | 1.14 ± 0.23 | 0.029 | 1.29 ± 0.26 | 1.22 ± 0.21 | 0.095 |
| Triglyceride | 1.35 (1.08, 2.11) | 1.29 (1.05, 2.16) | 0.236 | 1.65 (1.13, 2.33) | 1.61 (1.12, 1.96) | 0.095 | 1.54 (1.12, 1.96) | 1.47 (1.02, 1.99) | 0.042 | 1.65 (1.01, 2.16) | 1.68 (1.24, 2.24) | 0.507 |
| Ultrasound of hepatobiliary system | ||||||||||||
| MASLD, n (%) | 0.50 | 1.0 | 0.50 | 1.0 | ||||||||
| Normal | 7 (18.4) | 9 (23.7) | 3 (7.1) | 3 (7.1) | 2 (5.4) | 4 (10.8) | 3 (8.3) | 3 (8.3) | ||||
| Grade 1 | 6 (15.8) | 5 (13.2) | 10 (23.8) | 10 (23.8) | 8 (21.6) | 6 (16.2) | 6 (16.7) | 6 (16.7) | ||||
| Grade 2 | 23 (60.5) | 22 (57.9) | 26 (61.9) | 26 (61.9) | 21 (56.8) | 21 (56.8) | 23 (63.9) | 23 (63.9) | ||||
| Grade 3 | 2 (5.3) | 2 (5.3) | 3 (7.1) | 3 (7.1) | 6 (16.2) | 6 (16.2) | 4 (11.1) | 4 (11.1) | ||||
Numerical variables are presented as the mean ± standard deviation or median (IQR), categorical variables are defined as absolute count and percentage
BP: blood pressure; BMI: body mass index; circ: circumference; MASLD: metabolic-associated steatotic liver disease
Effect of treatment on cardiovascular parameters
The effect of treatment on cardiovascular parameters in all study subjects is presented in Table 4. Only group 3 demonstrated significant improvement in the 24 h average and nocturnal heart rates. Groups 1 and 4 showed deterioration of the short-term HRV parameters over 16 weeks. Although there was significant improvement of FMD in all groups, only groups 2 and 3 had significant increment of the number of patients who achieved normal FMD at the end of study visits.
Table 4.
Cardiovascular parameter changes before and after treatment in all cohort
| Variables | Group 1 n = 36 | Group 2 n = 41 | Group 3 n = 39 | Group 4 n = 37 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before | After | p | Before | After | p | Before | After | p | Before | After | p | |
| hsCRP | 6.8 ± 9.9 | 6.5 ± 5.8 | 0.903 | 6.3 ± 5.6 | 5.5 ± 5.0 | 0.050 | 7.2 ± 5.6 | 7.6 ± 5.3 | 0.587 | 8.4 ± 9.6 | 6.5 ± 6.5 | 0.017 |
| Long term HRV, ms | ||||||||||||
| ASDNN | 49.7 ± 21.4 | 52.9 ± 21.2 | 0.228 | 48.7 ± 15.6 | 53.0 ± 16.1 | 0.053 | 47.5 ± 14.8 | 48.6 ± 15.6 | 0.700 | 44.4 ± 17.1 | 44.3 ± 17.3 | 0.946 |
| SDANN | 105.5 ± 45.6 | 114.1 ± 39.6 | 0.065 | 104.0 ± 39.2 | 109.2 ± 44.3 | 0.258 | 96.5 ± 34.0 | 102.5 ± 33.3 | 0.078 | 100.7 ± 27.6 | 102.1 ± 31.2 | 0.750 |
| SDNN | 118.9 ± 48.4 | 128.6 ± 42.5 | 0.072 | 116.9 ± 37.8 | 123.2 ± 40.4 | 0.194 | 111.9 ± 37.6 | 115.9 ± 32.3 | 0.387 | 111.9 ± 30.9 | 114.1 ± 33.9 | 0.637 |
| Short term HRV | ||||||||||||
| SDNN, ms | 17.9 (11.1, 25.0) | 12.5 (7.8, 21.8) | 0.026 | 20.2 (13.4, 30.2) | 15.2 (8.8, 28.2) | 0.208 | 16.8 (11.6, 28.5) | 16.4 (10.1, 31.0) | 0.694 | 17.0 (11.8, 21.8) | 11.0 (8.5, 18.8) | 0.005 |
| Mean RR, ms | 787.8 ± 145.0 | 748.8 ± 147.5 | 0.010 | 786.7 ± 155.4 | 777.6 ± 133.6 | 0.620 | 761.4 ± 149.0 | 786.8 ± 147.0 | 0.146 | 753.7 ± 127.5 | 735.1 ± 134.3 | 0.238 |
| RMSSD, ms | 16.5 (8.0, 25.3) | 10.5 (6.0, 19.0) | 0.032 | 17.5 (10.5, 29.8) | 16.0 (7.5, 29.0) | 0.142 | 15.5 (9.5, 22.0) | 13.0 (9.0, 20.0) | 0.803 | 15.5 (10.0, 22.8) | 10.0 (7.0, 14.8) | 0.007 |
| PNS index | − 1.3 ± 1.0 | − 1.7 ± 0.9 | 0.003 | − 1.1 ± 1.3 | − 1.2 ± 1.1 | 0.555 | − 1.3 ± 1.1 | − 1.3 ± 0.9 | 0.935 | − 1.5 ± 0.9 | − 1.7 ± 0.9 | 0.051 |
| SNS index | 3.0 ± 2.8 | 4.0 ± 3.5 | 0.006 | 2.8 ± 3.2 | 3.0 ± 2.6 | 0.555 | 3.3 ± 3.2 | 3.1 ± 2.3 | 0.527 | 3.4 ± 3.0 | 4.4 ± 3.1 | 0.019 |
| Stress index | 22.1 ± 10.0 | 26.4 ± 14.3 | 0.016 | 21.7 ± 13.9 | 23.9 ± 12.6 | 0.390 | 24.4 ± 15.3 | 23.9 ± 10.2 | 0.817 | 23.4 ± 12.0 | 27.8 ± 10.4 | 0.035 |
