Abstract
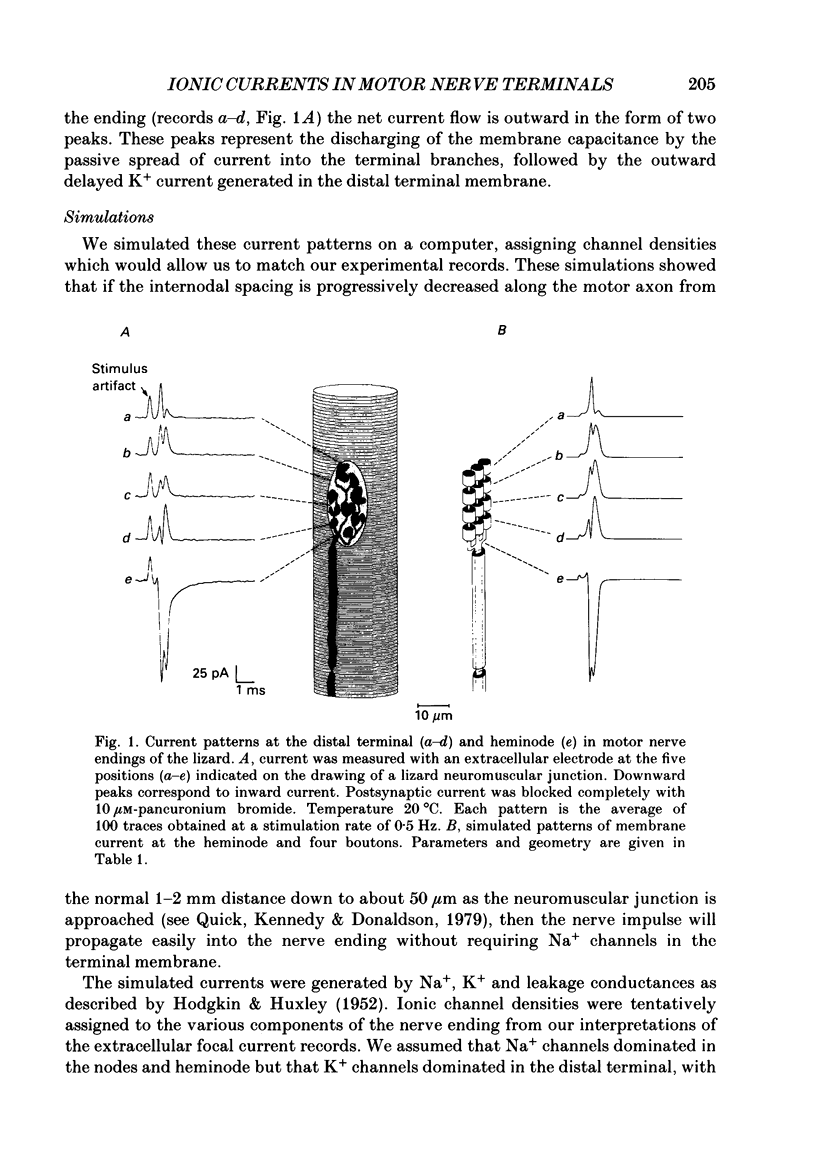
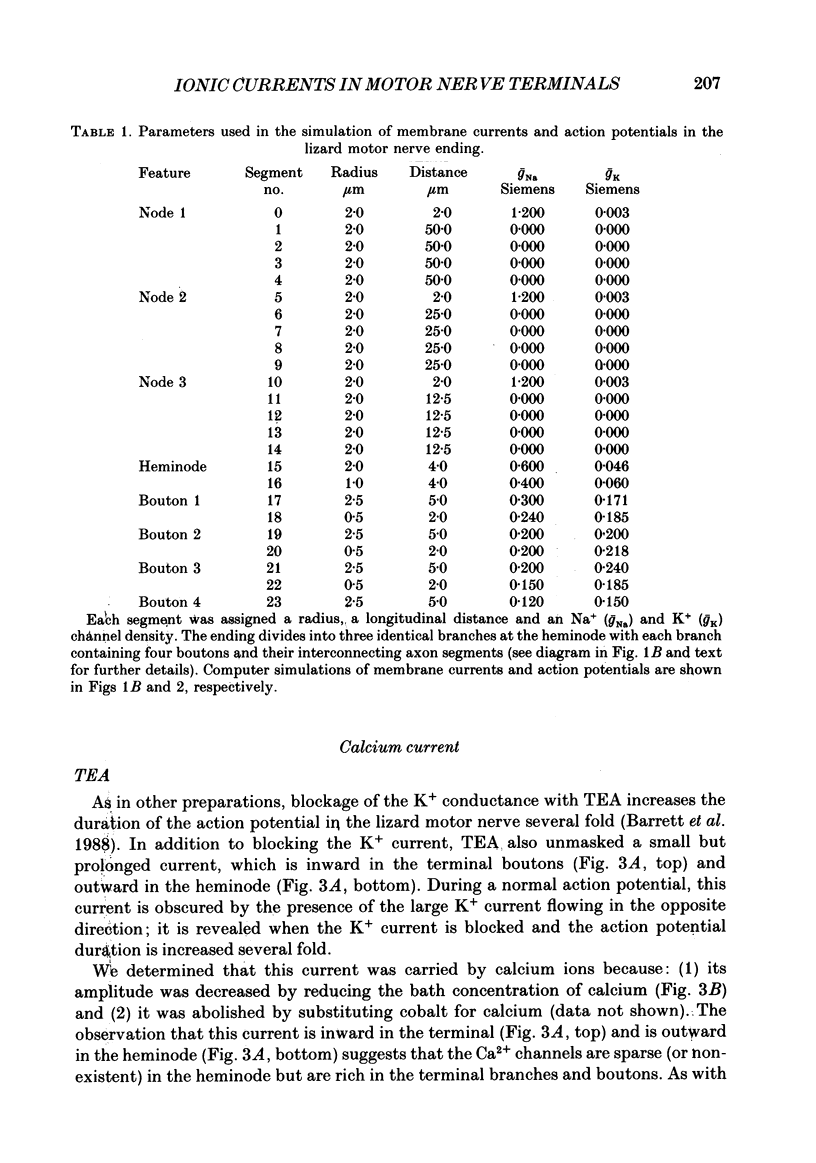
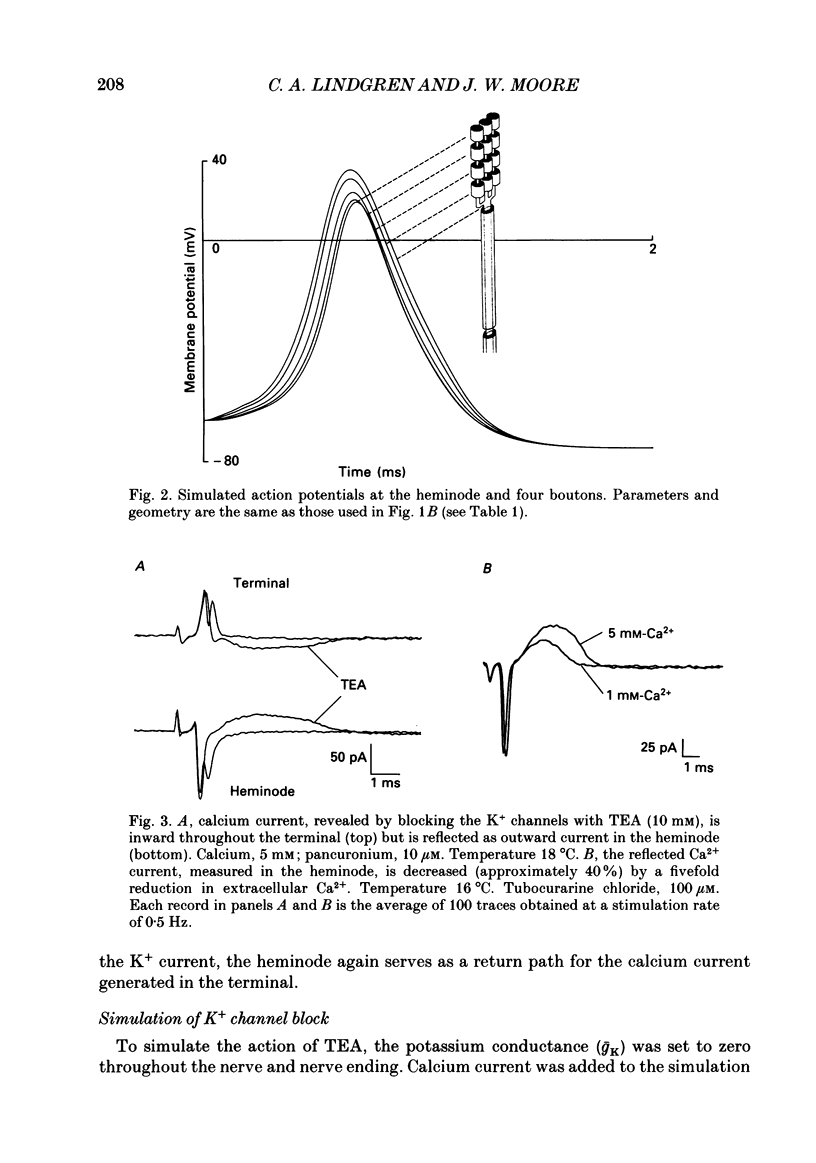
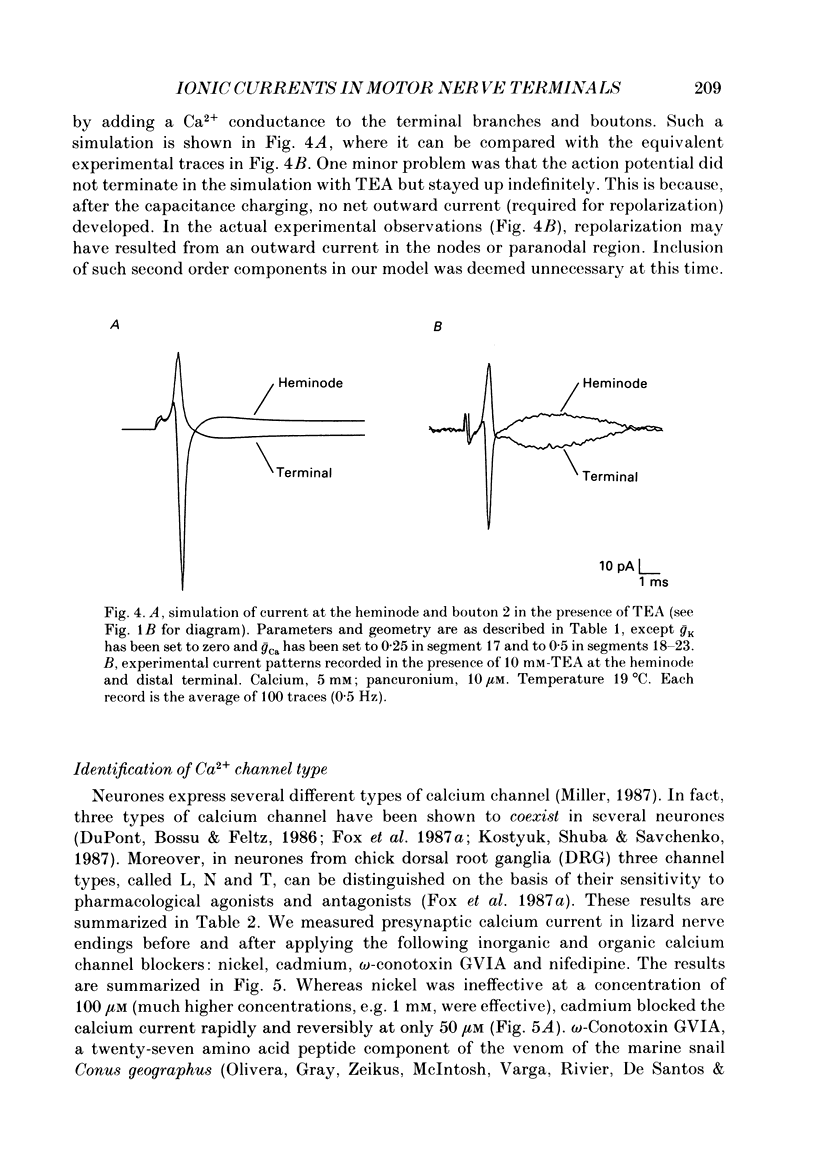
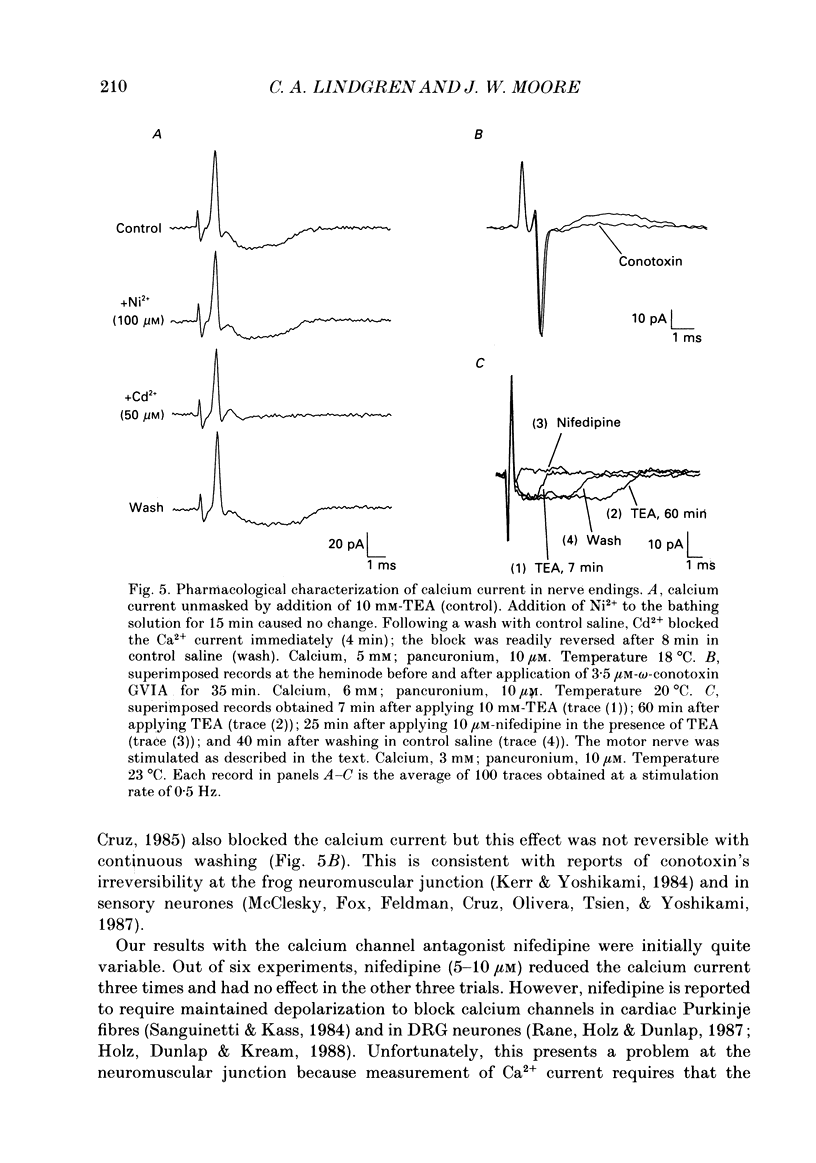
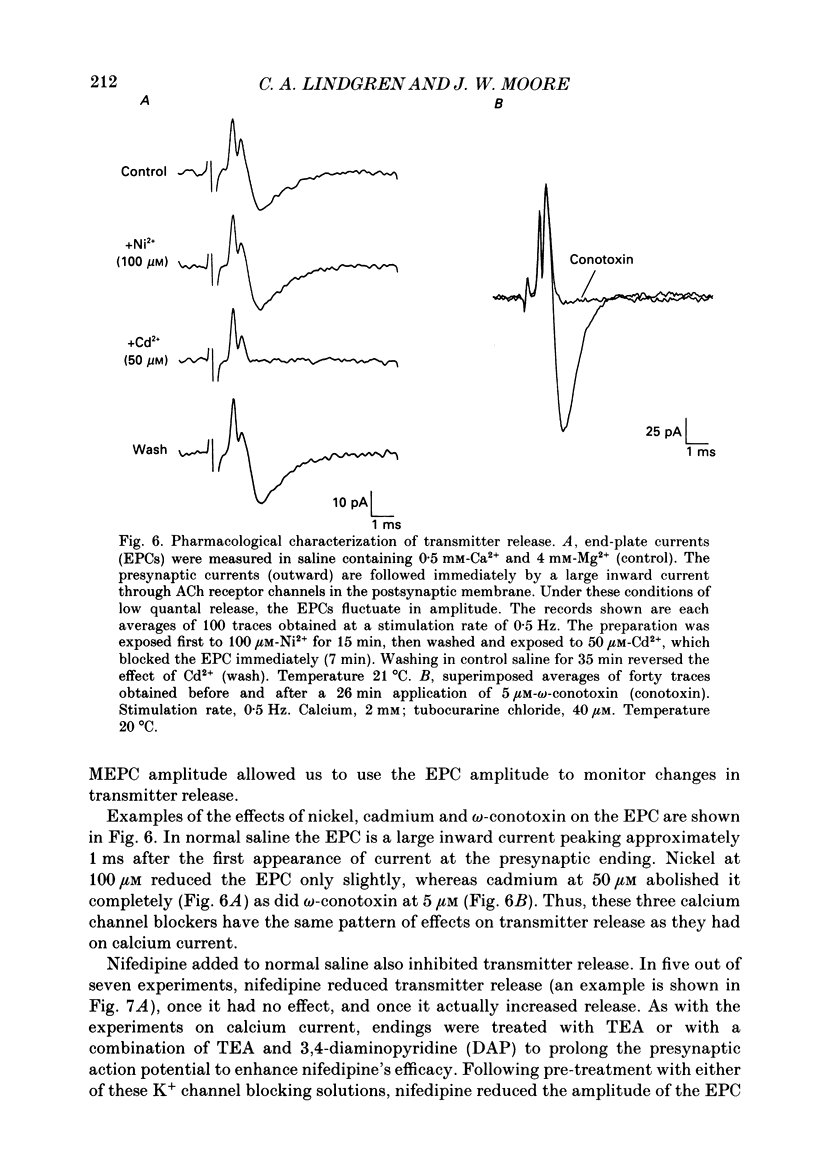
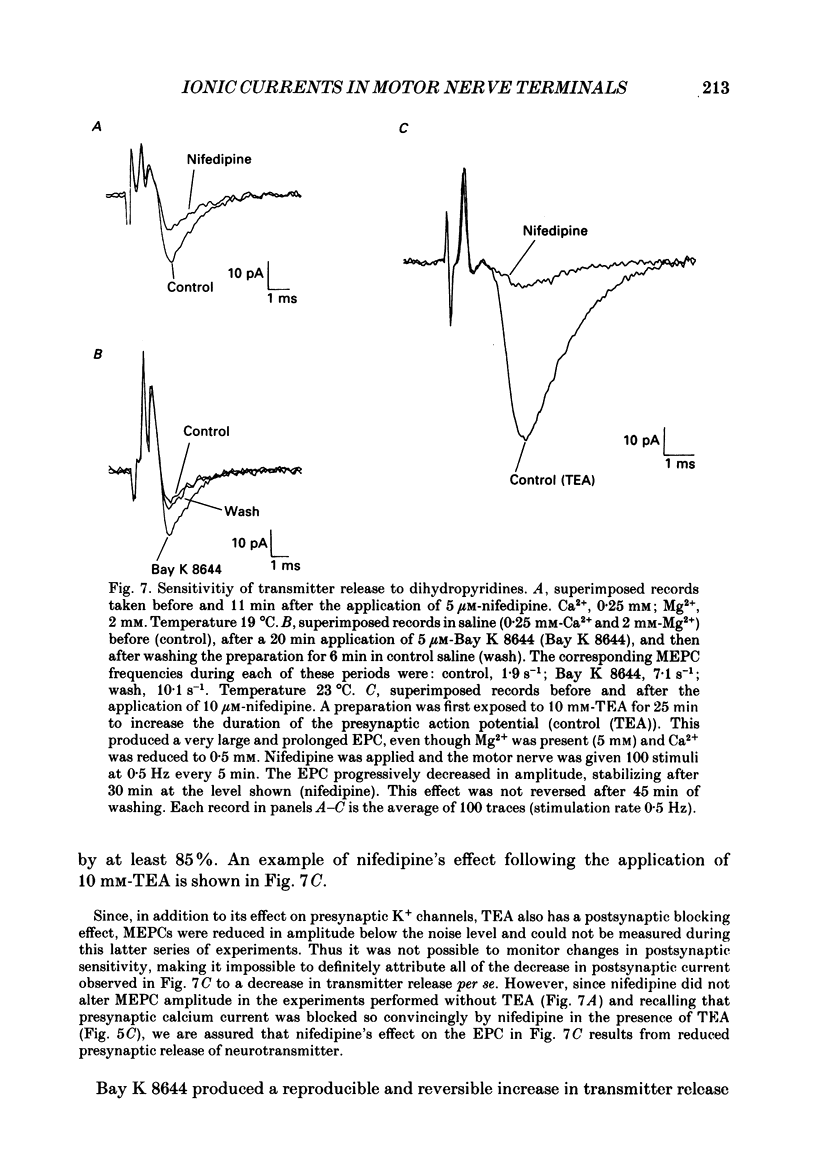
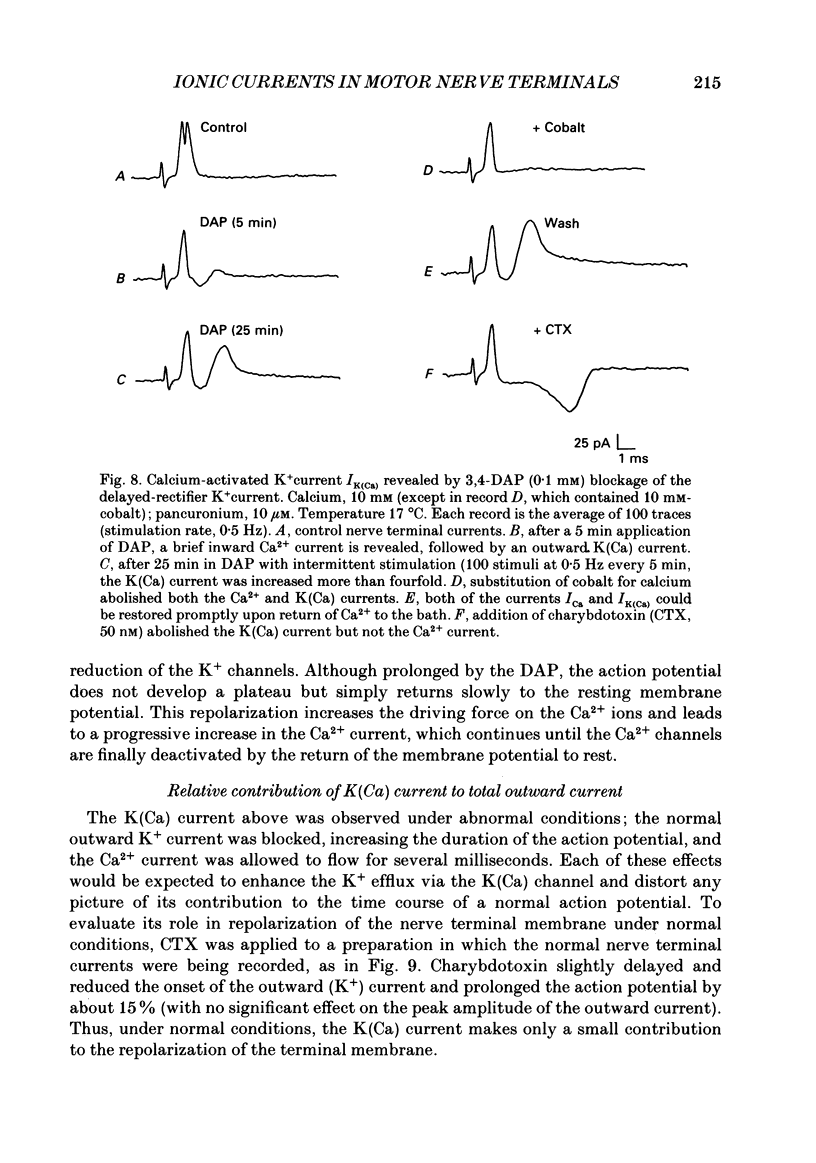
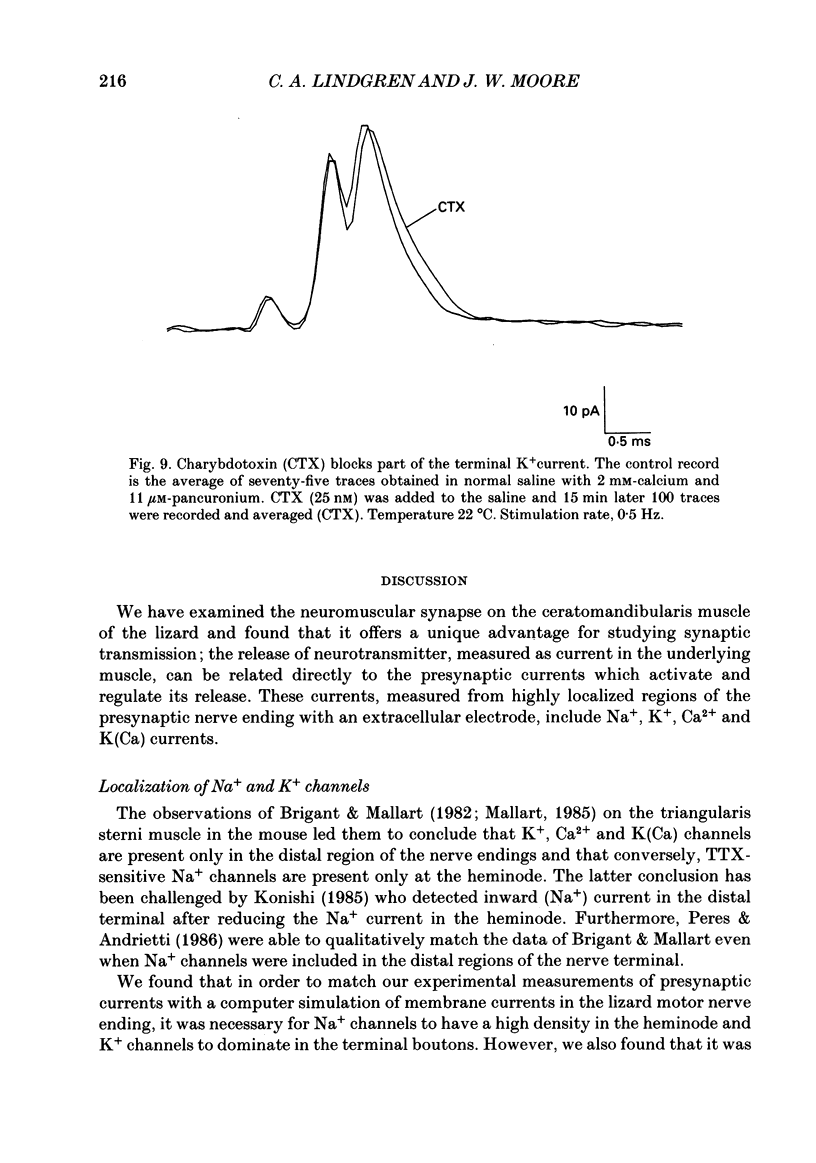
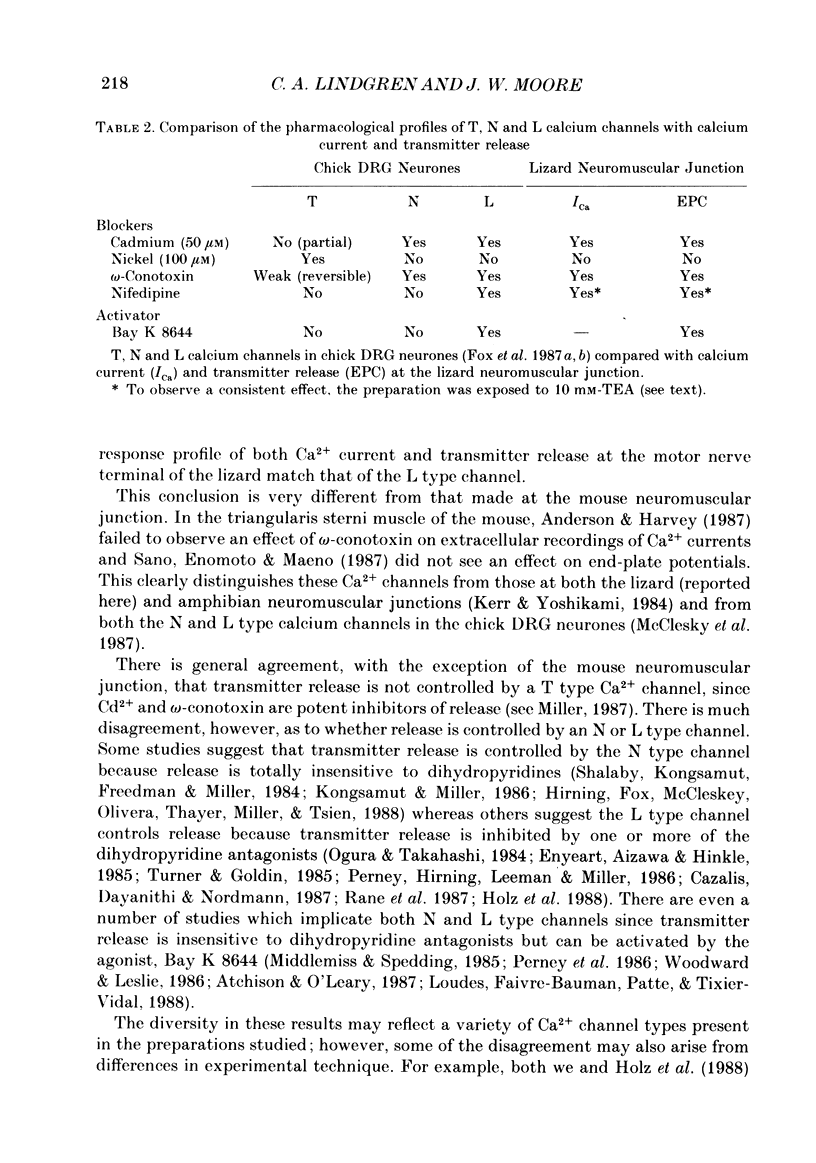
1. Ionic currents associated with the invasion of an action potential into the motor nerve ending of the lizard, Anolis carolinensis, were measured with a focal extracellular electrode at several locations along the nerve ending. 2. These experimentally observed currents could be matched with computer simulations of action potential propagation into the nerve ending. They revealed that while Na+ channels are the major ionic current pathway in the heminode, K+ channels provide the major pathway in the terminal branches and boutons. 3. Calcium current in the presynaptic ending was unmasked by the application of tetraethylammonium (TEA). This current was blocked by: (a) cadmium, (b) omega-conotoxin GVIA and (c) nifedipine, but was unaffected by nickel at concentrations less than or equal to 100 microM. Nifedipine's action became more definitive when the duration of the action potential was greatly extended by pre-treatment with TEA. The effect of Bay K 8644 was inconsistent. 4. Transmitter release, as measured by postsynaptic current, had a pharmacological response profile similar to that of the Ca2+ current, with the exception that transmitter release was increased reliably and reversibly by Bay K 8644. 5. This pharmacological response profile is identical to that of the L type Ca2+ channel identified by Fox, Nowycky & Tsien (1987 alpha) in chick dorsal root ganglion neurones. We saw no evidence for more than a single type of Ca2+ channel in lizard motor nerve endings. 