Abstract
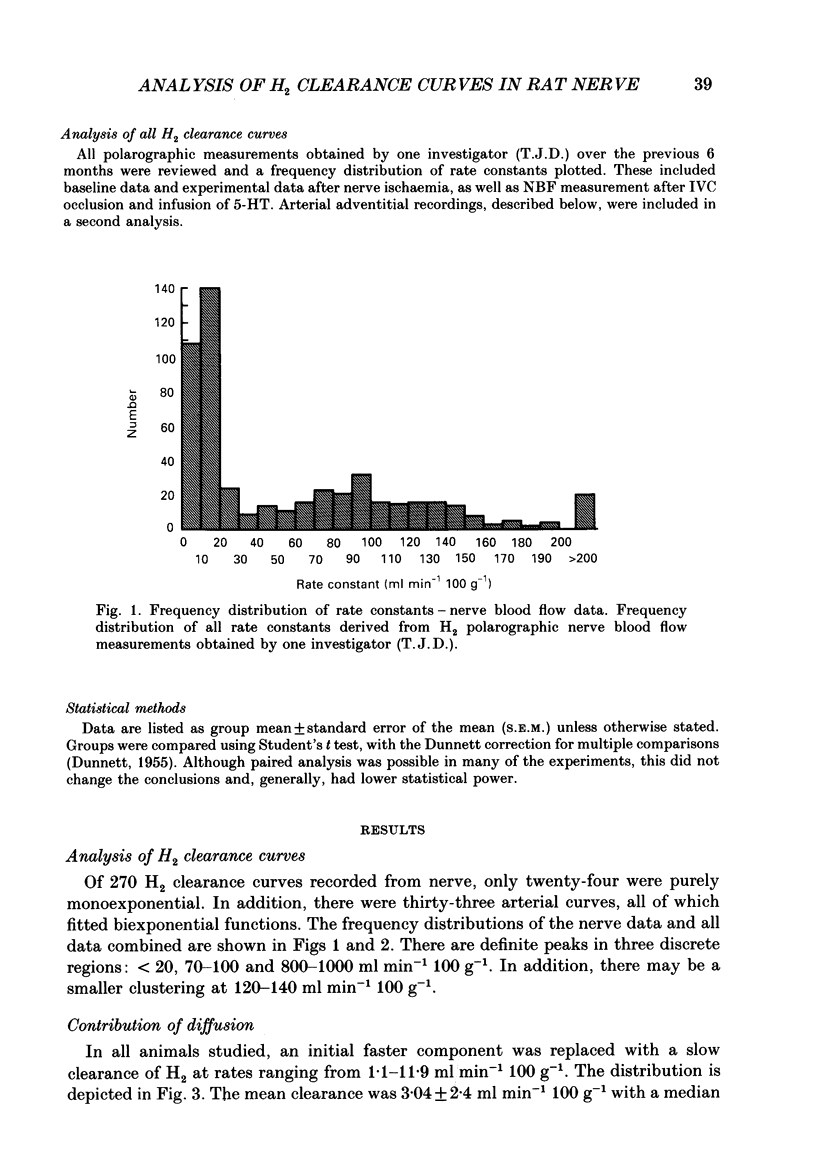
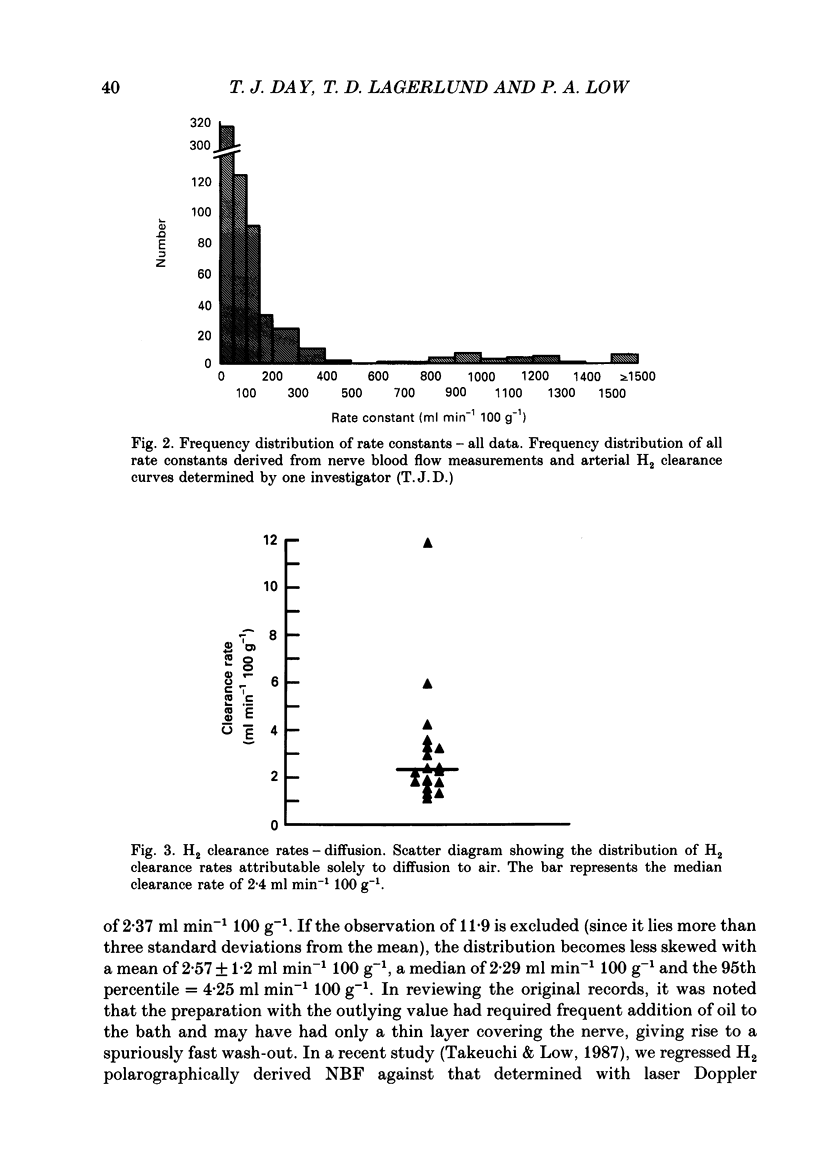
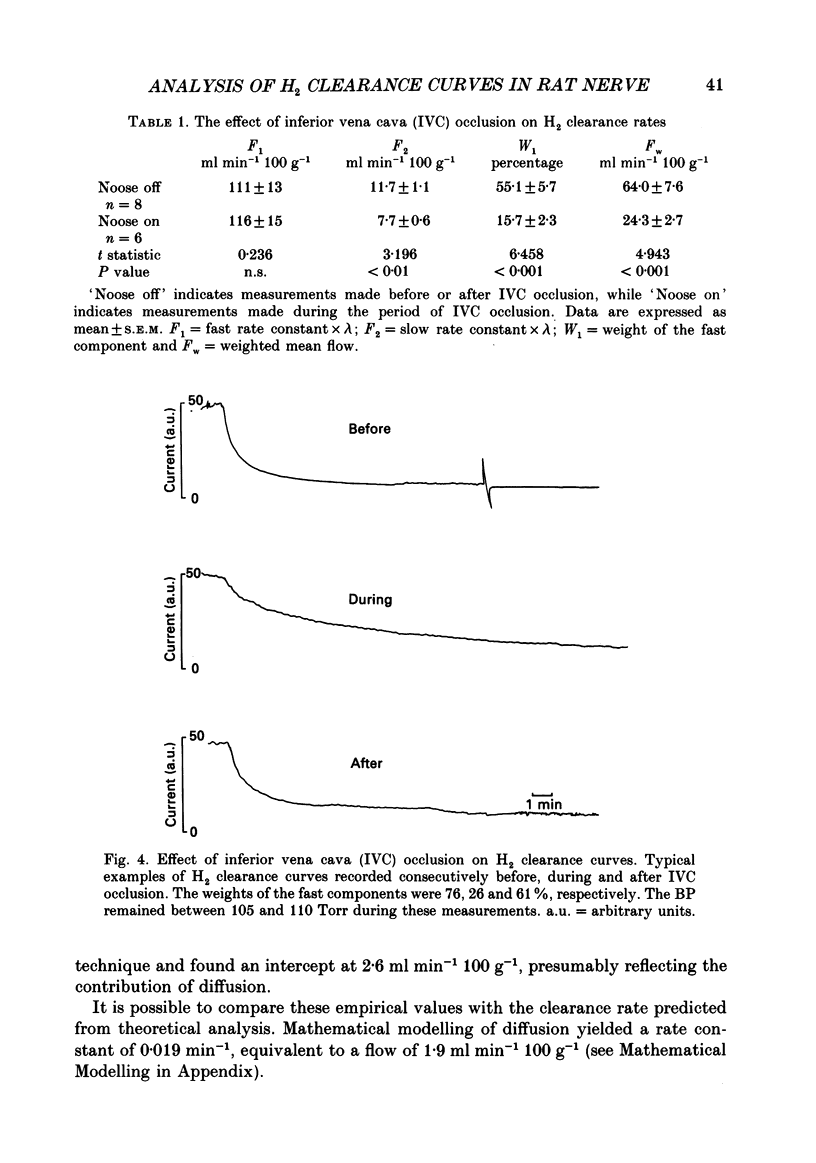
1. By use of the H2 clearance technique, blood flow was measured in the sciatic nerve of healthy, anaesthetized Sprague-Dawley rats at rest, during inferior vena cava occlusion and during 5-hydroxytryptamine infusion. The purpose was to clarify the mechanisms underlying the biexponential curves which are commonly obtained using this technique. 2. An analysis of the frequency distribution of rate constants of 270 nerve and thirty-three arterial samples indicated that H2 clearance rates cluster below 20 ml min-1 100 g-1 and between 70 and 100 ml min-1 100 g-1. This suggests that at least two compartments are present. 