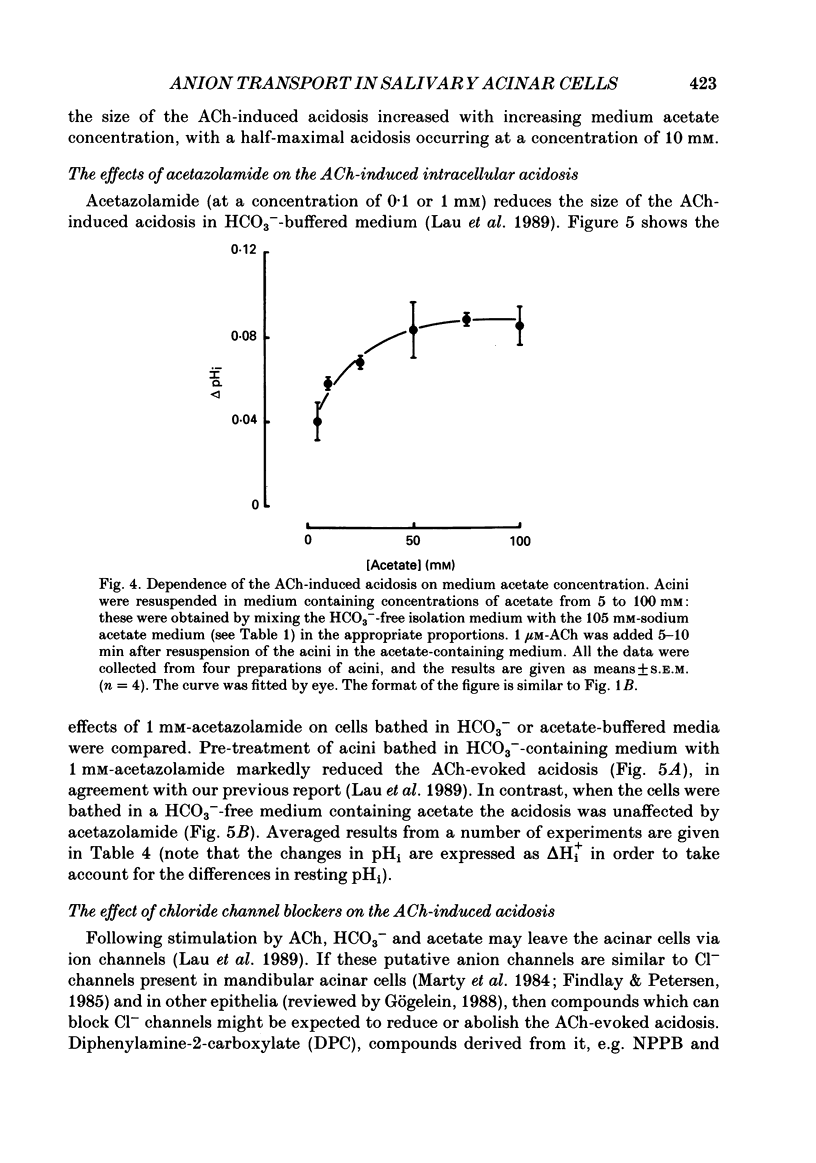
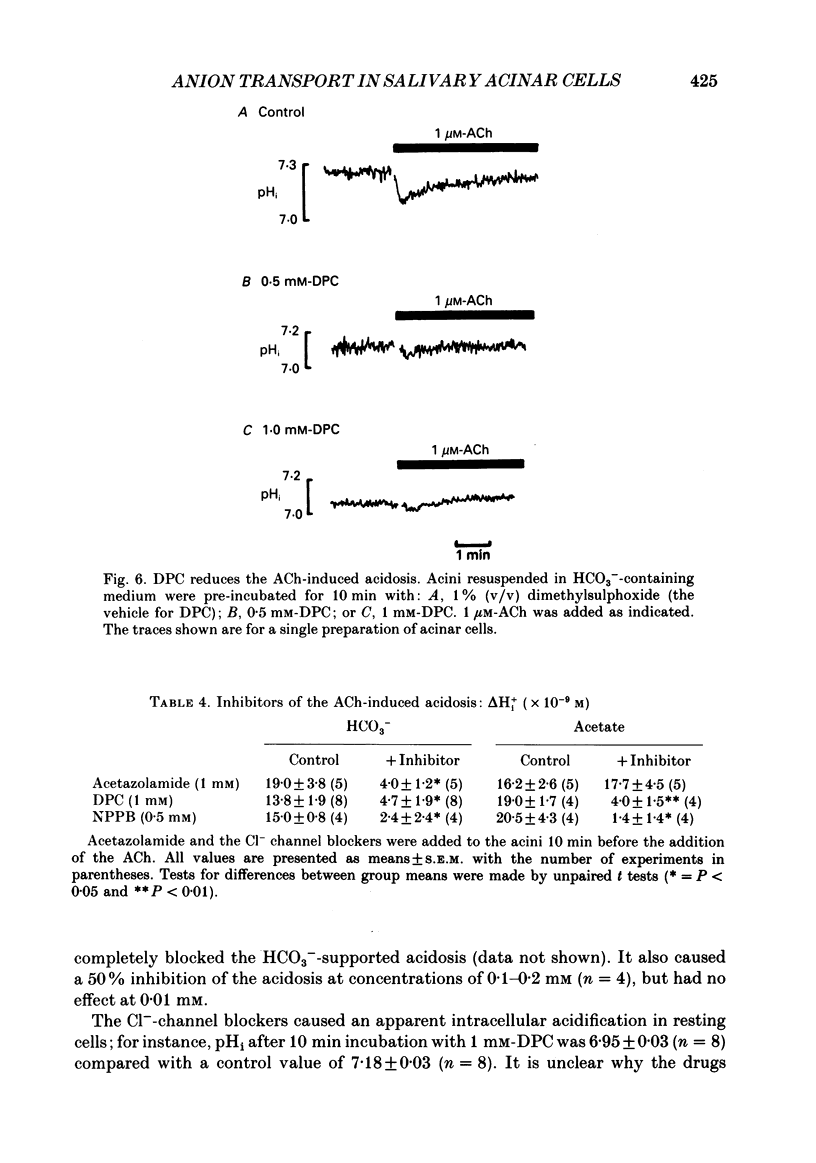
Abstract
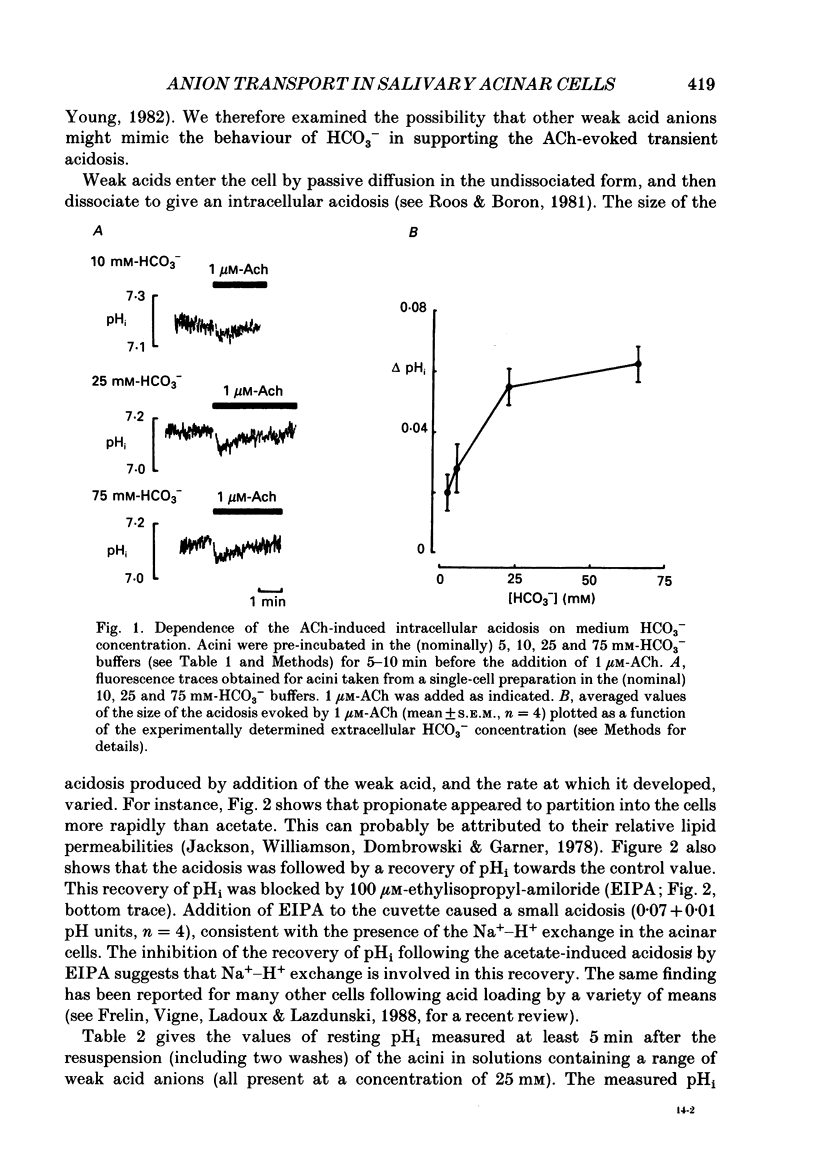
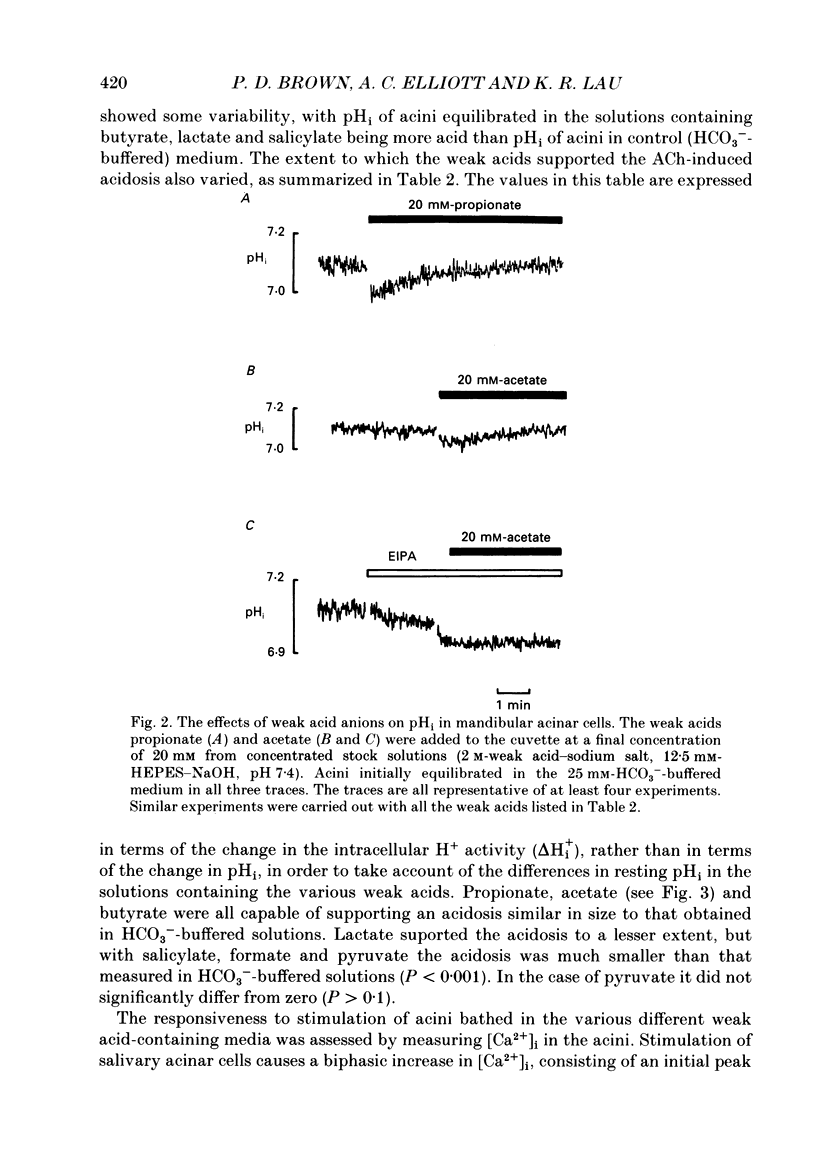
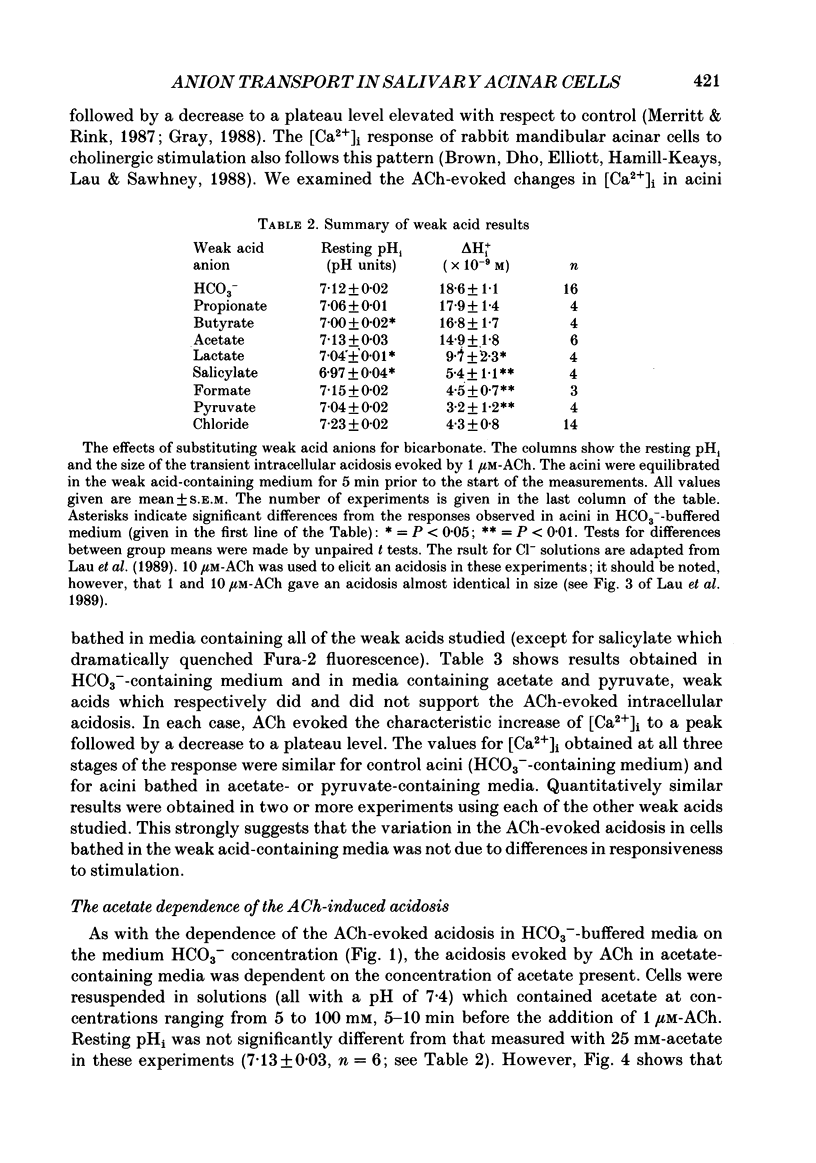
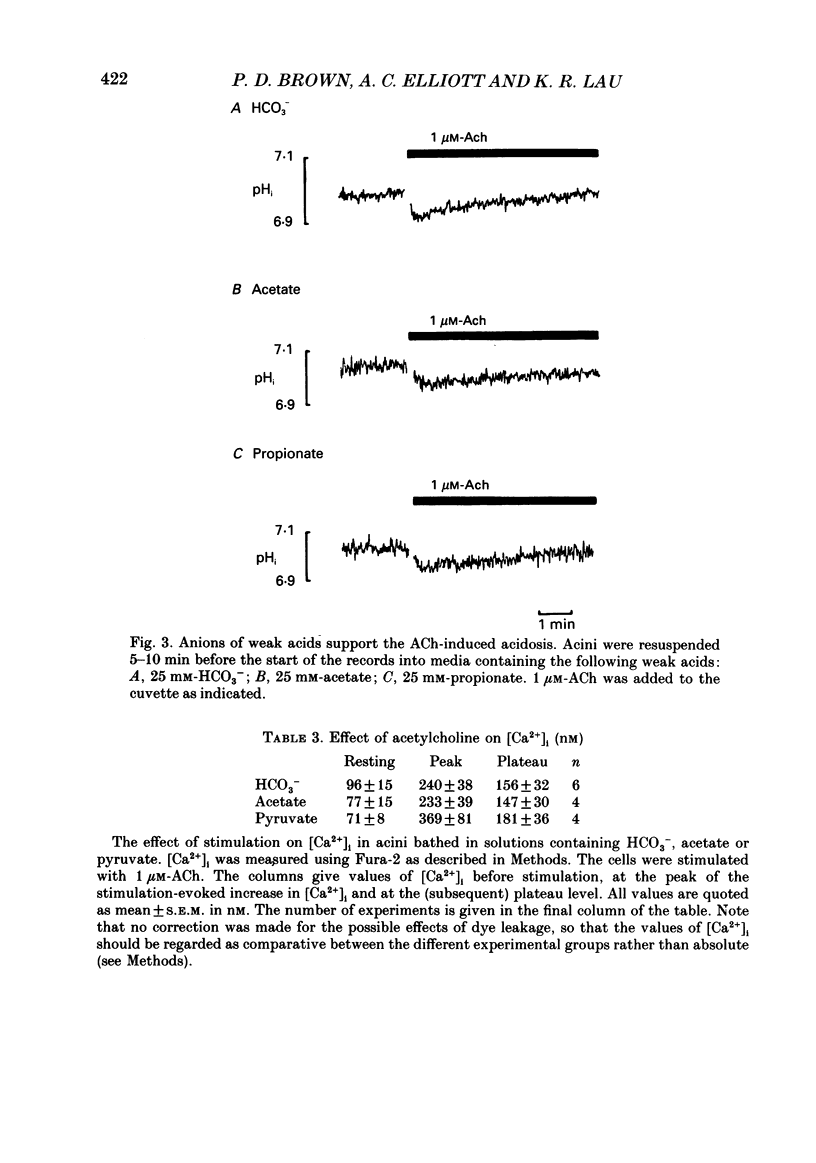
1. Intracellular pH (pHi) was measured using the fluorescent pH-sensitive dye 2',7'-bis(carboxyethyl)-5(6)-carboxyfluorescein in acini isolated from the rabbit mandibular salivary gland. 2. Stimulation of the acinar cells with acetylcholine (ACh) evoked an intracellular acidosis, the size of which was dependent on the HCO3-concentration in the bathing medium. A half-maximal acidosis was observed at approximately 10 mM-HCO3-. ACh also evoked an acidosis in HCO3(-)-free solutions containing acetate; a half-maximal acidosis was observed at about 10 mM-acetate. 3. Propionate, lactate and butyrate were also able to support the ACh-evoked acidosis to varying extents. In contrast, formate, pyruvate and salicylate did not support the ACh-induced acidosis to any great extent. 