Abstract
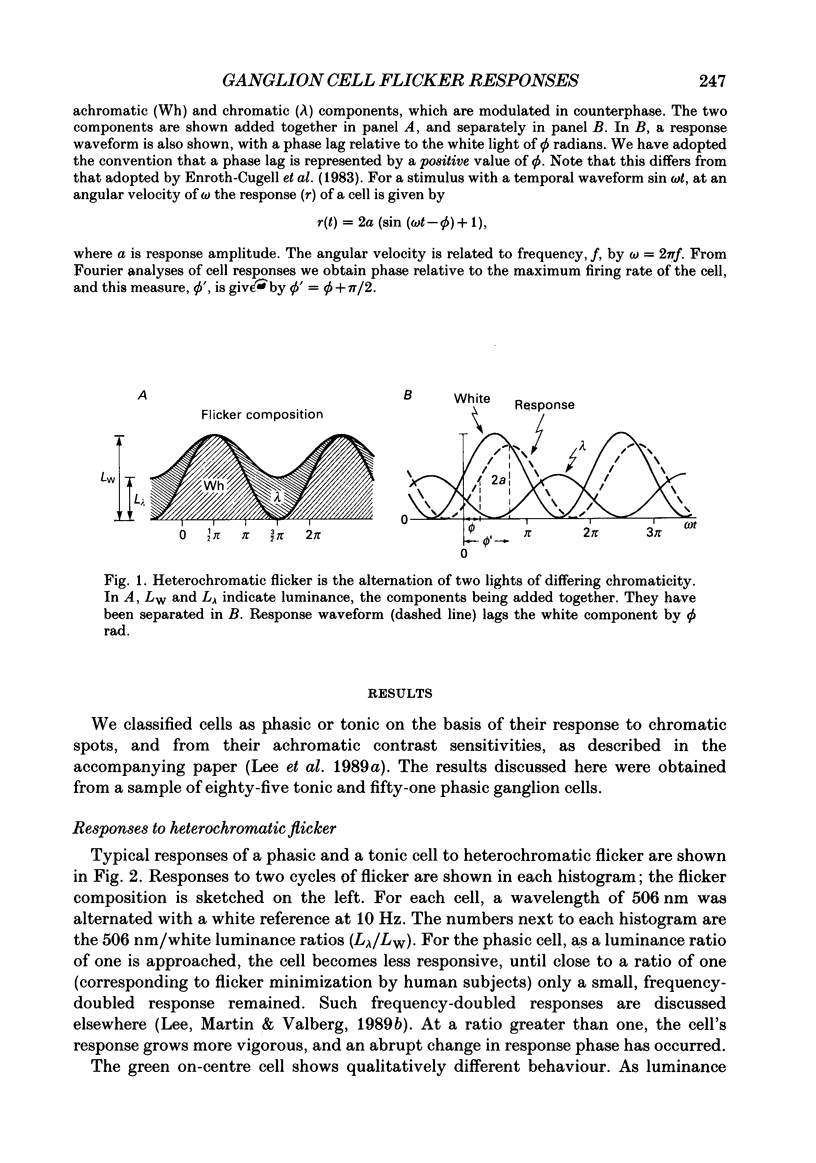
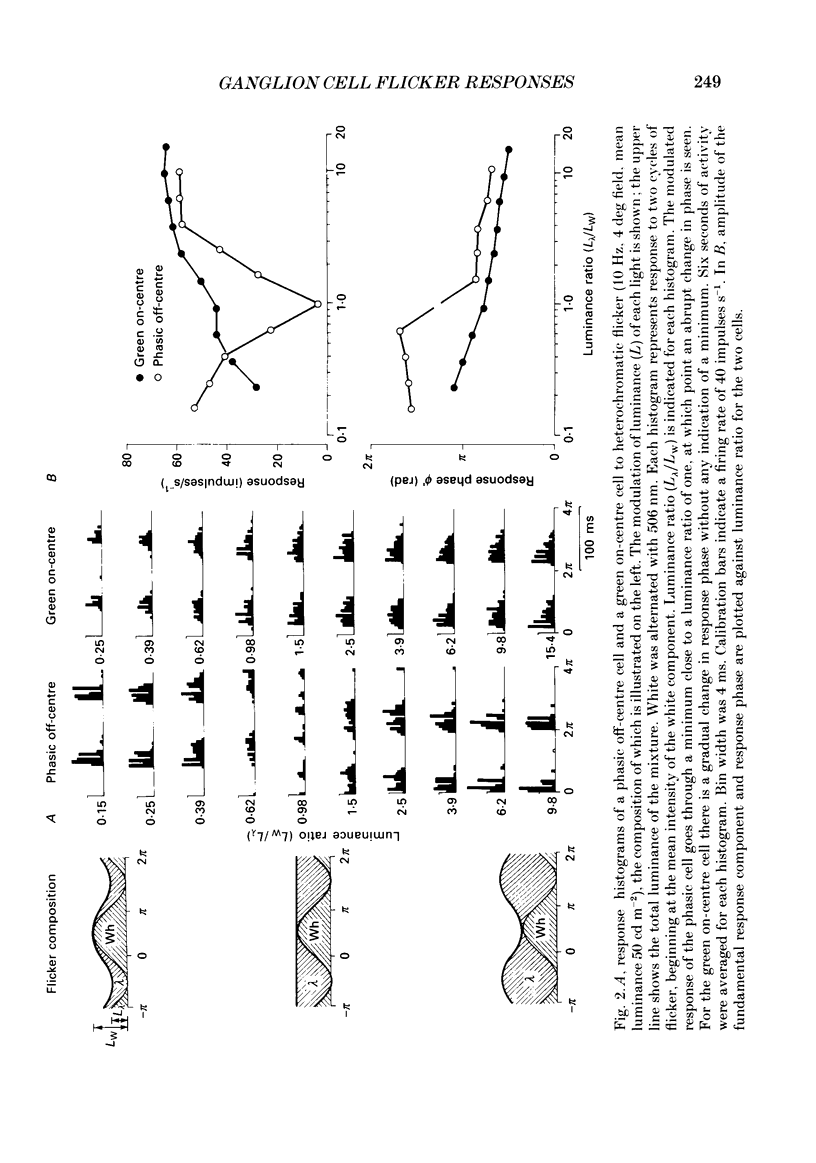
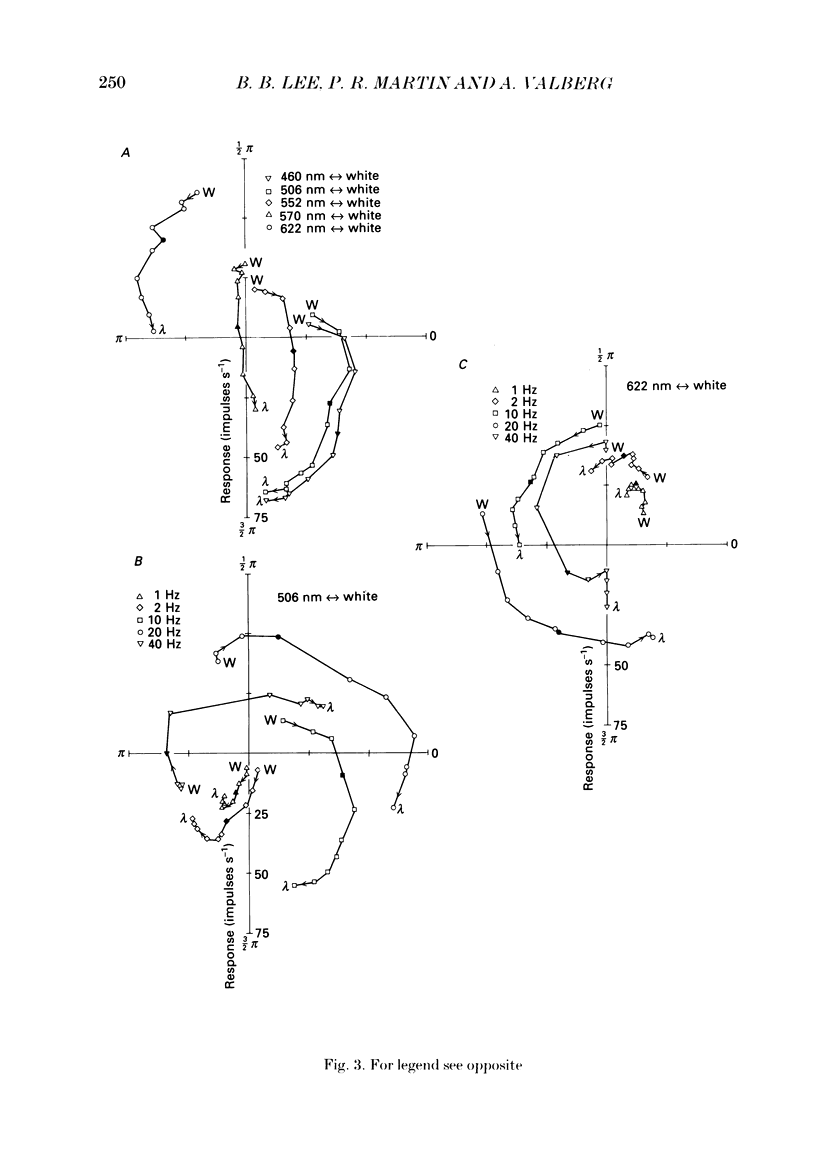
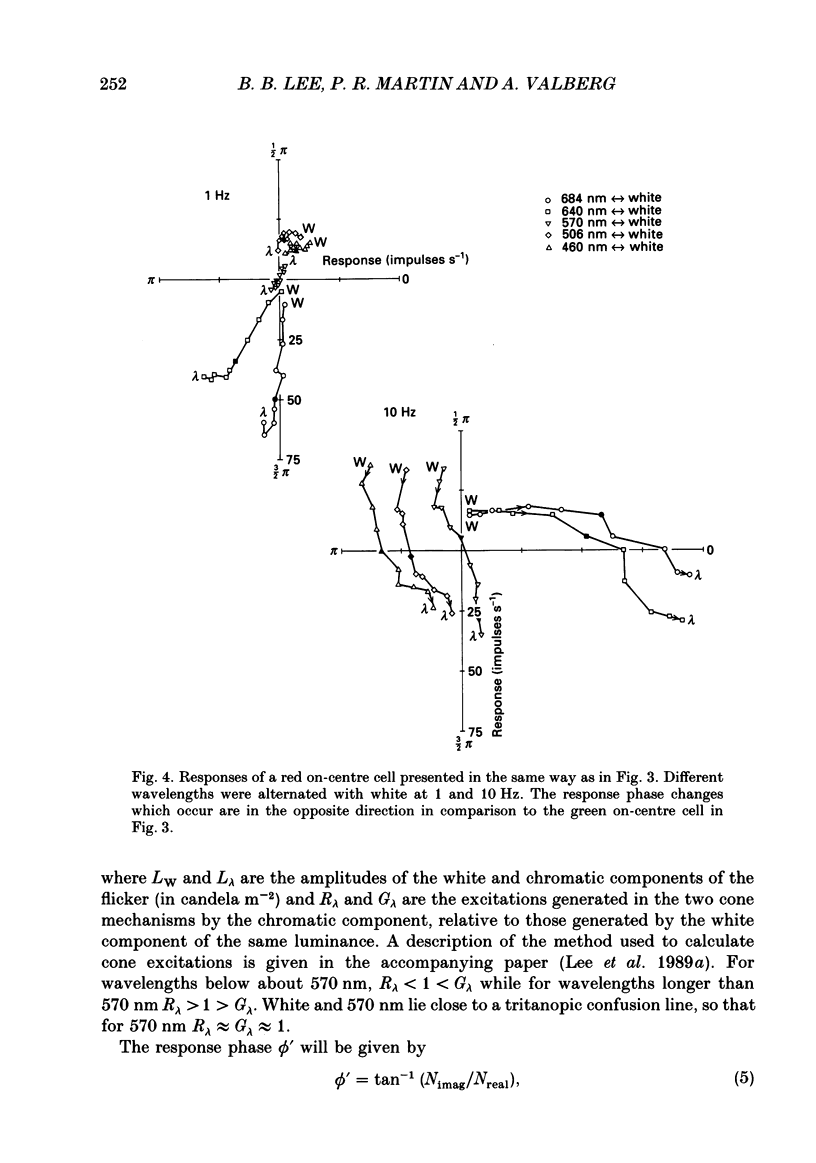
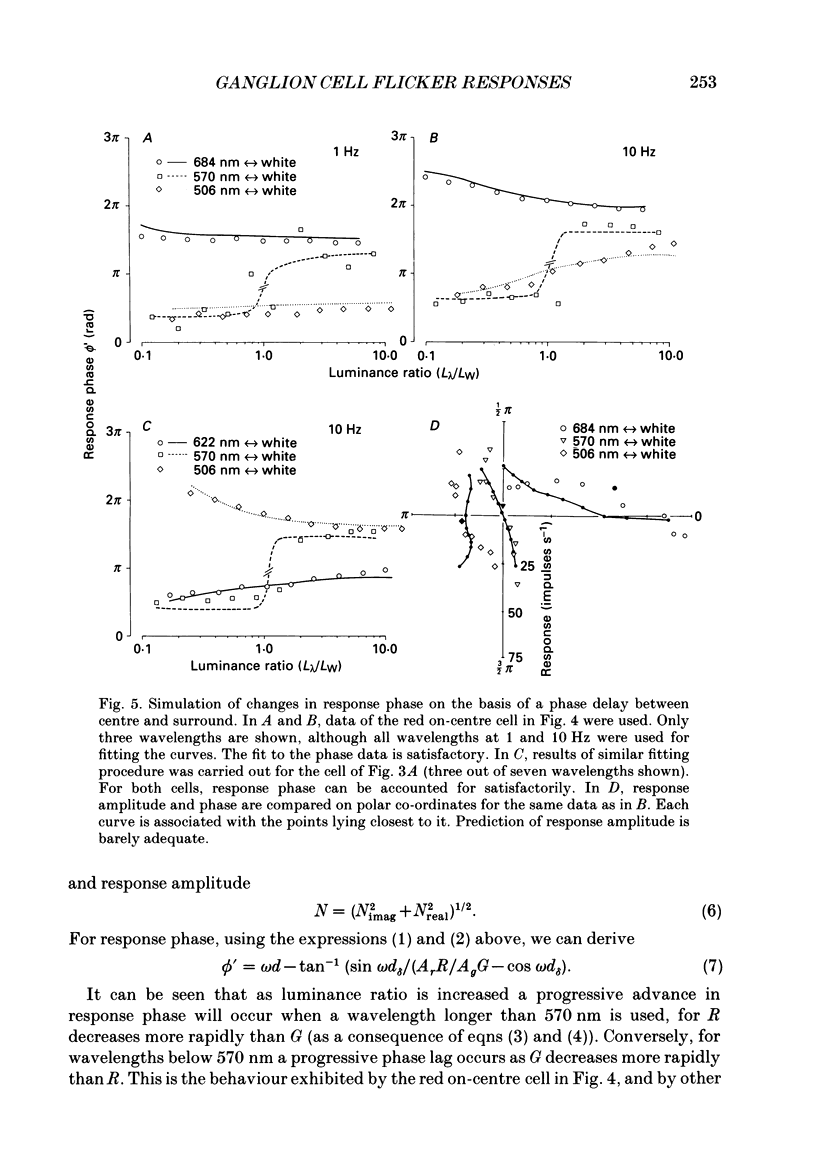
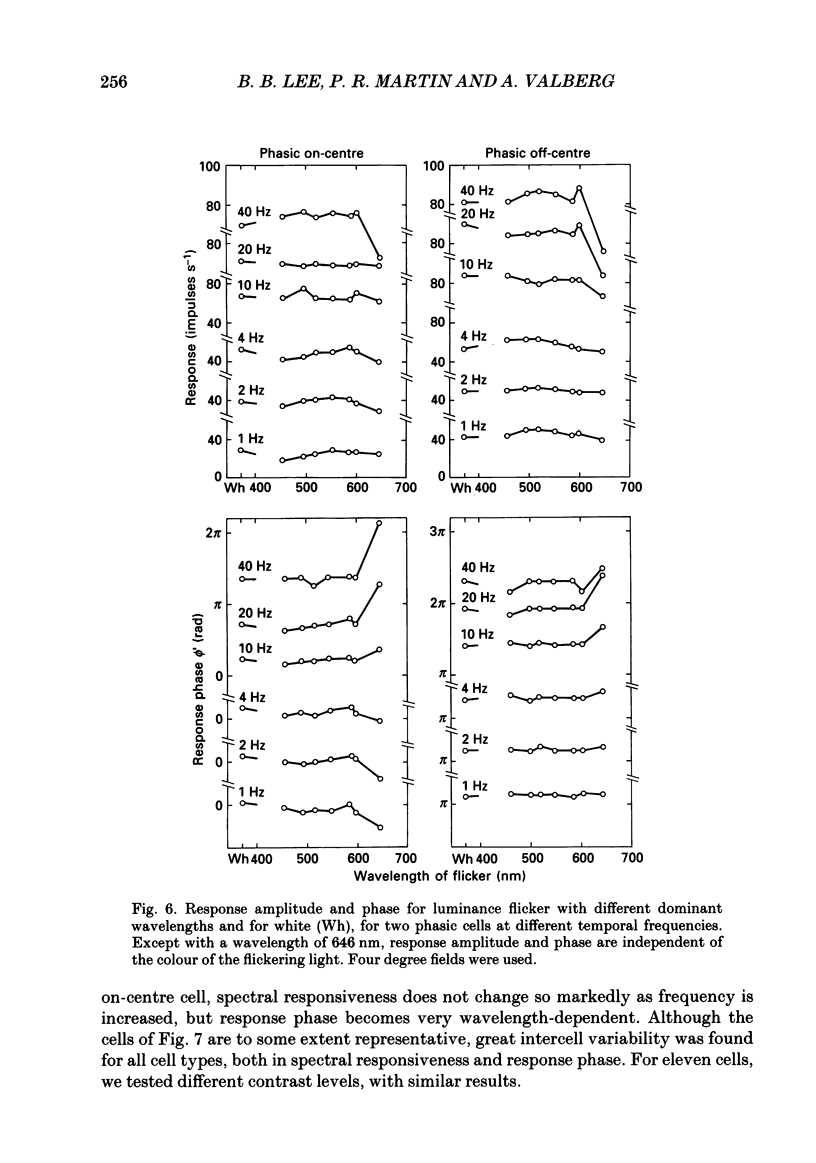
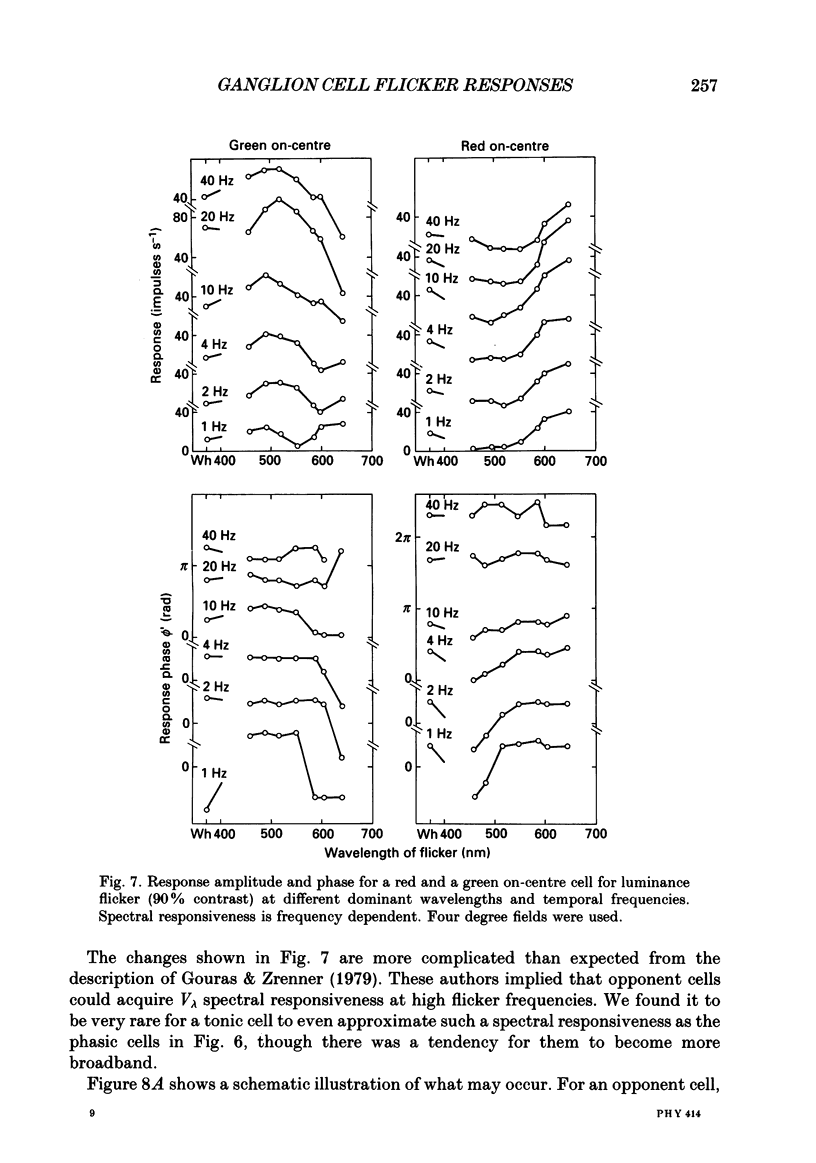
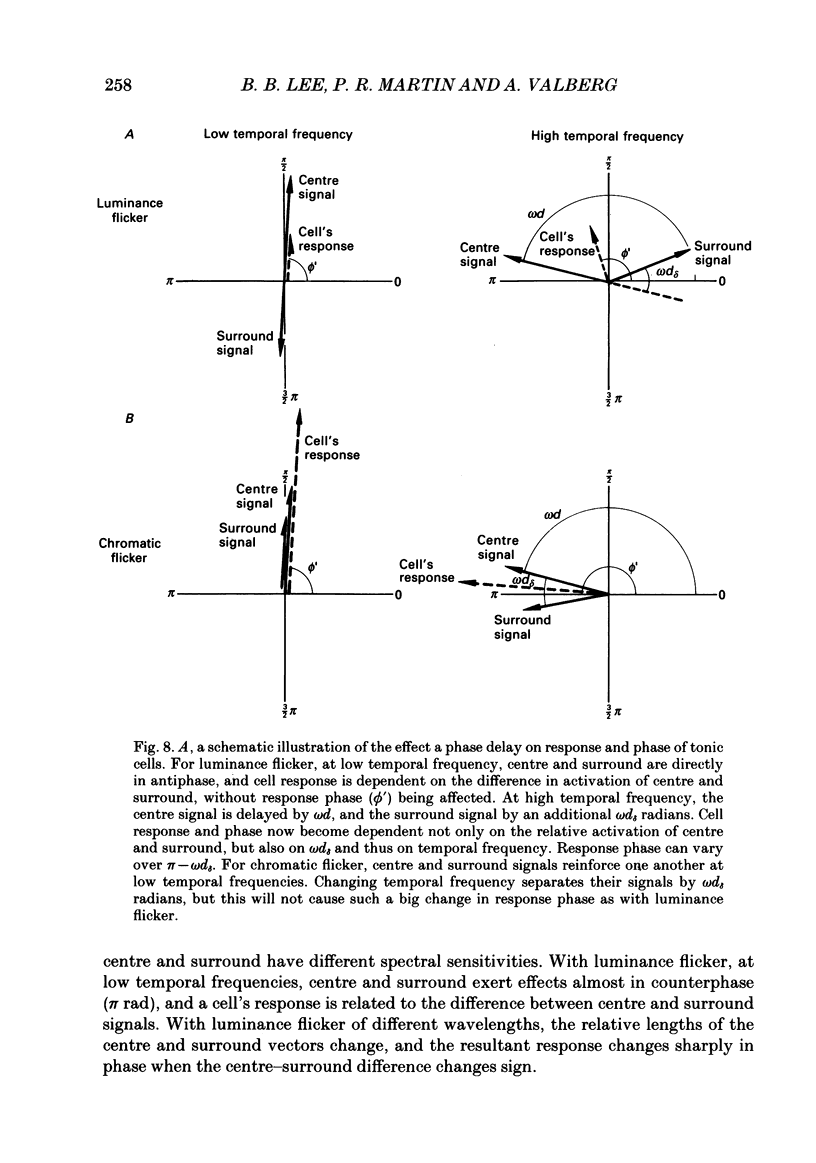
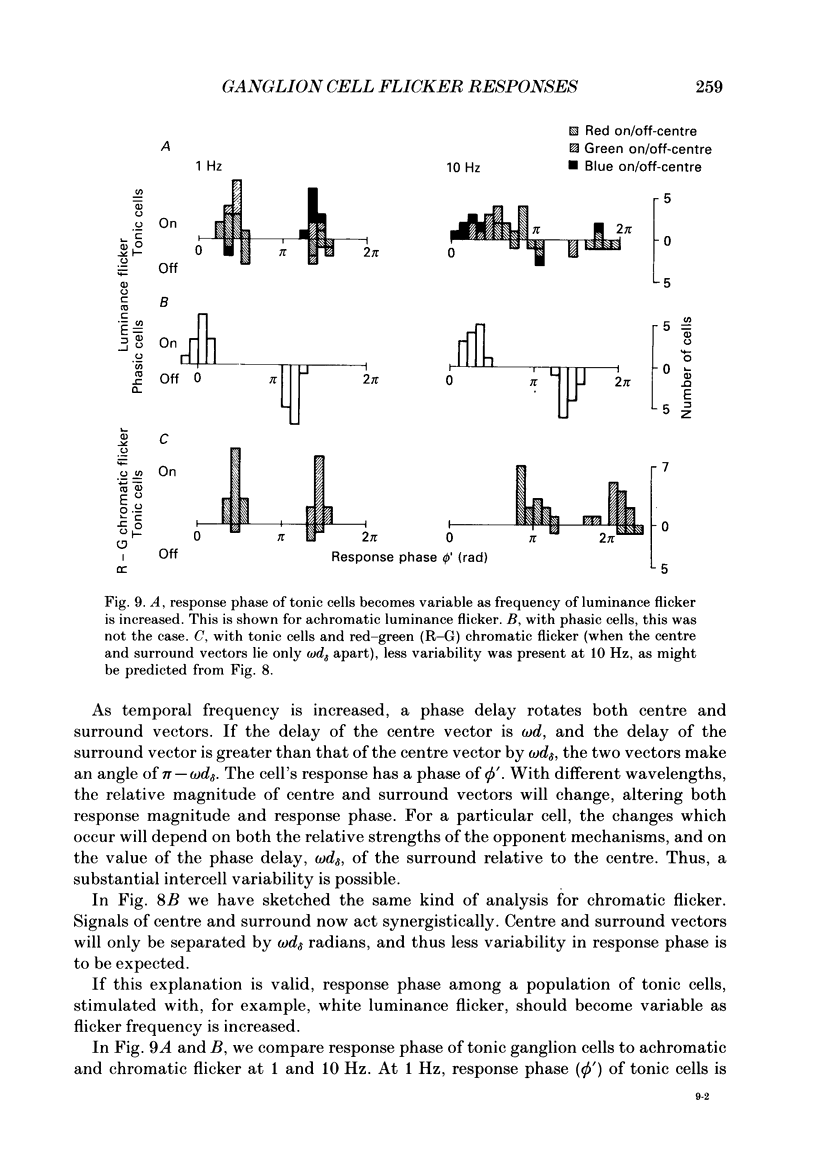
1. We have measured responses of macaque retinal ganglion cells to a uniform flickering field, with variation in luminance, chromaticity or both (heterochromatic flicker). 2. With heterochromatic flicker, as the luminance ratio of the flicker components was varied, phasic ganglion cell activity went through a minimum and an abrupt phase change close to equal luminance. Tonic ganglion cell responses underwent a gradual phase