6. A calcium-activated K+ current IK(Ca) was revealed by application of 3,4-diaminopyridine (DAP), a delayed-rectifier K+ channel blocker. This K(Ca) current was blocked by TEA, charybdotoxin and by substitution of cobalt for extracellular calcium.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARMSTRONG C. M., BINSTOCK L. ANOMALOUS RECTIFICATION IN THE SQUID GIANT AXON INJECTED WITH TETRAETHYLAMMONIUM CHLORIDE. J Gen Physiol. 1965 May;48:859–872. doi: 10.1085/jgp.48.5.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson A. J., Harvey A. L. Omega-conotoxin does not block the verapamil-sensitive calcium channels at mouse motor nerve terminals. Neurosci Lett. 1987 Nov 23;82(2):177–180. doi: 10.1016/0304-3940(87)90125-x. [DOI] [PubMed] [Google Scholar]
- Anderson A. J., Harvey A. L., Rowan E. G., Strong P. N. Effects of charybdotoxin, a blocker of Ca2+-activated K+ channels, on motor nerve terminals. Br J Pharmacol. 1988 Dec;95(4):1329–1335. doi: 10.1111/j.1476-5381.1988.tb11772.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atchison W. D., O'Leary S. M. BAY K 8644 increases release of acetylcholine at the murine neuromuscular junction. Brain Res. 1987 Sep 1;419(1-2):315–319. doi: 10.1016/0006-8993(87)90599-3. [DOI] [PubMed] [Google Scholar]
- Augustine G. J., Charlton M. P., Horn R. Role of calcium-activated potassium channels in transmitter release at the squid giant synapse. J Physiol. 1988 Apr;398:149–164. doi: 10.1113/jphysiol.1988.sp017035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustine G. J., Charlton M. P., Smith S. J. Calcium action in synaptic transmitter release. Annu Rev Neurosci. 1987;10:633–693. doi: 10.1146/annurev.ne.10.030187.003221. [DOI] [PubMed] [Google Scholar]
