Abstract
OBJECTIVE: To study the effect of lithium administration on brain choline/creatine (Cho/Cr) ratios in healthy volunteers. DESIGN: Double-blind, placebo-controlled, prospective study. SETTING: The Nuclear Magnetic Resonance Research Unit at the University of Alberta. PARTICIPANTS: Sixteen healthy volunteers, recruited through advertisements. Subjects were excluded if they had a physical illness, or a personal or family history of psychiatric illness. The study period was from Feb. 6, 1996, to Mar. 21, 1996. INTERVENTIONS: Subjects received a baseline proton magnetic resonance spectroscopy (1H MRS) scan, and then were instructed to take either lithium (1,200 mg) or placebo at night for 7 days. On Day 8, the subjects returned for a second 1H MRS scan. Study participants were seen by a physician at the beginning and at the end of the experiment, and had access to the physician throughout the study period. OUTCOME MEASURES: Ratios of Cho/Cr measured in the temporal lobes by 1H MRS. RESULTS: There were no significant differences in the Cho/Cr ratios between the 2 groups on the test day (placebo 0.748 [standard deviation 0.29] versus lithium 0.811 [SD 0.25]; F = 0.147, p = 0.72), and there was no significant change from baseline in either group (0.003 above baseline for placebo; 0.056 above baseline for lithium; F = 1.21, p = 0.32). CONCLUSIONS: Lithium administration to healthy volunteers does not alter the Cho/Cr ratio in temporal lobe as measured by 1H MRS. The result concurs with reports that differences in Cho/Cr ratios observed in patients with bipolar disorder are likely specific to the illness, and are not the result of lithium therapy. Hence, alterations in choline function are not involved in the clinical effectiveness of lithium.
Full text
PDF

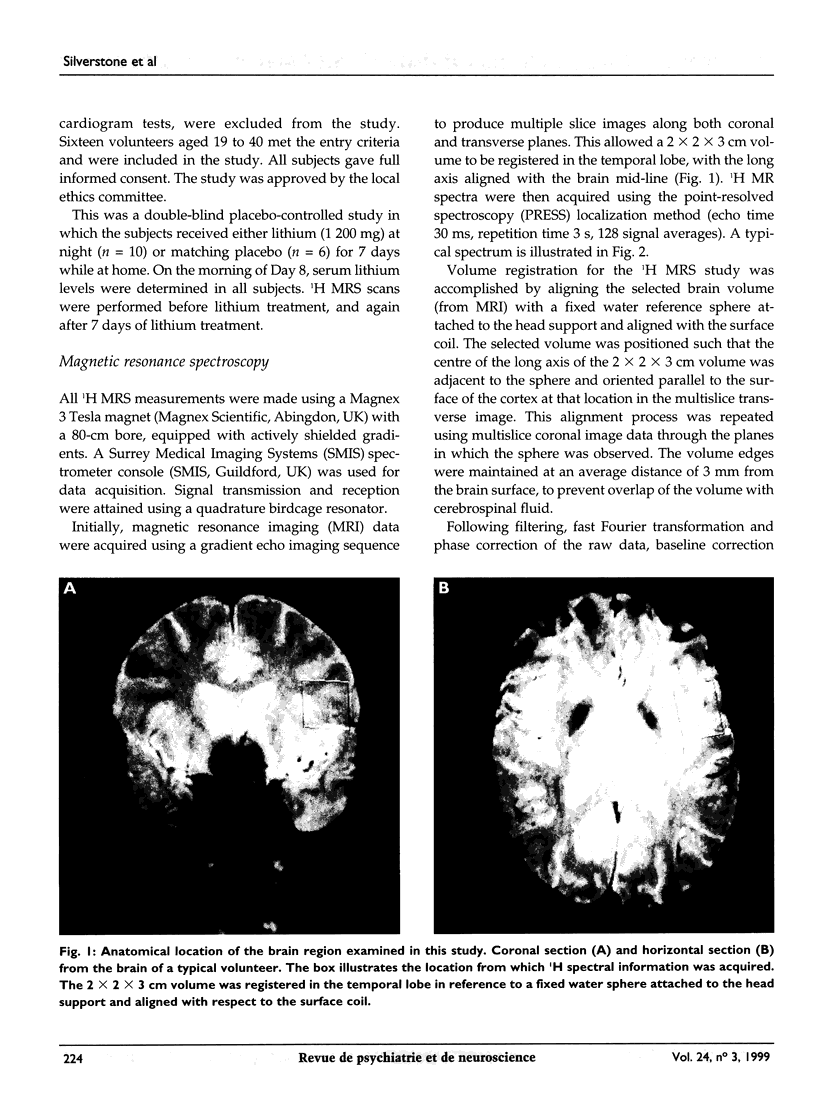
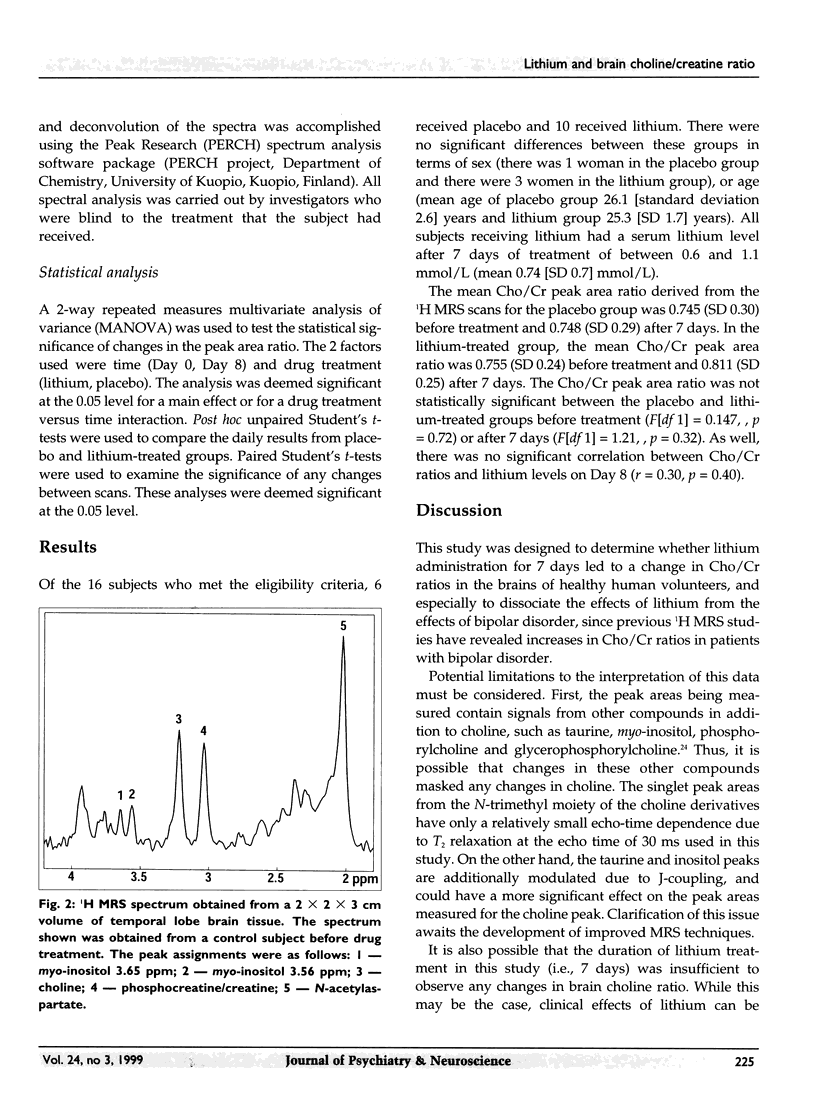

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berridge M. J., Irvine R. F. Inositol phosphates and cell signalling. Nature. 1989 Sep 21;341(6239):197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- Buckley P. F., Moore C., Long H., Larkin C., Thompson P., Mulvany F., Redmond O., Stack J. P., Ennis J. T., Waddington J. L. 1H-magnetic resonance spectroscopy of the left temporal and frontal lobes in schizophrenia: clinical, neurodevelopmental, and cognitive correlates. Biol Psychiatry. 1994 Dec 15;36(12):792–800. doi: 10.1016/0006-3223(94)90591-6. [DOI] [PubMed] [Google Scholar]