change without any minimum close to equal luminance. For red on-centre cells, when wavelengths above 570 nm were altered with white, a progressive phase advance occurred as luminance ratio (L lambda/LW) was increased. With wavelengths below 570 nm a progressive phase lag occurred. For green on-centre cells, the opposite pattern was found. For all tonic cells, the higher the temporal frequency, the more rapidly did such phase changes occur. A simple model incorporating a centre-surround delay of 3-8 ms could quantitatively account for these changes. 3. With luminance flicker of different dominant wavelengths, amplitudes and phase of responses of phasic ganglion cells were independent of wavelength at all frequencies. The amplitude and phase of the responses of tonic ganglion cells was very dependent on wavelength, as well as on flicker frequency. Their characteristics hardly ever resembled results from phasic cells. 4. For achromatic flicker, response phase of tonic cells at or above 10 Hz was variable, probably due to the centre-surround delay. Such variability was not seen among phasic cells. 5. An interesting implication of these results is that the ability of tonic ganglion cells to unambiguously signal rapid chromatic or spatial change is limited.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Crook J. M., Lee B. B., Tigwell D. A., Valberg A. Thresholds to chromatic spots of cells in the macaque geniculate nucleus as compared to detection sensitivity in man. J Physiol. 1987 Nov;392:193–211. doi: 10.1113/jphysiol.1987.sp016776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Monasterio F. M., Gouras P. Functional properties of ganglion cells of the rhesus monkey retina. J Physiol. 1975 Sep;251(1):167–195. doi: 10.1113/jphysiol.1975.sp011086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derrington A. M., Krauskopf J., Lennie P. Chromatic mechanisms in lateral geniculate nucleus of macaque. J Physiol. 1984 Dec;357:241–265. doi: 10.1113/jphysiol.1984.sp015499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derrington A. M., Lennie P. Spatial and temporal contrast sensitivities of neurones in lateral geniculate nucleus of macaque. J Physiol. 1984 Dec;357:219–240. doi: 10.1113/jphysiol.1984.sp015498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreher B., Fukada Y., Rodieck R. W. Identification, classification and anatomical segregation of cells with X-like and Y-like properties in the lateral geniculate nucleus of old-world primates. J Physiol. 