- Barrett E. F., Barrett J. N. Intracellular recording from vertebrate myelinated axons: mechanism of the depolarizing afterpotential. J Physiol. 1982 Feb;323:117–144. doi: 10.1113/jphysiol.1982.sp014064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett E. F., Morita K., Scappaticci K. A. Effects of tetraethylammonium on the depolarizing after-potential and passive properties of lizard myelinated axons. J Physiol. 1988 Aug;402:65–78. doi: 10.1113/jphysiol.1988.sp017194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigant J. L., Mallart A. Presynaptic currents in mouse motor endings. J Physiol. 1982 Dec;333:619–636. doi: 10.1113/jphysiol.1982.sp014472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazalis M., Dayanithi G., Nordmann J. J. Hormone release from isolated nerve endings of the rat neurohypophysis. J Physiol. 1987 Sep;390:55–70. doi: 10.1113/jphysiol.1987.sp016686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont J. L., Bossu J. L., Feltz A. Effect of internal calcium concentration on calcium currents in rat sensory neurones. Pflugers Arch. 1986 Apr;406(4):433–435. doi: 10.1007/BF00590950. [DOI] [PubMed] [Google Scholar]
- Ellisman M. H., Rash J. E., Staehelin L. A., Porter K. R. Studies of excitable membranes. II. A comparison of specializations at neuromuscular junctions and nonjunctional sarcolemmas of mammalian fast and slow twitch muscle fibers. J Cell Biol. 1976 Mar;68(3):752–774. doi: 10.1083/jcb.68.3.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enyeart J. J., Aizawa T., Hinkle P. M. Dihydropyridine Ca2+ antagonists: potent inhibitors of secretion from normal and transformed pituitary cells. Am J Physiol. 1985 May;248(5 Pt 1):C510–C519. doi: 10.1152/ajpcell.1985.248.5.C510. [DOI] [PubMed] [Google Scholar]