| 24 h heart rate, bpm | ||||||||||||
| Min HR | 50.3 ± 8.9 | 48.7 ± 7.9 | 0.217 | 49.1 ± 9.5 | 47.8 ± 8.4 | 0.282 | 49.9 ± 9.5 | 49.4 ± 6.8 | 0.643 | 52.8 ± 9.0 | 51.6 ± 8.2 | 0.223 |
| Max HR | 123.3 ± 16.3 | 122.1 ± 16.3 | 0.592 | 124.8 ± 13.7 | 122.5 ± 13.2 | 0.206 | 122.7 ± 14.3 | 121.7 ± 14.8 | 0.630 | 127.6 ± 13.7 | 125.2 ± 13.1 | 0.110 |
| Avg HR | 80.6 ± 11.1 | 78.6 ± 10.5 | 0.085 | 80.8 ± 9.8 | 78.7 ± 9.6 | 0.114 | 81.9 ± 9.3 | 79.4 ± 9.5 | 0.008 | 84.1 ± 9.4 | 83.9 ± 9.8 | 0.866 |
| Day HR | 84.2 ± 12.3 | 84.0 ± 11.7 | 0.874 | 85.1 ± 9.9 | 83.1 ± 11.3 | 0.182 | 85.6 ± 9.2 | 84.2 ± 9.8 | 0.232 | 88.1 ± 10.7 | 88.6 ± 11.2 | 0.735 |
| Noct. HR | 71.2 ± 13.0 | 69.0 ± 10.7 | 0.141 | 71.9 ± 11.9 | 69.3 ± 11.0 | 0.103 | 72.9 ± 10.3 | 70.0 ± 9.0 | 0.003 | 74.4 ± 10.1 | 74.5 ± 10.5 | 0.943 |
| Dipping, % | 15.3 ± 11.1 | 17.5 ± 9.6 | 0.148 | 15.6 ± 9.3 | 16.2 ± 10.6 | 0.678 | 14.8 ± 6.9 | 16.5 ± 8.0 | 0.189 | 15.4 ± 7.4 | 15.6 ± 9.0 | 0.890 |
| Non-dipper, n (%) | 9 (24.3) | 6 (16.2) | 0.375 | 11 (27.5) | 9 (22.5) | 0.754 | 9 (23.1) | 7 (18.4) | 0.754 | 10 (27.8) | 9 (25.0) | 1.0 |
| Renin–angiotensin–aldosterone system parameters | ||||||||||||
| PAC, ng/dL | 8.96 (5.48, 12.10) | 10.45 (7.58, 12.78) | 0.113 | 9.36 (6.66, 11.40) | 7.62 (4.96, 10.85) | 0.464 | 10.10 (6.83, 13.55) | 9.78 (6.70, 12.93) | 0.252 | 8.82 (6.54, 11.30) | 7.89 (6.76, 11.15) | 0.934 |
| PRC, μIU/mL | 13.03 (7.10, 23.67) | 15.50 (9.75, 35.31) | 0.523 | 13.50 (6.88, 20.91) | 13.30 (5.67, 26.42) | 0.979 | 15.27 (6.02, 23.32) | 12.77 (5.05, 29.20) | 0.492 | 14.76 (7.45, 24.72) | 14.67 (8.16, 23.91) | 0.789 |
| Endothelial function | ||||||||||||
| FMD, % | 6.17 ± 1.31 | 6.70 ± 1.04 | < 0.001 | 5.92 ± 1.80 | 6.61 ± 1.71 | 0.004 | 6.12 ± 1.50 | 6.60 ± 1.25 | 0.010 | 6.06 ± 1.21 | 6.51 ± 0.83 | 0.001 |
| > 7.1% | 6 (16.7) | 11 (30.6) | 0.125 | 6 (14.6) | 12 (29.3) | 0.031 | 6 (15.4) | 13 (33.3) | 0.016 | 3 (8.1) | 7 (18.9) | 0.125 |
Numerical variables are presented as the mean ± standard deviation or median (IQR), categorical variables are defined as absolute count and percentage
hsCRP: high-sensitivity C-reactive protein; ASDNN: the mean of the 5 min SDNN calculated over 24 h; SDANN: the standard deviation of the average NN interval calculated over 5 min; SDNN: standard deviation of the NN interval; RMSSD: the square root of the mean of the sum of the squares of differences between adjacent NN intervals; PNS: parasympathetic nervous system; SNS: sympathetic nervous system; min: minimum; max: maximum; avg: average; noct: nocturnal; PAC: plasma aldosterone concentration; PRC: plasma renin concentration; FMD: flow mediated dilatation; 25(OH)D: 25-hydroxyvitamin D
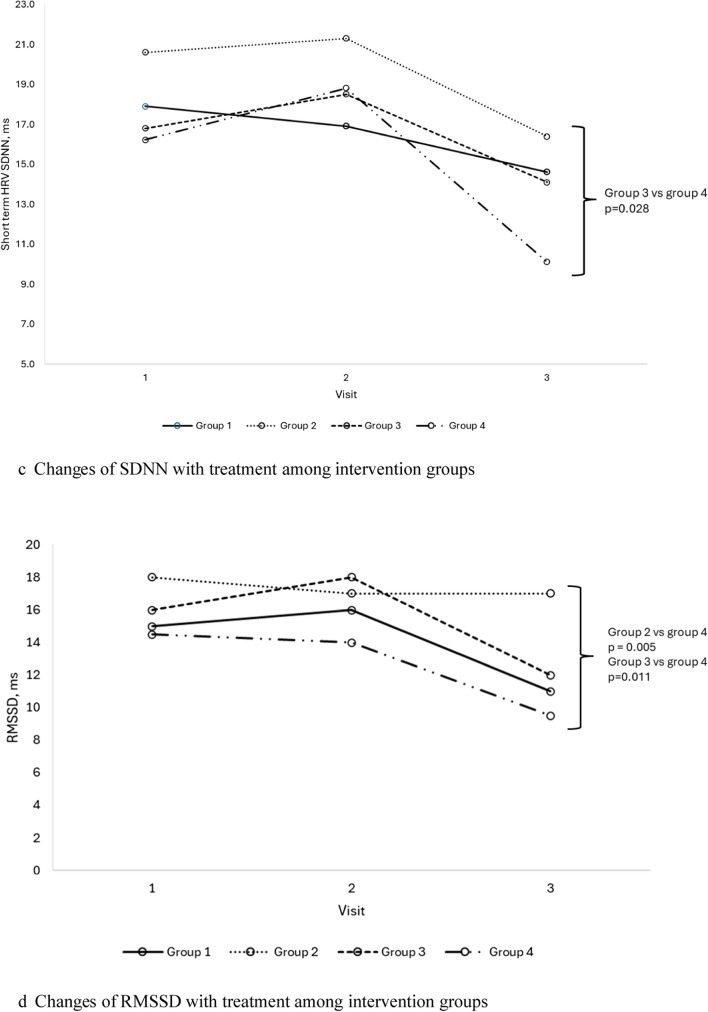
Subgroup analysis demonstrated significant improvement in ASDNN and average heart rate in group 2; and significant improvement in mean RR and 24 h average and nocturnal heart rates in group 3 (Table 5). There were also significant differences noted in SNS index, PNS index, SDNN, RMSSD, and average heart rate at the end of study visit between groups 2 and 3 with group 4 (Fig. 1). Specifically, group 3 demonstrated significantly lower SNS index, and higher PNS index and SDNN, whilst groups 2 and 3 showed significantly higher RMSSD and lower average heart rate, at the end of study visit compared to group 4. Gender-based analysis of group 3 revealed that females exhibited more pronounced cardiovascular improvements compared to males (Supp Table 4).