4. Acetazolamide greatly reduced the size of the acidosis in HCO3(-)-buffered medium, but had no effect in acetate-buffered medium, suggesting that the inhibitory effect of acetazolamide was due to a specific inhibition of carbonic anhydrase activity. 5. The Cl- channel blockers diphenylamine-2-carboxylic acid (DPC, 1 mM) and 5-nitro-2-(3-phenylpropylamino)-benzoic acid (0.5 mM) abolished the ACh-evoked acidosis in both HCO3(-) -and acetate-buffered media. 6. The data are consistent with the presence in the acinar cell of relatively non-specific anion channels sensitive to DPC and its derivatives. Such channels, activated on stimulation with ACh, would allow HCO3- and other weak acid ions to leave the cell, leading to the observed acidosis. The existence of such channels, located in the apical membrane, could explain why HCO3- or acetate can sustain fluid secretion in the intact perfused rabbit mandibular gland in the absence of Cl-.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bormann J., Hamill O. P., Sakmann B. Mechanism of anion permeation through channels gated by glycine and gamma-aminobutyric acid in mouse cultured spinal neurones. J Physiol. 1987 Apr;385:243–286. doi: 10.1113/jphysiol.1987.sp016493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case R. M., Conigrave A. D., Favaloro E. J., Novak I., Thompson C. H., Young J. A. The role of buffer anions and protons in secretion by the rabbit mandibular salivary gland. J Physiol. 1982 Jan;322:273–286. doi: 10.1113/jphysiol.1982.sp014037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case R. M., Hotz J., Hutson D., Scratcherd T., Wynne R. D. Electrolyte secretion by the isolated cat pancreas during replacement of extracellular bicarbonate by organic anions and chloride by inorganic anions. J Physiol. 1979 Jan;286:563–576. doi: 10.1113/jphysiol.1979.sp012637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case R. M., Hunter M., Novak I., Young J. A. The anionic basis of fluid secretion by the rabbit mandibular salivary gland. J Physiol. 1984 Apr;349:619–630. doi: 10.1113/jphysiol.1984.sp015177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findlay I., Petersen O. H. Acetylcholine stimulates a Ca2+-dependent C1- conductance in mouse lacrimal acinar cells. Pflugers Arch. 1985 Mar;403(3):328–330. doi: 10.1007/BF00583609. [DOI] [PubMed] [Google Scholar]