- Finkelstein A., Zimmerberg J., Cohen F. S. Osmotic swelling of vesicles: its role in the fusion of vesicles with planar phospholipid bilayer membranes and its possible role in exocytosis. Annu Rev Physiol. 1986;48:163–174. doi: 10.1146/annurev.ph.48.030186.001115. [DOI] [PubMed] [Google Scholar]
- Fox A. P., Nowycky M. C., Tsien R. W. Kinetic and pharmacological properties distinguishing three types of calcium currents in chick sensory neurones. J Physiol. 1987 Dec;394:149–172. doi: 10.1113/jphysiol.1987.sp016864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox A. P., Nowycky M. C., Tsien R. W. Single-channel recordings of three types of calcium channels in chick sensory neurones. J Physiol. 1987 Dec;394:173–200. doi: 10.1113/jphysiol.1987.sp016865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. The selective inhibition of delayed potassium currents in nerve by tetraethylammonium ion. J Gen Physiol. 1967 May;50(5):1287–1302. doi: 10.1085/jgp.50.5.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirning L. D., Fox A. P., McCleskey E. W., Olivera B. M., Thayer S. A., Miller R. J., Tsien R. W. Dominant role of N-type Ca2+ channels in evoked release of norepinephrine from sympathetic neurons. Science. 1988 Jan 1;239(4835):57–61. doi: 10.1126/science.2447647. [DOI] [PubMed] [Google Scholar]
- Hodgkin A. L. Evidence for electrical transmission in nerve: Part I. J Physiol. 1937 Jul 15;90(2):183–210. doi: 10.1113/jphysiol.1937.sp003507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holz G. G., 4th, Dunlap K., Kream R. M. Characterization of the electrically evoked release of substance P from dorsal root ganglion neurons: methods and dihydropyridine sensitivity. J Neurosci. 1988 Feb;8(2):463–471. doi: 10.1523/JNEUROSCI.08-02-00463.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATZ B., MILEDI R. PROPAGATION OF ELECTRIC ACTIVITY IN MOTOR NERVE TERMINALS. Proc R Soc Lond B Biol Sci. 1965 Feb 16;161:453–482. doi: 10.1098/rspb.1965.0015. [DOI] [PubMed] [Google Scholar]