Table 5.
Cardiovascular parameter changes with treatment in subgroup analysis*
| Variables | Group 1 n = 33 | Group 2 n = 29 | Group 3 n = 32 | Group 4 n = 33 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before | After | p | Before | After | p | Before | After | p | Before | After | p | |
| hsCRP | 6.8 ± 10.1 | 6.9 ± 5.9 | 0.952 | 6.2 ± 5.3 | 5.3 ± 4.8 | 0.056 | 7.0 ± 5.0 | 7.9 ± 5.7 | 0.244 | 9.3 ± 9.9 | 7.1 ± 6.6 | 0.018 |
| Long term HRV, ms | ||||||||||||
| ASDNN | 50.7 ± 21.8 | 53.5 ± 21.8 | 0.174 | 48.4 ± 15.6 | 54.9 ± 16.2 | 0.013 | 48.2 ± 15.1 | 49.9 ± 16.3 | 0.612 | 44.2 ± 17.8 | 44.5 ± 18.2 | 0.871 |
| SDANN | 105.4 ± 44.7 | 113.3 ± 39.5 | 0.149 | 101.4 ± 36.8 | 109.8 ± 41.1 | 0.150 | 98.0 ± 32.4 | 104.5 ± 28.6 | 0.094 | 99.2 ± 28.4 | 100.4 ± 30.7 | 0.794 |
| SDNN | 119.6 ± 48.0 | 128.7 ± 42.9 | 0.117 | 114.0 ± 36.4 | 124.2 ± 37.5 | 0.078 | 114.0 ± 37.5 | 118.7 ± 28.9 | 0.393 | 110.8 ± 32.3 | 113.0 ± 33.8 | 0.643 |
| Short term HRV | ||||||||||||
| SDNN, ms | 18.0 (11.2, 26.8) | 14.6 (7.4, 22.1) | 0.057 | 20.6 (13.7, 29.2) | 16.4 (9.2, 25.4) | 0.264 | 19.0 (10.2, 29.0) | 15.5 (10.8, 30.2) | 0.927 | 16.2 (11.6, 23.2) | 10.1 (8.1, 16.8) | 0.003 |
| Mean RR, ms | 788.9 ± 156.2 | 753.4 ± 152.8 | 0.027 | 796.2 ± 150.9 | 802.0 ± 131.2 | 0.799 | 767.2 ± 143.8 | 806.8 ± 146.7 | 0.011 | 738.2 ± 124.9 | 714.8 ± 122.2 | 0.146 |
| RMSSD, ms | 18.0 (8.0, 24.5) | 11.0 (6.0, 19.0) | 0.077 | 18.0 (12.5, 30.0) | 17.0 (8.8, 27.3) | 0.157 | 16.0 (8.0, 22.0) | 12.5 (9.0, 27.8) | 0.686 | 14.5 (10.0, 22.8) | 9.5 (5.5, 13.0) | 0.007 |
| PNS index | − 1.2 ± 1.3 | − 1.5 ± 1.2 | 0.008 | − 1.1 ± 1.1 | − 1.1 ± 1.0 | 0.977 | − 1.2 ± 0.9 | − 1.2 ± 1.0 | 0.374 | − 1.3 ± 1.6 | − 1.7 ± 1.1 | 0.025 |
| SNS index | 3.0 ± 2.9 | 3.9 ± 3.6 | 0.018 | 2.3 ± 2.4 | 2.8 ± 2.4 | 0.447 | 3.2 ± 3.0 | 2.8 ± 2.3 | 0.438 | 3.6 ± 3.1 | 4.7 ± 3.1 | 0.017 |
| Stress index | 23.1 ± 12.4 | 27.3 ± 17.4 | 0.028 | 21.9 ± 14.0 | 26.7 ± 19.2 | 0.128 | 23.6 ± 14.4 | 23.2 ± 10.3 | 0.825 | 25.0 ± 13.6 | 30.5 ± 13.6 | 0.019 |
| 24 h heart rate, bpm | ||||||||||||
| Min. HR | 50.1 ± 9.2 | 48.6 ± 8.2 | 0.248 | 49.3 ± 9.8 | 46.8 ± 7.0 | 0.058 | 48.9 ± 9.7 | 48.7 ± 6.8 | 0.849 | 53.4 ± 9.3 | 51.9 ± 8.5 | 0.170 |
| Max. HR | 124.1 ± 16.8 | 122.5 ± 17.0 | 0.495 | 123.2 ± 15.7 | 119.4 ± 13.8 | 0.091 | 121.7 ± 15.1 | 120.8 ± 15.1 | 0.699 | 128.9 ± 13.7 | 126.6 ± 13.0 | 0.160 |
| Avg. HR | 80.9 ± 11.2 | 79.3 ± 11.1 | 0.207 | 79.4 ± 9.9 | 76.1 ± 8.5 | 0.037 | 80.5 ± 8.4 | 77.7 ± 7.8 | 0.002 | 84.3 ± 9.8 | 84.2 ± 9.8 | 0.910 |
| Day HR | 84.3 ± 12.5 | 84.7 ± 12.5 | 0.760 | 83.7 ± 9.9 | 80.2 ± 11.3 | 0.068 | 84.6 ± 8.9 | 83.0 ± 8.7 | 0.118 | 88.2 ± 11.1 | 88.9 ± 11.3 | 0.616 |
| Noct. HR | 71.6 ± 12.8 | 69.3 ± 10.2 | 0.124 | 71.1 ± 12.2 | 67.2 ± 8.9 | 0.050 | 71.6 ± 9.5 | 68.9 ± 7.7 | 0.010 | 74.8 ± 10.4 | 74.7 ± 10.3 | 0.962 |
| Dipping, % | 14.8 ± 11.1 | 17.8 ± 8.8 | 0.082 | 15.1 ± 9.5 | 15.6 ± 9.9 | 0.805 | 15.3 ± 6.7 | 16.8 ± 6.9 | 0.251 | 14.9 ± 7.6 | 15.5 ± 9.2 | 0.747 |
| Non-dipper, n (%) | 8 (23.5) | 5 (14.7) | 0.375 | 8 (28.6) | 6 (21.4) | 0.727 | 6 (18.8) | 4 (12.5) | 0.727 | 10 (30.3) | 8 (24.2) | 0.774 |
| Renin–angiotensin–aldosterone system parameters | ||||||||||||
| PAC, ng/dL | 8.95 (5.37, 12.30) | 10.50 (7.62, 12.65) | 0.062 | 7.84 (6.54, 11.40) | 6.83 (4.37, 10.20) | 0.206 | 9.71 (6.08, 13.20) | 9.92 (6.64, 13.20) | 0.948 | 8.75 (6.44, 11.30) | 7.82 (6.76, 11.15) | 0.893 |
| PRC, μIU/mL | 12.79 (5.93, 22.60) | 15.97 (9.92, 33.02) | 0.231 | 12.12 (5.38, 17.55) | 11.60 (4.96, 22.83) | 0.785 | 10.05 (4.85, 23.52) | 11.85 (5.84, 32.10) | 0.075 | 15.12 (10.17, 26.41) | 15.27 (9.09, 25.67) | 0.908 |
| Endothelial function | ||||||||||||