- Frelin C., Vigne P., Ladoux A., Lazdunski M. The regulation of the intracellular pH in cells from vertebrates. Eur J Biochem. 1988 May 16;174(1):3–14. doi: 10.1111/j.1432-1033.1988.tb14055.x. [DOI] [PubMed] [Google Scholar]
- Giraldez F., Sepúlveda F. V., Sheppard D. N. A chloride conductance activated by adenosine 3',5'-cyclic monophosphate in the apical membrane of Necturus enterocytes. J Physiol. 1988 Jan;395:597–623. doi: 10.1113/jphysiol.1988.sp016937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray P. T. Oscillations of free cytosolic calcium evoked by cholinergic and catecholaminergic agonists in rat parotid acinar cells. J Physiol. 1988 Dec;406:35–53. doi: 10.1113/jphysiol.1988.sp017367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Jackson M. J., Williamson A. M., Dombrowski W. A., Garner D. E. Intestinal transport of weak electrolytes: Determinants of influx at the luminal surface. J Gen Physiol. 1978 Mar;71(3):301–327. doi: 10.1085/jgp.71.3.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaila K., Voipio J. Postsynaptic fall in intracellular pH induced by GABA-activated bicarbonate conductance. Nature. 1987 Nov 12;330(6144):163–165. doi: 10.1038/330163a0. [DOI] [PubMed] [Google Scholar]
- Lau K. R., Case R. M. Evidence for apical chloride channels in rabbit mandibular salivary glands. A chloride-selective microelectrode study. Pflugers Arch. 1988 Jun;411(6):670–675. doi: 10.1007/BF00580864. [DOI] [PubMed] [Google Scholar]
- Lau K. R., Elliott A. C., Brown P. D. Acetylcholine-induced intracellular acidosis in rabbit salivary gland acinar cells. Am J Physiol. 1989 Feb;256(2 Pt 1):C288–C295. doi: 10.1152/ajpcell.1989.256.2.C288. [DOI] [PubMed] [Google Scholar]
- Maren T. H. Use of inhibitors in physiological studies of carbonic anhydrase. Am J Physiol. 1977 Apr;232(4):F291–F297. doi: 10.1152/ajprenal.1977.232.4.F291. [DOI] [PubMed] [Google Scholar]
- Marty A., Tan Y. P., Trautmann A. Three types of calcium-dependent channel in rat lacrimal glands. J Physiol. 