3. The contribution of diffusion was studied by recording H2 clearance immediately following circulatory arrest. Slow clearance rates (median = 2.4 ml min-1 100 g-1) were observed, indicating that diffusion is not likely to contribute significantly to nutritive flow under most situations. 4. The contribution of arteriovenous shunts to H2 clearance was assessed by determining H2 clearance during inferior vena cava occlusion and the infusion of 5-hydroxytryptamine. Both manoeuvres caused abolition of, or a significant reduction in the weight of, the fast component which indicates that this compartment is closely related to arteriovenous shunts in nerve. 5. By use of a multi-compartmental model, it was shown that H2 clearance should follow a multi-exponential course, where the weights of the components reflect the relative volumes of each compartment and the exponents represent the relative flow (i.e. flow per unit volume) in each compartment. 6. By use of other mathematical models, estimates were made for the clearance rates attributable to polarographic oxidation of H2 at the tip of the microelectrode (0.2 ml min-1 100 g-1) and to diffusion to air (2 ml min-1 100 g-1). The latter estimate is very close to the measured value of 2.4 ml min-1 100 g-1. 7. These findings indicate that it is possible to separately assess nutritive and non-nutritive flow by application of biexponential analysis to H2 clearance curves. The data suggest that the fast component of a H2 clearance curve is closely associated with arteriovenous shunts, while the slower component is likely to represent capillary flow. Processes such as diffusion to air or oxidation of H2 by the electrode are very slow and therefore are unlikely to distort the assessment of blood flow by using this technique.