| FMD, % | 6.18 ± 1.37 | 6.70 ± 1.09 | < 0.001 | 5.89 ± 1.51 | 6.55 ± 1.93 | 0.006 | 6.05 ± 1.64 | 6.45 ± 1.18 | 0.028 | 6.2 ± 1.2 | 6.6 ± 0.8 | 0.003 |
| > 7.1% | 6 (18.2) | 11 (33.3) | 0.125 | 5 (17.2) | 8 (27.6) | 0.250 | 4 (12.5) | 9 (28.1) | 0.063 | 3 (9.1) | 7 (21.2) | 0.125 |
Numerical variables are presented as the mean ± standard deviation or median (IQR), categorical variables are defined as absolute count and percentage
hsCRP: high-sensitivity C-reactive protein; ASDNN: the mean of the 5 min SDNN calculated over 24 h; SDANN: the standard deviation of the average NN interval calculated over 5 min; SDNN: standard deviation of the NN interval; RMSSD: the square root of the mean of the sum of the squares of differences between adjacent NN intervals; PNS: parasympathetic nervous system; SNS: sympathetic nervous system; min: minimum; max: maximum; avg: average; noct: nocturnal; PAC: plasma aldosterone concentration; PRC: plasma renin concentration; FMD: flow mediated dilatation; 25(OH)D: 25-hydroxyvitamin D
*Patients who had 25(OH)D < 30 ng/mL at baseline and achieved 25(OH)D > 30 ng/mL at the end of visit
Fig. 1.
a Changes of sympathetic nervous system index with treatment among intervention groups. b Changes of parasympathetic nervous system index with treatment among intervention groups. c Changes of SDNN with treatment among intervention groups. d Changes of RMSSD with treatment among intervention groups. e Changes of average heart rate with treatment among intervention groups
ESS and QoL scores
Table 6 outlines patient-reported outcomes in ESS and QoL. Groups 1, 2, and 3 reported significant improvements in the QoL scores, except in the domain of social relationships. However, only group 3 exhibited significant reduction in ESS.
Table 6.
ESS and quality of life changes before and after treatment
| Variables | Group 1 n = 36 | Group 2 n = 41 | Group 3 n = 39 | Group 4 n = 37 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before | After | p | Before | After | p | Before | After | p | Before | After | p | |
| ESS | 8.9 ± 5.0 | 8.0 ± 4.8 | 0.259 | 8.9 ± 5.7 | 8.6 ± 5.8 | 0.722 | 10.2 ± 4.7 | 7.6 ± 5.2 | 0.004 | 9.4 ± 5.7 | 8.6 ± 4.8 | 0.254 |
| WHOQoL | ||||||||||||
| Quality of Life | 3.49 ± 0.77 | 3.97 ± 0.69 | < 0.001 | 3.39 ± 0.70 | 3.78 ± 0.57 | 0.002 | 3.40 ± 0.81 | 3.88 ± 0.69 | 0.004 | 3.61 ± 0.72 | 3.73 ± 0.80 | 0.347 |
| General Health | 2.97 ± 0.87 | 3.24 ± 0.72 | 0.048 | 3.02 ± 0.69 | 3.34 ± 0.83 | 0.008 | 2.95 ± 0.64 | 3.60 ± 0.87 | < 0.001 | 3.11 ± 0.97 | 3.27 ± 0.96 | 0.245 |
| Domain 1 | 12.2 ± 1.7 | 13.2 ± 1.6 | 0.003 | 12.5 ± 1.7 | 13.1 ± 2.0 | 0.012 | 12.7 ± 1.6 | 13.4 ± 2.2 | 0.014 | 12.8 ± 1.7 | 13.2 ± 1.4 | 0.252 |
| Domain 2 | 12.8 ± 1.9 | 13.4 ± 1.8 | 0.033 | 12.5 ± 1.6 | 13.5 ± 1.9 | < 0.001 | 13.1 ± 2.1 | 13.7 ± 2.4 | 0.075 | 13.1 ± 1.7 | 13.3 ± 1.9 | 0.497 |
| Domain 3 | 14.4 ± 2.5 | 15.1 ± 2.8 | 0.202 | 13.9 ± 2.8 | 14.1 ± 2.6 | 0.751 | 13.9 ± 2.9 | 14.6 ± 3.5 | 0.217 | 14.5 ± 3.4 | 14.5 ± 2.6 | 1.0 |
| Domain 4 | 14.1 ± 2.2 | 14.6 ± 1.6 | 0.076 | 13.7 ± 2.1 | 14.5 ± 2.1 | 0.007 | 14.1 ± 1.9 | 15.0 ± 2.9 | 0.032 | 14.6 ± 1.8 | 15.0 ± 1.7 | 0.044 |
Numerical variables are presented as the mean ± standard deviation
ESS: Epworth Sleepiness Scale; WHOQoL: World Health Organization Quality of Life; Domain 1: physical health; Domain 2: psychological health; Domain 3: social relationships; Domain 4: environmental health
Adverse events
Three patients in group 1 developed genitourinary infection during the study period, which subsequently resolved with treatment. There were more patients in group 3 who developed other infections as shown in Supp Table 5. None of the patients who received Dapagliflozin developed ketoacidosis.