1976 Jun;258(2):433–452. doi: 10.1113/jphysiol.1976.sp011429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enroth-Cugell C., Robson J. G., Schweitzer-Tong D. E., Watson A. B. Spatio-temporal interactions in cat retinal ganglion cells showing linear spatial summation. J Physiol. 1983 Aug;341:279–307. doi: 10.1113/jphysiol.1983.sp014806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frishman L. J., Freeman A. W., Troy J. B., Schweitzer-Tong D. E., Enroth-Cugell C. Spatiotemporal frequency responses of cat retinal ganglion cells. J Gen Physiol. 1987 Apr;89(4):599–628. doi: 10.1085/jgp.89.4.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouras P. Antidromic responses of orthodromically identified ganglion cells in monkey retina. J Physiol. 1969 Oct;204(2):407–419. doi: 10.1113/jphysiol.1969.sp008920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouras P., Zrenner E. Enchancement of luminance flicker by color-opponent mechanisms. Science. 1979 Aug 10;205(4406):587–589. doi: 10.1126/science.109925. [DOI] [PubMed] [Google Scholar]
- Julesz B., Schumer R. A. Early visual perception. Annu Rev Psychol. 1981;32:575–627. doi: 10.1146/annurev.ps.32.020181.003043. [DOI] [PubMed] [Google Scholar]
- Kelly D. H., van Norren D. Two-band model of heterochromatic flicker. J Opt Soc Am. 1977 Aug;67(8):1081–1091. doi: 10.1364/josa.67.001081. [DOI] [PubMed] [Google Scholar]