- Kerr L. M., Yoshikami D. A venom peptide with a novel presynaptic blocking action. Nature. 1984 Mar 15;308(5956):282–284. doi: 10.1038/308282a0. [DOI] [PubMed] [Google Scholar]
- Kirsch G. E., Narahashi T. 3,4-diaminopyridine. A potent new potassium channel blocker. Biophys J. 1978 Jun;22(3):507–512. doi: 10.1016/S0006-3495(78)85503-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kongsamut S., Miller R. J. Nerve growth factor modulates the drug sensitivity of neurotransmitter release from PC-12 cells. Proc Natl Acad Sci U S A. 1986 Apr;83(7):2243–2247. doi: 10.1073/pnas.83.7.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. S., Tsien R. W. Mechanism of calcium channel blockade by verapamil, D600, diltiazem and nitrendipine in single dialysed heart cells. Nature. 1983 Apr 28;302(5911):790–794. doi: 10.1038/302790a0. [DOI] [PubMed] [Google Scholar]
- Loudes C., Faivre-Bauman A., Patte C., Tixier-Vidal A. Involvement of DHP voltage-sensitive calcium channels and protein kinase C in thyroliberin (TRH) release by developing hypothalamic neurons in culture. Brain Res. 1988 Jul 26;456(2):324–332. doi: 10.1016/0006-8993(88)90235-1. [DOI] [PubMed] [Google Scholar]
- Mallart A. A calcium-activated potassium current in motor nerve terminals of the mouse. J Physiol. 1985 Nov;368:577–591. doi: 10.1113/jphysiol.1985.sp015877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCleskey E. W., Fox A. P., Feldman D. H., Cruz L. J., Olivera B. M., Tsien R. W., Yoshikami D. Omega-conotoxin: direct and persistent blockade of specific types of calcium channels in neurons but not muscle. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4327–4331. doi: 10.1073/pnas.84.12.4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middlemiss D. N., Spedding M. A functional correlate for the dihydropyridine binding site in rat brain. Nature. 1985 Mar 7;314(6006):94–96. doi: 10.1038/314094a0. [DOI] [PubMed] [Google Scholar]
- Miller C., Moczydlowski E., Latorre R., Phillips M. Charybdotoxin, a protein inhibitor of single Ca2+-activated K+ channels from mammalian skeletal muscle. Nature. 1985 Jan 24;313(6000):316–318. doi: 10.1038/313316a0. [DOI] [PubMed] [Google Scholar]
- Miller R. J. Multiple calcium channels and neuronal function. Science. 1987 Jan 2;235(4784):46–52. doi: 10.1126/science.2432656. [DOI] [PubMed] [Google Scholar]
- Moore J. W., Joyner R. W., Brill M. H., Waxman S. D., Najar-Joa M. Simulations of conduction in uniform myelinated fibers. Relative sensitivity to changes in nodal and internodal parameters. Biophys J. 1978 Feb;21(2):147–160. doi: 10.1016/S0006-3495(78)85515-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogura A., Takahashi M. Differential effect of a dihydropyridine derivative to Ca2+ entry pathways in neuronal preparations. Brain Res. 1984 Jun 3;301(2):323–330. doi: 10.1016/0006-8993(84)91101-6. [DOI] [PubMed] [Google Scholar]
- Olivera B. M., Gray W. R., Zeikus R., McIntosh J. M., Varga J., Rivier J., de Santos V., Cruz L. J. Peptide neurotoxins from fish-hunting cone snails. Science. 1985 Dec 20;230(4732):1338–1343. doi: 10.1126/science.4071055. [DOI] [PubMed] [Google Scholar]
- Peres A., Andrietti F. Computer reconstruction of the spread of excitation in nerve terminals with inhomogeneous channel distribution. Eur Biophys J. 1986;13(4):235–243. doi: 10.1007/BF00260370. [DOI] [PubMed] [Google Scholar]