Discussion
This study demonstrated three pertinent findings. Firstly, untreated OSA has deleterious effects on the cardiovascular system over time, highlighting the progressive nature of metabolic dysfunction in OSA patients. Secondly, the combination of SGLT2i and vitamin D3 provide a synergistic benefit in metabolic improvement and prevention of cardiac autonomic function deterioration, particularly in females. Thirdly, the use of this combination therapy led to significant improvements in both sleepiness symptoms as well as QoL. To the best of our knowledge, this is the first study evaluating the synergistic effect of combining SGLT2i and vitamin D3 in cardio-metabolic parameters, sleepiness symptoms, and QoL in a group of OSA patients with high cardiovascular risks.
In untreated OSA patients, cardio-metabolic parameters often continue to worsen due to insulin resistance, intermittent hypoxia, and sympathetic overactivity [36]. Despite an improvement in hsCRP in the control arm, which may reflect the natural variation rather than a true anti-inflammatory effect, the persistent autonomic dysregulation and cardiovascular stress explain the lack of improvement in metabolic parameters and deterioration in HRV over time, highlighting the progressive impact of untreated OSA on autonomic and metabolic health.
The extra-glycemic benefits of SGLT-2i have been well-demonstrated. Apart from lowering blood glucose with minimal adverse effects of hypoglycemia, SGLT2i led to reductions in body weight, visceral fat, and BP [37]. Nevertheless, the lack of impact on cardiac autonomic function as demonstrated in this study suggests that improving metabolic factors alone may not be adequate to protect against cardiac autonomic dysregulation in OSA. In fact, SGLT2i alone could potentially worsen HRV due to volume depletion and subsequent RAAS activation [38], which may lead to increases in SNS activity and worsened HRV. This is demonstrated by an increment in PRC and PAC only in the SGLT2i arm, despite not achieving statistically significant differences.
Although vitamin D3 led to some improvements in metabolic parameters, it was not as robust as SGLT2i treatment. Existing meta-analyses demonstrated contradicting results on the effect of vitamin D3 supplementation on BP, which could be contributed by the various doses and short duration of treatment [39–41]. In the current study, vitamin D3 led to improvement in BP among those who achieved vitamin D sufficiency at the end of follow up. Furthermore, vitamin D3 use was demonstrated to exert protective effect on cardiac autonomic function by preventing the deterioration in HRV expected in untreated OSA, which could be attributed to the direct effects of vitamin D3 on the physiology of the cardiomyocytes [42].
In addition to achieving comparable metabolic improvements to the SGLT2i monotherapy group, the combination of SGLT2i and vitamin D3 effectively prevented the deterioration of cardiac autonomic function, which was otherwise observed in the control group and the SGLT2i monotherapy arm. This finding suggests a synergistic effect of the combined treatment on both metabolic and cardiovascular autonomic function. The protective role of vitamin D in reducing oxidative stress and preserving cardiac autonomic function [43, 44], together with the ability of SGLT2i to improve glycemic control, metabolic parameters, and reduce fluid overload, may work in concert to mitigate cardio-metabolic dysfunction seen in untreated OSA. Moreover, only the combination treatment resulted in significant reduction in nocturnal heart rate, along with a decrease in the 24 h average heart rate. Elevated nocturnal heart rate, often referred to as a “non-dipper” profile, has been associated with increased risk of cerebrovascular and cardiovascular diseases, as well as subclinical inflammation [45–47]. The lack of significant results when comparing non-dippers to dippers following treatment may be attributed to the fact that most patients were already classified as dippers at baseline. With fewer non-dippers to observe changes in response to treatment, the statistical power to detect significant differences between the groups was reduced. This baseline distribution may have limited the ability to demonstrate a meaningful shift in dipping status as a treatment effect.
This study demonstrated that the female patients who received the combination treatment of SGLT-2i and vitamin D3 exhibited greater improvements in both metabolic and cardiovascular parameters compared to their male counterparts. These findings suggest potential gender-specific differences in response to the combination therapy. Estrogen is known to exert cardioprotective effects and play a key role in metabolic regulation [48, 49], which may have contributed to the more pronounced benefits observed in the female patients. Additionally, gender differences in vitamin D metabolism and gene expression may have also played a role in modulating these outcomes [50].
SGLT2i monotherapy has been demonstrated to improve QoL among patients with heart failure [51] and type 2 diabetes [52], but presents conflicting findings regarding the impact on ESS in OSA patients with type 2 diabetes [53, 54]. Furthermore, the sole study evaluating the effects of vitamin D supplementation on ESS in OSA patients reported no significant improvement in sleepiness symptom following treatment [55]. There is no data on the influence of vitamin D supplementation on QoL among OSA patients. Similarly, in other disease entities, this area remains under-researched [56]. Our study demonstrates that, while all three intervention arms showed improvements in QoL scores, only the combination therapy group achieved significant improvements in both QoL and ESS scores. While no statistically significant differences were observed between the groups at baseline, the slightly higher starting ESS in the combination arm could have provided more room for improvement in the symptoms, contributing to the observed reduction. Nonetheless, the consistent improvements observed across multiple outcomes suggest that the combination treatment likely offered meaningful therapeutic benefits. This highlights the potential of this treatment combination as a more comprehensive approach to managing hypertensive OSA with metabolic dysfunction, addressing multiple aspects in cardiac health, metabolic function, sleepiness symptoms, and QoL.