- Charles H. C., Lazeyras F., Krishnan K. R., Boyko O. B., Payne M., Moore D. Brain choline in depression: in vivo detection of potential pharmacodynamic effects of antidepressant therapy using hydrogen localized spectroscopy. Prog Neuropsychopharmacol Biol Psychiatry. 1994 Nov;18(7):1121–1127. doi: 10.1016/0278-5846(94)90115-5. [DOI] [PubMed] [Google Scholar]
- Domino E. F., Sharp R. R., Lipper S., Ballast C. L., Delidow B., Bronzo M. R. NMR chemistry analysis of red blood cell constituents in normal subjects and lithium-treated psychiatric patients. Biol Psychiatry. 1985 Dec;20(12):1277–1283. doi: 10.1016/0006-3223(85)90112-x. [DOI] [PubMed] [Google Scholar]
- Hamakawa H., Kato T., Murashita J., Kato N. Quantitative proton magnetic resonance spectroscopy of the basal ganglia in patients with affective disorders. Eur Arch Psychiatry Clin Neurosci. 1998;248(1):53–58. doi: 10.1007/s004060050017. [DOI] [PubMed] [Google Scholar]
- Jope R. S., Jenden D. J., Ehrlich B. E., Diamond J. M. Choline accumulates in erythrocytes during lithium therapy. N Engl J Med. 1978 Oct 12;299(15):833–834. [PubMed] [Google Scholar]
- Jope R. S., Williams M. B. Lithium and brain signal transduction systems. Biochem Pharmacol. 1994 Feb 9;47(3):429–441. doi: 10.1016/0006-2952(94)90172-4. [DOI] [PubMed] [Google Scholar]
- Kato T., Hamakawa H., Shioiri T., Murashita J., Takahashi Y., Takahashi S., Inubushi T. Choline-containing compounds detected by proton magnetic resonance spectroscopy in the basal ganglia in bipolar disorder. J Psychiatry Neurosci. 1996 Jul;21(4):248–254. [PMC free article] [PubMed] [Google Scholar]
- Leiva D. B. The neurochemistry of mania: a hypothesis of etiology and rationale for treatment. Prog Neuropsychopharmacol Biol Psychiatry. 1990;14(3):423–429. doi: 10.1016/0278-5846(90)90030-k. [DOI] [PubMed] [Google Scholar]
- Manji H. K., Lenox R. H. Long-term action of lithium: a role for transcriptional and posttranscriptional factors regulated by protein kinase C. Synapse. 1994 Jan;16(1):11–28. doi: 10.1002/syn.890160103. [DOI] [PubMed] [Google Scholar]
- Manji H. K., Potter W. Z., Lenox R. H. Signal transduction pathways. Molecular targets for lithium's actions. Arch Gen Psychiatry. 1995 Jul;52(7):531–543. doi: 10.1001/archpsyc.1995.03950190013003. [DOI] [PubMed] [Google Scholar]