- Perney T. M., Hirning L. D., Leeman S. E., Miller R. J. Multiple calcium channels mediate neurotransmitter release from peripheral neurons. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6656–6659. doi: 10.1073/pnas.83.17.6656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proske U., Vaughan P. Histological and electrophysiological investigation of lizard skeletal muscle. J Physiol. 1968 Dec;199(3):495–509. doi: 10.1113/jphysiol.1968.sp008665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quick D. C., Kennedy W. R., Donaldson L. Dimensions of myelinated nerve fibers near the motor and sensory terminals in cat tenuissimus muscles. Neuroscience. 1979;4(8):1089–1096. doi: 10.1016/0306-4522(79)90190-8. [DOI] [PubMed] [Google Scholar]
- Rane S. G., Holz G. G., 4th, Dunlap K. Dihydropyridine inhibition of neuronal calcium current and substance P release. Pflugers Arch. 1987 Aug;409(4-5):361–366. doi: 10.1007/BF00583789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revenko S. V., Timin E. N., Khodorov B. I. Osobennosti provedeniia nervnykh impul'sov iz mielinizirovannoi chasti aksona v bezmiakotnuiu terminal'. Biofizika. 1973 Nov-Dec;18(6):1074–1078. [PubMed] [Google Scholar]
- Sanguinetti M. C., Kass R. S. Voltage-dependent block of calcium channel current in the calf cardiac Purkinje fiber by dihydropyridine calcium channel antagonists. Circ Res. 1984 Sep;55(3):336–348. doi: 10.1161/01.res.55.3.336. [DOI] [PubMed] [Google Scholar]
- Sanguinetti M. C., Krafte D. S., Kass R. S. Voltage-dependent modulation of Ca channel current in heart cells by Bay K8644. J Gen Physiol. 1986 Sep;88(3):369–392. doi: 10.1085/jgp.88.3.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano K., Enomoto K., Maeno T. Effects of synthetic omega-conotoxin, a new type Ca2+ antagonist, on frog and mouse neuromuscular transmission. Eur J Pharmacol. 1987 Sep 11;141(2):235–241. doi: 10.1016/0014-2999(87)90268-8. [DOI] [PubMed] [Google Scholar]