Our study is not without limitations. Firstly, we included only hypertensive OSA patients who were obese, which may limit the generalizability of the findings to other groups, such as normotensive OSA patients, or those with normal BMI. Besides, the 16-week study period may not have been long enough to capture the full range of physiological and metabolic changes associated with the interventions, hence limiting the ability to assess the long-term durability of the observed effects, particularly cardiovascular benefits, which may evolve over a longer duration. Moreover, despite controlling for some variables, other unmeasured factors such as sleep quality and lifestyle changes could have influenced the outcomes, potentially confounding the effects of the interventions. In addition, individual responses to the interventions could vary, which may mask specific benefits that could have been more apparent with each intervention.
Nevertheless, this is the first study investigating the combination of SGLT2i and vitamin D3 in improving cardio-metabolic parameters, daytime somnolence, and QoL in OSA patients with high cardiovascular risks, contributing to the growing body of evidence examining the complex interactions in these aspects. The randomized controlled 4-arm study design helped minimize bias and enabled the comparison of multiple treatment strategies, offering valuable insights into the relative effectiveness of each approach. This facilitates a more exquisite understanding of how different interventions influence various clinical outcomes in patients with OSA. Additionally, by evaluating multiple aspects, including metabolic and cardiovascular parameters alongside to sleepiness symptoms and QoL, this study provides a comprehensive overview of the effects of each treatment. The investigation into the HRV offered valuable insights into cardiac autonomic function, a crucial indicator of cardiovascular risk, particularly in patients with OSA. While our study was not designed to directly investigate the underlying mechanisms of the improvement observed with the combination therapy for heart disease, kidney failure, and autonomic function, the study findings suggest a potential role for this combination therapy in helping to mitigate key risk factors associated with cardiovascular and renal complications. Future research should focus on elucidating the precise mechanistic pathways involved, potentially paving the way for more targeted and effective treatment strategies.
Conclusions
This study, the first to evaluate multiple outcomes in relation to metabolic and cardiovascular health alongside patient-reported outcomes relevant to hypertensive obese OSA patients, highlights the beneficial synergistic effects of combining vitamin D3 and SGLT2i. This combination not only improves key metabolic parameters but also helps mitigate the deterioration of cardiac autonomic function, while reducing daytime somnolence and enhancing QoL, particularly in female patients. These findings underscore the potential of this combination therapy in addressing the metabolic and cardiovascular challenges, ultimately improving overall health outcomes in these patients. Further long-term studies are essential to confirm the durability of these benefits and to better understand the underlying mechanisms driving the observed improvements.
Supplementary Information
Acknowledgements
We would like to thank the Director General of Health Malaysia for his permission to publish this article.
Abbreviations
- OSA
Obstructive sleep apnea
- QoL
Quality of life
- CPAP
Continuous positive airway pressure
- RAAS
Renin–angiotensin–aldosterone system
- VDD
Vitamin D deficiency
- SGLT2i
Sodium glucose transporter-2 inhibitor
- BP
Blood pressure
- BMI
Body mass index
- WHO
World Health Organization
- AHI
Apnoea hypopnea index
- 25(OH)D
25-Hydroxyvitamin D
- MASLD
Metabolic-associated steatotic liver disease
- hsCRP
High-sensitivity C-reactive protein
- HRV
Heart rate variability
- SDNN
Standard deviation of the NN interval
- ASDNN
Mean of the 5-min SDNN calculated over 24 h
- SDANN
The standard deviation of the average NN interval calculated over 5 min
- RMSSD
Square root of the mean of the sum of the squares of differences between adjacent NN intervals
- PNS
Parasympathetic nervous system
- SNS
Sympathetic nervous system
- PAC
Plasma aldosterone concentration
- PRC
Plasma renin concentration
- FMD
Flow-mediated dilatation
- ESS
Epworth Sleepiness Scale
Author contributions
HHL and NS conceived and designed the work; HHL, SPT, AJK, MCY, AS, CSC, NMAM, BBCT were involved in data acquisition; HHL and AT analysed the data; HHL and NS interpreted the data. HHL drafted the work, while NS, AS, and FHST substantively revised it. All authors approved the submitted version and agreed to be accountable for the work.
Funding
Open Access funding provided by Universiti Malaysia Sarawak. This study received funding from UNIMAS IMPACT Research Grant (F05/IMPACT/2122/2021) and the National University of Malaysia (UKM) grant (FF-2022–066). The sponsors had no role in the design or conduct of this paper.
Availability of data and materials
The data that supports the findings of this study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
The study was conducted in accordance with the principles outlined in the Declaration of Helsinki. Ethical approval was provided by Medical Research Ethical Committee of Malaysia (NMRR-21-1472-60812) and UNIMAS Medical Ethics Committee (UNIMAS/TNC(PI)/09-65/01). All patients gave written informed consent.
Consent for publication
Not applicable.