Full text
PDF




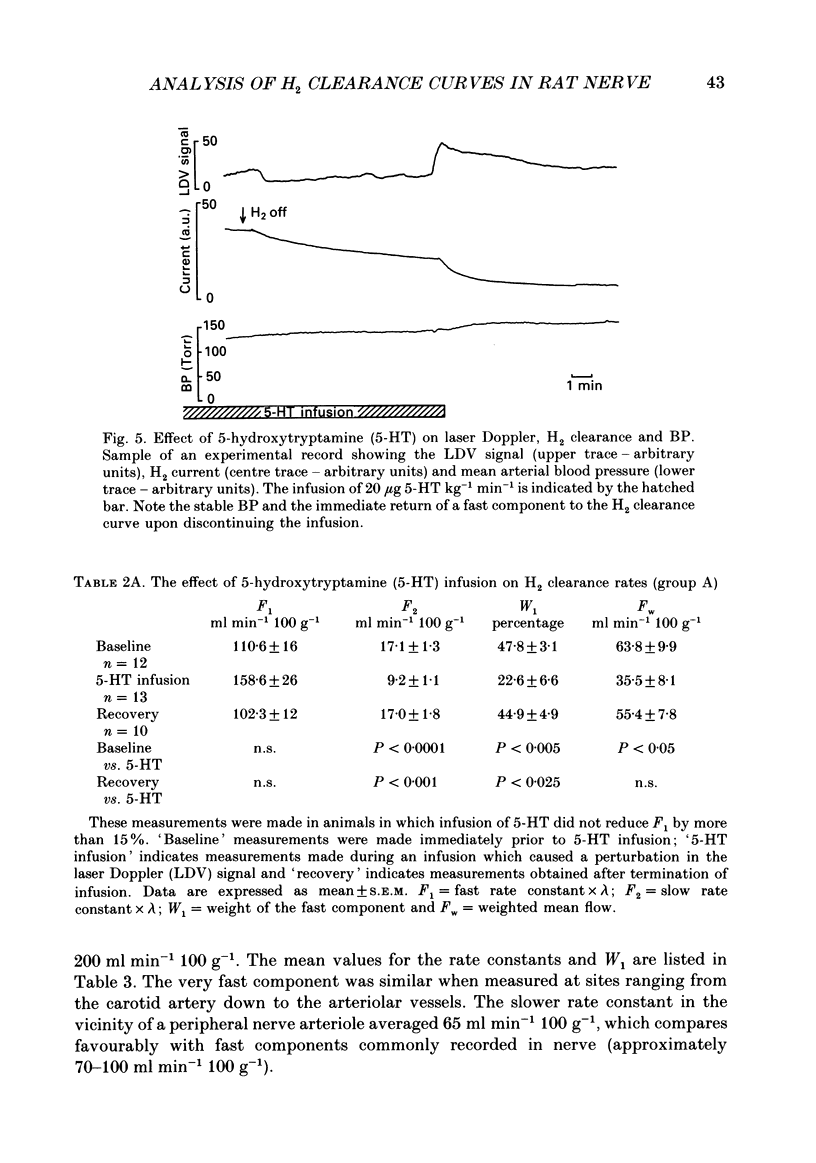
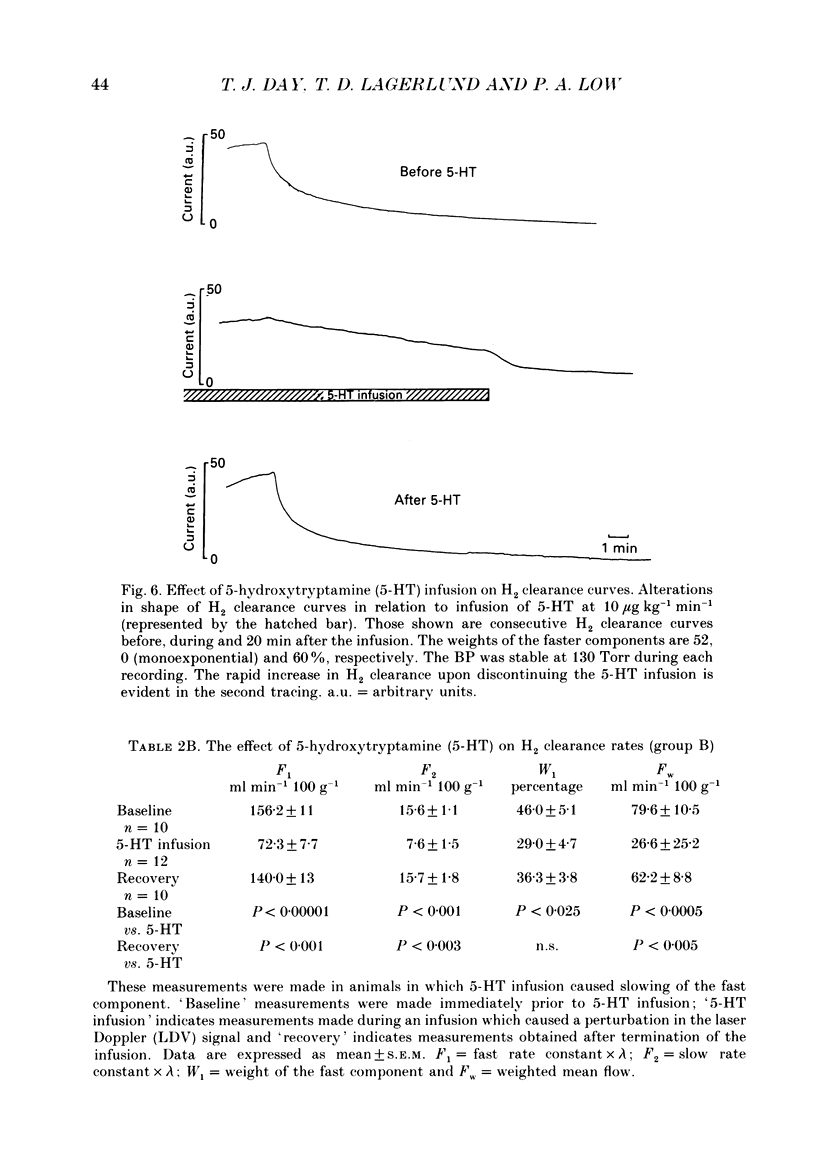