- Lee B. B., Martin P. R., Valberg A. Sensitivity of macaque retinal ganglion cells to chromatic and luminance flicker. J Physiol. 1989 Jul;414:223–243. doi: 10.1113/jphysiol.1989.sp017685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B. B., Martin P. R., Valberg A. The physiological basis of heterochromatic flicker photometry demonstrated in the ganglion cells of the macaque retina. J Physiol. 1988 Oct;404:323–347. doi: 10.1113/jphysiol.1988.sp017292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B. B., Valberg A., Tigwell D. A., Tryti J. An account of responses of spectrally opponent neurons in macaque lateral geniculate nucleus to successive contrast. Proc R Soc Lond B Biol Sci. 1987 Apr 22;230(1260):293–314. doi: 10.1098/rspb.1987.0021. [DOI] [PubMed] [Google Scholar]
- Schiller P. H., Malpeli J. G. Functional specificity of lateral geniculate nucleus laminae of the rhesus monkey. J Neurophysiol. 1978 May;41(3):788–797. doi: 10.1152/jn.1978.41.3.788. [DOI] [PubMed] [Google Scholar]
- Valberg A., Lee B. B., Tigwell D. A. Neurones with strong inhibitory S-cone inputs in the macaque lateral geniculate nucleus. Vision Res. 1986;26(7):1061–1064. doi: 10.1016/0042-6989(86)90040-4. [DOI] [PubMed] [Google Scholar]
- Valberg A., Lee B. B., Tryti J. Simulation of responses of spectrally-opponent neurones in the macaque lateral geniculate nucleus to chromatic and achromatic light stimuli. Vision Res. 1987;27(6):867–882. doi: 10.1016/0042-6989(87)90003-4. [DOI] [PubMed] [Google Scholar]
- de Monasterio F. M. Properties of concentrically organized X and Y ganglion cells of macaque retina. J Neurophysiol. 1978 Nov;41(6):1394–1417. doi: 10.1152/jn.1978.41.6.1394. [DOI] [PubMed] [Google Scholar]