- Peet M., Pratt J. P. Lithium. Current status in psychiatric disorders. Drugs. 1993 Jul;46(1):7–17. doi: 10.2165/00003495-199346010-00002. [DOI] [PubMed] [Google Scholar]
- Prien R. F., Kupfer D. J., Mansky P. A., Small J. G., Tuason V. B., Voss C. B., Johnson W. E. Drug therapy in the prevention of recurrences in unipolar and bipolar affective disorders. Report of the NIMH Collaborative Study Group comparing lithium carbonate, imipramine, and a lithium carbonate-imipramine combination. Arch Gen Psychiatry. 1984 Nov;41(11):1096–1104. doi: 10.1001/archpsyc.1983.01790220086014. [DOI] [PubMed] [Google Scholar]
- Sharma R., Venkatasubramanian P. N., Bárány M., Davis J. M. Proton magnetic resonance spectroscopy of the brain in schizophrenic and affective patients. Schizophr Res. 1992 Oct;8(1):43–49. doi: 10.1016/0920-9964(92)90059-e. [DOI] [PubMed] [Google Scholar]
- Stoll A. L., Cohen B. M., Hanin I. Erythrocyte choline concentrations in psychiatric disorders. Biol Psychiatry. 1991 Feb 15;29(4):309–321. doi: 10.1016/0006-3223(91)90216-9. [DOI] [PubMed] [Google Scholar]
- Stoll A. L., Renshaw P. F., Sachs G. S., Guimaraes A. R., Miller C., Cohen B. M., Lafer B., Gonzalez R. G. The human brain resonance of choline-containing compounds is similar in patients receiving lithium treatment and controls: an in vivo proton magnetic resonance spectroscopy study. Biol Psychiatry. 1992 Nov 15;32(10):944–949. doi: 10.1016/0006-3223(92)90184-2. [DOI] [PubMed] [Google Scholar]
- Stoll A. L., Sachs G. S., Cohen B. M., Lafer B., Christensen J. D., Renshaw P. F. Choline in the treatment of rapid-cycling bipolar disorder: clinical and neurochemical findings in lithium-treated patients. Biol Psychiatry. 1996 Sep 1;40(5):382–388. doi: 10.1016/0006-3223(95)00423-8. [DOI] [PubMed] [Google Scholar]
- Uney J. B., Marchbanks R. M., Reynolds G. P., Perry R. H. Lithium prophylaxis inhibits choline transport in post-mortem brain. Lancet. 1986 Aug 23;2(8504):458–458. doi: 10.1016/s0140-6736(86)92162-8. [DOI] [PubMed] [Google Scholar]
- el-Mallakh R. S., Wyatt R. J. The Na,K-ATPase hypothesis for bipolar illness. Biol Psychiatry. 1995 Feb 15;37(4):235–244. doi: 10.1016/0006-3223(94)00201-D. [DOI] [PubMed] [Google Scholar]