- Shalaby I. A., Kongsamut S., Freedman S. B., Miller R. J. The effects of dihydropyridines on neurotransmitter release from cultured neuronal cells. Life Sci. 1984 Sep 17;35(12):1289–1295. doi: 10.1016/0024-3205(84)90100-0. [DOI] [PubMed] [Google Scholar]
- Stanley E. F., Ehrenstein G. A model for exocytosis based on the opening of calcium-activated potassium channels in vesicles. Life Sci. 1985 Nov 25;37(21):1985–1995. doi: 10.1016/0024-3205(85)90029-3. [DOI] [PubMed] [Google Scholar]
- Turner T. J., Goldin S. M. Calcium channels in rat brain synaptosomes: identification and pharmacological characterization. High affinity blockade by organic Ca2+ channel blockers. J Neurosci. 1985 Mar;5(3):841–849. doi: 10.1523/JNEUROSCI.05-03-00841.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walrond J. P., Reese T. S. Structure of axon terminals and active zones at synapses on lizard twitch and tonic muscle fibers. J Neurosci. 1985 May;5(5):1118–1131. doi: 10.1523/JNEUROSCI.05-05-01118.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman S. G., Brill M. H. Conduction through demyelinated plaques in multiple sclerosis: computer simulations of facilitation by short internodes. J Neurol Neurosurg Psychiatry. 1978 May;41(5):408–416. doi: 10.1136/jnnp.41.5.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman S. G., Ritchie J. M. Organization of ion channels in the myelinated nerve fiber. Science. 1985 Jun 28;228(4707):1502–1507. doi: 10.1126/science.2409596. [DOI] [PubMed] [Google Scholar]
- Woodward J. J., Leslie S. W. Bay K 8644 stimulation of calcium entry and endogenous dopamine release in rat striatal synaptosomes antagonized by nimodipine. Brain Res. 1986 Apr 9;370(2):397–400. doi: 10.1016/0006-8993(86)90502-0. [DOI] [PubMed] [Google Scholar]