Images in this article
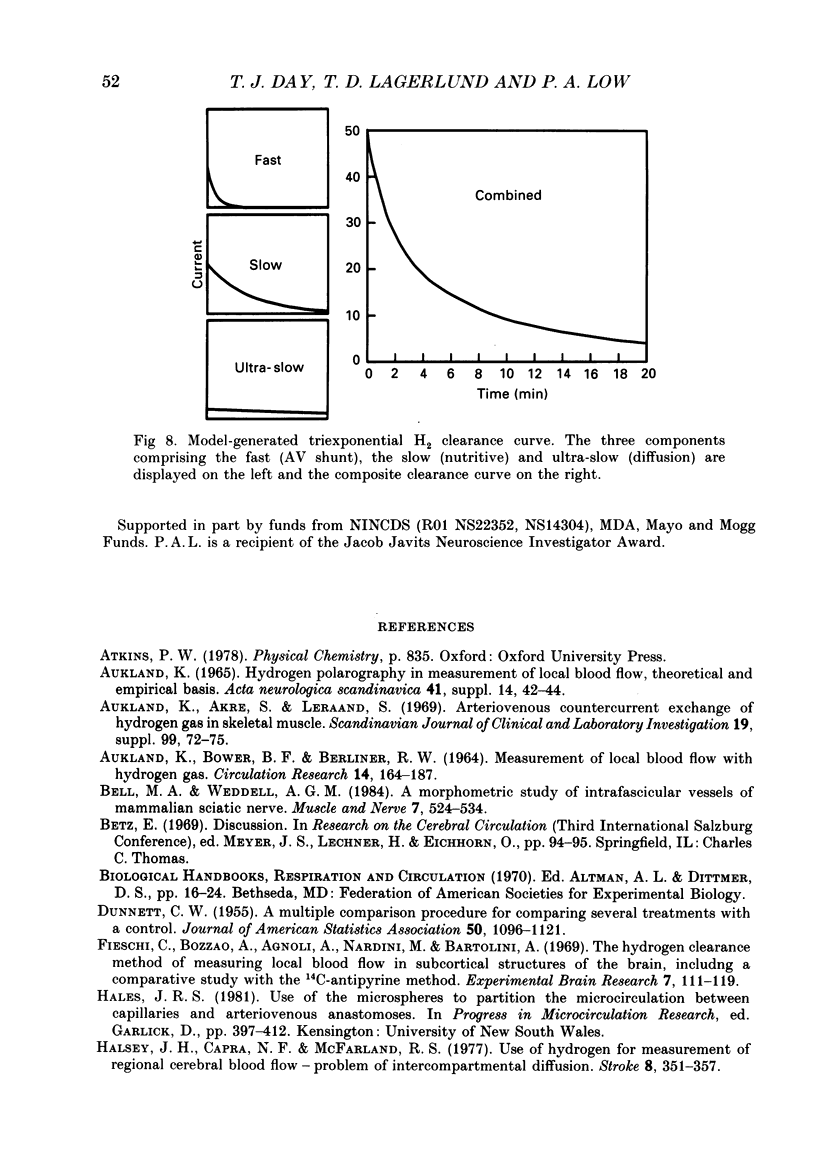
Selected References
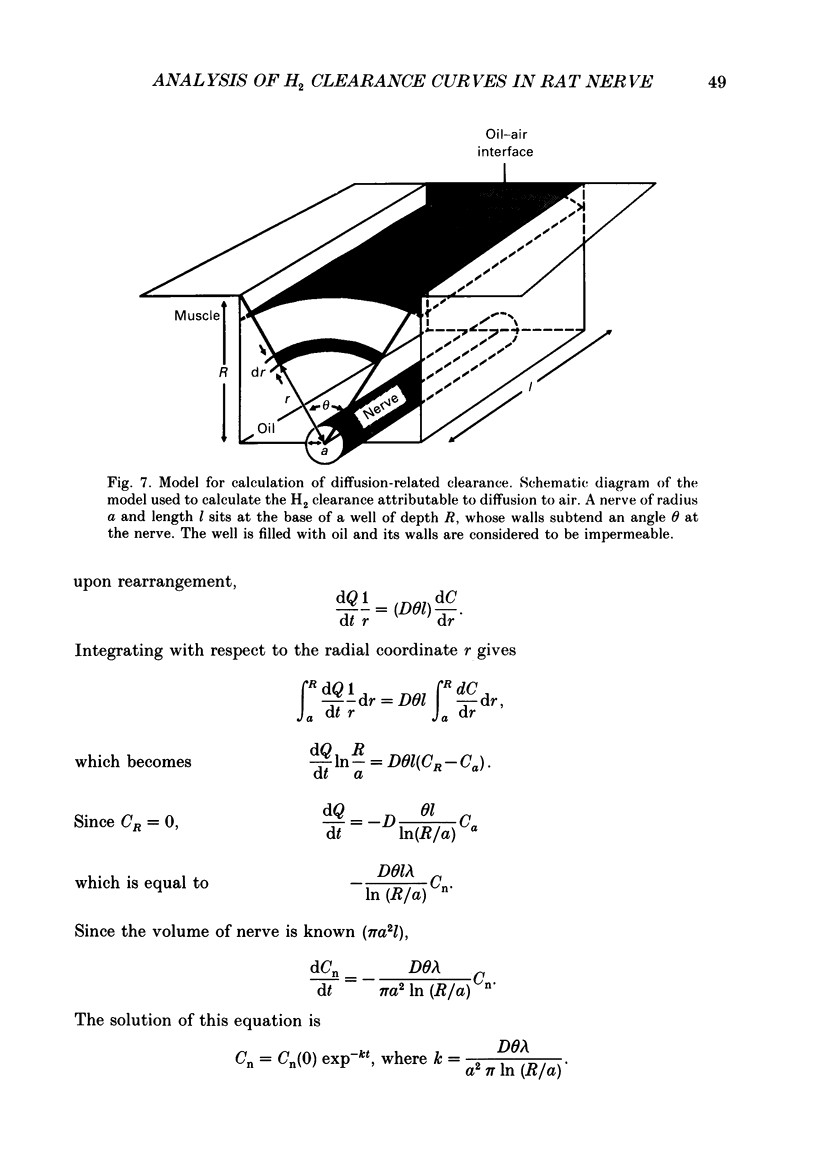
These references are in PubMed. This may not be the complete list of references from this article.