Competing interests
None declared.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Heinzer R, Vat S, Marques-Vidal P, Marti-Soler H, Andries D, Tobback N, et al. Prevalence of sleep-disordered breathing in the general population: the HypnoLaus study. Lancet Respir Med. 2015;3(4):310–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zdravkovic M, Popadic V, Klasnja S, Milic N, Rajovic N, Divac A, et al. Obstructive sleep apnea and cardiovascular risk: the role of dyslipidemia, inflammation, and obesity. Front Pharmacol. 2022;13: 898072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Floras JS. Sleep apnea and cardiovascular disease: an enigmatic risk factor. Circ Res. 2018;122(12):1741–64. [DOI] [PubMed] [Google Scholar]
- 4.Lal C, Weaver TE, Bae CJ, Strohl KP. Excessive daytime sleepiness in obstructive sleep apnea. Mechanisms and clinical management. Ann Am Thorac Soc. 2021;18(5):757–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bjornsdottir E, Keenan BT, Eysteinsdottir B, Arnardottir ES, Janson C, Gislason T, et al. Quality of life among untreated sleep apnea patients compared with the general population and changes after treatment with positive airway pressure. J Sleep Res. 2015;24(3):328–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rotenberg BW, Murariu D, Pang KP. Trends in CPAP adherence over 20 years of data collection: a flattened curve. J Otolaryngol Head Neck Surg. 2016;45(1):43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McEvoy RD, Antic NA, Heeley E, Luo Y, Ou Q, Zhang X, et al. CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med. 2016;375(10):919–31. [DOI] [PubMed] [Google Scholar]
- 8.Shiina K. Obstructive sleep apnea—related hypertension: a review of the literature and clinical management strategy. Hypertens Res. 2024;47(11):3085–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pop D, Dădârlat-Pop A, Tomoaia R, Zdrenghea D, Caloian B. Updates on the renin–angiotensin–aldosterone system and the cardiovascular continuum. Biomedicines. 2024;12(7):1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Joseph JJ, Echouffo-Tcheugui JB, Kalyani RR, Yeh HC, Bertoni AG, Effoe VS, et al. Aldosterone, Renin, cardiovascular events, and all-cause mortality among African Americans: the Jackson heart study. JACC Heart Fail. 2017;5(9):642–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Md Isa Z, Mohd Nordin NR, Mahmud MH, Hashim S. An update on Vitamin D deficiency status in Malaysia. Nutrients. 2022. 10.3390/nu14030567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Loh HH, Sukor N. Obstructive sleep apnea and vitamin D level: has the dust settled? Clin Respir J. 2023. 10.1111/crj.13593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Loh HH, Lim QH, Kang WH, Yee A, Yong MC, Sukor N. Obstructive sleep apnea and Vitamin D: an updated systematic review and meta-analysis. Hormones. 2023. 10.1007/s42000-023-00481-3. [DOI] [PubMed] [Google Scholar]
- 14.Marquina C, Mousa A, Scragg R, de Courten B. Vitamin D and cardiometabolic disorders: a review of current evidence, genetic determinants and pathomechanisms. Obes Rev. 2019;20(2):262–77. [DOI] [PubMed] [Google Scholar]
- 15.Mazidi M, Karimi E, Rezaie P, Vatanparast H. The impact of Vitamin D supplement intake on vascular endothelial function; a systematic review and meta-analysis of randomized controlled trials. Food Nutr Res. 2017;61(1):1273574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maestro B, Campion J, Davila N, Calle C. Stimulation by 1,25-dihydroxyvitamin D3 of insulin receptor expression and insulin responsiveness for glucose transport in U-937 human promonocytic cells. Endocr J. 2000;47(4):383–91. [DOI] [PubMed] [Google Scholar]
- 17.Li YC. Chapter 45—Vitamin D and the Renin-angiotensin system. In: Feldman D, editor. Vitamin D. 4th ed. Cambridge: Academic Press; 2018. p. 825–47. [Google Scholar]
- 18.Mozos I, Marginean O. Links between Vitamin D deficiency and cardiovascular diseases. Biomed Res Int. 2015;2015: 109275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu J, Atkins A, Downes M, Wei Z. Vitamin D in diabetes: uncovering the sunshine hormone’s role in glucose metabolism and beyond. Nutrients. 2023. 10.3390/nu15081997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Radkhah N, Zarezadeh M, Jamilian P, Ostadrahimi A. The effect of Vitamin D supplementation on lipid profiles: an umbrella review of meta-analyses. Adv Nutr. 2023;14(6):1479–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krajewska M, Witkowska-Sedek E, Ruminska M, Stelmaszczyk-Emmel A, Sobol M, Majcher A, et al. Vitamin D effects on selected anti-inflammatory and pro-inflammatory markers of obesity-related chronic inflammation. Front Endocrinol (Lausanne). 2022;13: 920340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tentolouris A, Vlachakis P, Tzeravini E, Eleftheriadou I, Tentolouris N. SGLT2 inhibitors: a review of their antidiabetic and cardioprotective effects. Int J Environ Res Public Health. 2019. 10.3390/ijerph16162965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380(4):347–57. [DOI] [PubMed] [Google Scholar]
- 24.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117–28. [DOI] [PubMed] [Google Scholar]
- 25.Dyck JRB, Sossalla S, Hamdani N, Coronel R, Weber NC, Light PE, et al. Cardiac mechanisms of the beneficial effects of SGLT2 inhibitors in heart failure: evidence for potential off-target effects. J Mol Cell Cardiol. 2022;167:17–31. [DOI] [PubMed] [Google Scholar]
- 26.Zhang R, Xie Q, Lu X, Fan R, Tong N. Research advances in the anti-inflammatory effects of SGLT inhibitors in type 2 diabetes mellitus. Diabetol Metab Syndr. 2024;16(1):99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kawasoe S, Maruguchi Y, Kajiya S, Uenomachi H, Miyata M, Kawasoe M, et al. Mechanism of the blood pressure-lowering effect of sodium-glucose cotransporter 2 inhibitors in obese patients with type 2 diabetes. BMC Pharmacol Toxicol. 2017;18(1):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Consultation WHOE. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363(9403):157–63. [DOI] [PubMed] [Google Scholar]
- 29.Kapur VK, Auckley DH, Chowdhuri S, Kuhlmann DC, Mehra R, Ramar K, et al. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American academy of sleep medicine clinical practice guideline. J Clin Sleep Med. 2017;13(3):479–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Evaluation, treatment, and prevention of Vitamin D deficiency: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911–30. [DOI] [PubMed] [Google Scholar]