- AUKLAND K., BOWER B. F., BERLINER R. W. MEASUREMENT OF LOCAL BLOOD FLOW WITH HYDROGEN GAS. Circ Res. 1964 Feb;14:164–187. doi: 10.1161/01.res.14.2.164. [DOI] [PubMed] [Google Scholar]
- Aukland K., Akre S., Leraand S. Arteriovenous counter-current exchange of hydrogen gas in skeletal muscle. Scand J Clin Lab Invest Suppl. 1967;99:72–75. [PubMed] [Google Scholar]
- Bell M. A., Weddell A. G. A morphometric study of intrafascicular vessels of mammalian sciatic nerve. Muscle Nerve. 1984 Sep;7(7):524–534. doi: 10.1002/mus.880070703. [DOI] [PubMed] [Google Scholar]
- Fieschi C., Bozzao L., Agnoli A., Nardini M., Bartolini A. The hydrogen method of measuring local blood flow in subcortical structures of the brain: including a comparative study with the 14C antipyrine method. Exp Brain Res. 1969;7(2):111–119. doi: 10.1007/BF00235437. [DOI] [PubMed] [Google Scholar]
- Halsey J. H., Jr, Capra N. F., McFarland R. S. Use of hydrogen for measurement of regional cerebral blood flow: problem of intercompartmental diffusion. Stroke. 1977 May-Jun;8(3):351–357. doi: 10.1161/01.str.8.3.351. [DOI] [PubMed] [Google Scholar]
- KETY S. S. The theory and applications of the exchange of inert gas at the lungs and tissues. Pharmacol Rev. 1951 Mar;3(1):1–41. [PubMed] [Google Scholar]
- Lawrence J. H., Loomis W. F., Tobias C. A., Turpin F. H. Preliminary observations on the narcotic effect of xenon with a review of values for solubilities of gases in water and oils. J Physiol. 1946 Dec 6;105(3):197–204. [PMC free article] [PubMed] [Google Scholar]
- Low P. A., Nukada H., Schmelzer J. D., Tuck R. R., Dyck P. J. Endoneurial oxygen tension and radial topography in nerve edema. Brain Res. 1985 Aug 19;341(1):147–154. doi: 10.1016/0006-8993(85)91482-9. [DOI] [PubMed] [Google Scholar]
- Low P. A., Schmelzer J. D., Ward K. K. The effect of age on energy metabolism and resistance to ischaemic conduction failure in rat peripheral nerve. J Physiol. 1986 May;374:263–271. doi: 10.1113/jphysiol.1986.sp016078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low P. A., Tuck R. R. Effects of changes of blood pressure, respiratory acidosis and hypoxia on blood flow in the sciatic nerve of the rat. J Physiol. 1984 Feb;347:513–524. doi: 10.1113/jphysiol.1984.sp015079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundborg G. Structure and function of the intraneural microvessels as related to trauma, edema formation, and nerve function. J Bone Joint Surg Am. 1975 Oct;57(7):938–948. [PubMed] [Google Scholar]
- McManis P. G., Low P. A. Factors affecting the relative viability of centrifascicular and subperineurial axons in acute peripheral nerve ischemia. Exp Neurol. 1988 Jan;99(1):84–95. doi: 10.1016/0014-4886(88)90129-x. [DOI] [PubMed] [Google Scholar]
- Meyer J. S., Fukuuchi Y., Kanda T., Shimarzu K., Hashi K. Regional cerebral blood flow measured by intracarotid injection of hydrogen. Comparison of regional vasomotor capacitance from cerebral infarction versus compression. Neurology. 1972 Jun;22(6):571–584. doi: 10.1212/wnl.22.6.571. [DOI] [PubMed] [Google Scholar]
- Myers R. R., Mizisin A. P., Powell H. C., Lampert P. W. Reduced nerve blood flow in hexachlorophene neuropathy: relationship to elevated endoneurial fluid pressure. J Neuropathol Exp Neurol. 1982 Jul;41(4):391–399. doi: 10.1097/00005072-198207000-00002. [DOI] [PubMed] [Google Scholar]
- Pasztor E., Symon L., Dorsch N. W., Branston N. M. The hydrogen clearance method in assessment of blood flow in cortex, white matter and deep nuclei of baboons. Stroke. 1973 Jul-Aug;4(4):556–567. doi: 10.1161/01.str.4.4.556. [DOI] [PubMed] [Google Scholar]
- Pearce R. A., Adams J. M. Measurement of rCBF by H2 clearance: theoretical analysis of diffusion effects. Stroke. 1982 May-Jun;13(3):347–355. doi: 10.1161/01.str.13.3.347. [DOI] [PubMed] [Google Scholar]
- SHERMAN J. L., Jr NORMAL ARTERIOVENOUS ANASTOMOSES. Medicine (Baltimore) 1963 Jul;42:247–267. doi: 10.1097/00005792-196307000-00001. [DOI] [PubMed] [Google Scholar]
- Saxena P. R., Forsyth R. P., Johnston B. M., De Werk A. Regional and systemic haemodynamic changes evoked by 5-hydroxytryptamine in awake and anaesthetized rabbits. Eur J Pharmacol. 1978 Jul 1;50(1):61–68. doi: 10.1016/0014-2999(78)90253-4. [DOI] [PubMed] [Google Scholar]
- Saxena P. R., Verdouw P. D. Redistribution by 5-hydroxytryptamine of carotid arterial blood at the expense of arteriovenous anastomotic blood flow. J Physiol. 1982 Nov;332:501–520. doi: 10.1113/jphysiol.1982.sp014427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena P. R., Verdouw P. D. Tissue blood flow and localization of arteriovenous anastomoses in pigs with microspheres of four different sizes. Pflugers Arch. 1985 Feb;403(2):128–135. doi: 10.1007/BF00584089. [DOI] [PubMed] [Google Scholar]
- Stosseck K. Hydrogen exchange through the pial vessel wall and its meaning for the determination of the local cerebral blood flow. Pflugers Arch. 1970;320(2):111–119. doi: 10.1007/BF00588546. [DOI] [PubMed] [Google Scholar]
- Takahashi H. Cardiovascular and sympathetic responses to intracarotid and intravenous injections of serotonin in rats. Naunyn Schmiedebergs Arch Pharmacol. 1985 May;329(3):222–226. doi: 10.1007/BF00501872. [DOI] [PubMed] [Google Scholar]
- Takeuchi M., Low P. A. Dynamic peripheral nerve metabolic and vascular responses to exsanguination. Am J Physiol. 1987 Oct;253(4 Pt 1):E349–E353. doi: 10.1152/ajpendo.1987.253.4.E349. [DOI] [PubMed] [Google Scholar]
- Tschetter T. H., Klassen A. C., Resch J. A., Meyer M. W. Blood flow in the central and peripheral nervous system of dogs using a particle distribution method. Stroke. 1970 Sep-Oct;1(5):370–374. doi: 10.1161/01.str.1.5.370. [DOI] [PubMed] [Google Scholar]
- Young W. H2 clearance measurement of blood flow: a review of technique and polarographic principles. Stroke. 1980 Sep-Oct;11(5):552–564. doi: 10.1161/01.str.11.5.552. [DOI] [PubMed] [Google Scholar]
- von Kummer R., Herold S. Hydrogen clearance method for determining local cerebral blood flow. I. Spatial resolution. J Cereb Blood Flow Metab. 1986 Aug;6(4):486–491. doi: 10.1038/jcbfm.1986.83. [DOI] [PubMed] [Google Scholar]
- von Kummer R., von Kries F., Herold S. Hydrogen clearance method for determining local cerebral blood flow. II. Effect of heterogeneity in cerebral blood flow. J Cereb Blood Flow Metab. 1986 Aug;6(4):492–498. doi: 10.1038/jcbfm.1986.84. [DOI] [PubMed] [Google Scholar]