- 31.Saadeh S, Younossi ZM, Remer EM, Gramlich T, Ong JP, Hurley M, et al. The utility of radiological imaging in nonalcoholic fatty liver disease. Gastroenterology. 2002;123(3):745–50. [DOI] [PubMed] [Google Scholar]
- 32.Roberts WL. CDC/AHA workshop on markers of inflammation and cardiovascular disease: application to clinical and public health practice: laboratory tests available to assess inflammation–performance and standardization: a background paper. Circulation. 2004;110(25):e572–6. [DOI] [PubMed] [Google Scholar]
- 33.Maruhashi T, Kajikawa M, Kishimoto S, Hashimoto H, Takaeko Y, Yamaji T, et al. Diagnostic criteria of flow-mediated vasodilation for normal endothelial function and nitroglycerin-induced vasodilation for normal vascular smooth muscle function of the brachial artery. J Am Heart Assoc. 2020;9(2): e013915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Trompenaars FJ, Masthoff ED, Van Heck GL, Hodiamont PP, De Vries J. The WHO quality of life assessment instrument (WHOQOL-100). Eur J Psychol Assess. 2006;22(3):207–15. [Google Scholar]
- 35.Milder TY, Stocker SL, Day RO, Greenfield JR. Potential safety issues with use of sodium-glucose cotransporter 2 inhibitors, particularly in people with type 2 diabetes and chronic kidney disease. Drug Saf. 2020;43(12):1211–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Drager LF, Togeiro SM, Polotsky VY, Lorenzi-Filho G. Obstructive sleep apnea: a cardiometabolic risk in obesity and the metabolic syndrome. J Am Coll Cardiol. 2013;62(7):569–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bonora BM, Avogaro A, Fadini GP. Extraglycemic effects of SGLT2 inhibitors: a review of the evidence. Diabetes Metab Syndr Obes. 2020;13:161–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peene B, Benhalima K. Sodium glucose transporter protein 2 inhibitors: focusing on the kidney to treat type 2 diabetes. Ther Adv Endocrinol Metab. 2014;5(5):124–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jensen NS, Wehland M, Wise PM, Grimm D. Latest knowledge on the role of Vitamin D in hypertension. Int J Mol Sci. 2023. 10.3390/ijms24054679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meng R, Radkhah N, Ghalichi F, Hamedi-Kalajahi F, Musazadeh V, Saleh SAK, et al. The impact of Vitamin D supplementation on improving blood pressure: evidence obtained from an umbrella meta-analysis. Clin Ther. 2023;45(10):e208–16. [DOI] [PubMed] [Google Scholar]
- 41.Witham MD, Nadir MA, Struthers AD. Effect of Vitamin D on blood pressure: a systematic review and meta-analysis. J Hypertens. 2009;27(10):1948–54. [DOI] [PubMed] [Google Scholar]
- 42.Cetin M, Kozdag G, Ural D, Kahraman G, Yilmaz I, Akay Y, et al. Could decreased Vitamin D levels be related with impaired cardiac autonomic functions in patients with chronic heart failure: an observational study. Anadolu Kardiyol Derg. 2014;14(5):434–41. [DOI] [PubMed] [Google Scholar]
- 43.Sepidarkish M, Farsi F, Akbari-Fakhrabadi M, Namazi N, Almasi-Hashiani A, Maleki Hagiagha A, et al. The effect of Vitamin D supplementation on oxidative stress parameters: a systematic review and meta-analysis of clinical trials. Pharmacol Res. 2019;139:141–52. [DOI] [PubMed] [Google Scholar]
- 44.Ziegler D, Buchholz S, Sohr C, Nourooz-Zadeh J, Roden M. Oxidative stress predicts progression of peripheral and cardiac autonomic nerve dysfunction over 6 years in diabetic patients. Acta Diabetol. 2015;52(1):65–72. [DOI] [PubMed] [Google Scholar]
- 45.Yano Y. Nocturnal heart rate and cerebrovascular disease. Hypertens Res. 2015;38(8):528–9. [DOI] [PubMed] [Google Scholar]
- 46.Garcia R, Warming PE, Narayanan K, Defaye P, Guedon-Moreau L, Blangy H, et al. Dynamic changes in nocturnal heart rate predict short-term cardiovascular events in patients using the wearable cardioverter-defibrillator: from the WEARIT-France cohort study. Europace. 2023;25:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yano Y, Kario K. Nocturnal heart rate and inflammation. J Clin Hypertens (Greenwich). 2014;16(12):862–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mahboobifard F, Pourgholami MH, Jorjani M, Dargahi L, Amiri M, Sadeghi S, et al. Estrogen as a key regulator of energy homeostasis and metabolic health. Biomed Pharmacother. 2022;156: 113808. [DOI] [PubMed] [Google Scholar]
- 49.Iorga A, Cunningham CM, Moazeni S, Ruffenach G, Umar S, Eghbali M. The protective role of estrogen and estrogen receptors in cardiovascular disease and the controversial use of estrogen therapy. Biol Sex Differ. 2017;8(1):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wierzbicka A, Oczkowicz M. Sex differences in Vitamin D metabolism, serum levels and action. Br J Nutr. 2022;128(11):2115–30. [DOI] [PubMed] [Google Scholar]
- 51.Gao M, Bhatia K, Kapoor A, Badimon J, Pinney SP, Mancini DM, et al. SGLT2 inhibitors, functional capacity, and quality of life in patients with heart failure: a systematic review and meta-analysis. JAMA Netw Open. 2024;7(4): e245135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ishii H, Nakajima H, Kamei N, Niiya T, Hiyoshi T, Hiramori Y, et al. Quality-of-life comparison of dapagliflozin versus dipeptidyl peptidase 4 inhibitors in patients with type 2 diabetes mellitus: a randomized controlled trial (J-BOND study). Diabetes Ther. 2020;11(12):2959–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Furukawa S, Miyake T, Senba H, Sakai T, Furukawa E, Yamamoto S, et al. The effectiveness of dapagliflozin for sleep-disordered breathing among Japanese patients with obesity and type 2 diabetes mellitus. Endocr J. 2018;65(9):953–61. [DOI] [PubMed] [Google Scholar]
- 54.Tang Y, Sun Q, Bai XY, Zhou YF, Zhou QL, Zhang M. Effect of dapagliflozin on obstructive sleep apnea in patients with type 2 diabetes: a preliminary study. Nutr Diabetes. 2019;9(1):32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kerley CP, Hutchinson K, Bramham J, McGowan A, Faul J, Cormican L. Vitamin D improves selected metabolic parameters but not neuropsychological or quality of life indices in OSA: a pilot study. J Clin Sleep Med. 2017;13(1):19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hoffmann MR, Senior PA, Mager DR. Vitamin D supplementation and health-related quality of life: a systematic review of the literature. J Acad Nutr Diet. 2015;115(3):406–18. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that supports the findings of this study are available from the corresponding author upon reasonable request.