1984 Dec;357:293–325. doi: 10.1113/jphysiol.1984.sp015501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama Y., Gallacher D. V., Petersen O. H. Voltage and Ca2+-activated K+ channel in baso-lateral acinar cell membranes of mammalian salivary glands. Nature. 1983 Apr 28;302(5911):827–829. doi: 10.1038/302827a0. [DOI] [PubMed] [Google Scholar]
- Melvin J. E., Kawaguchi M., Baum B. J., Turner R. J. A muscarinic agonist-stimulated chloride efflux pathway is associated with fluid secretion in rat parotid acinar cells. Biochem Biophys Res Commun. 1987 Jun 15;145(2):754–759. doi: 10.1016/0006-291x(87)91029-1. [DOI] [PubMed] [Google Scholar]
- Novak I., Greger R. Properties of the luminal membrane of isolated perfused rat pancreatic ducts. Effect of cyclic AMP and blockers of chloride transport. Pflugers Arch. 1988 May;411(5):546–553. doi: 10.1007/BF00582376. [DOI] [PubMed] [Google Scholar]
- Paradiso A. M., Negulescu P. A., Machen T. E. Na+-H+ and Cl(-)-OH-(HCO3-) exchange in gastric glands. Am J Physiol. 1986 Apr;250(4 Pt 1):G524–G534. doi: 10.1152/ajpgi.1986.250.4.G524. [DOI] [PubMed] [Google Scholar]
- Petersen K. U., Wood J. R., Schulze G., Heintze K. Stimulation of gallbladder fluid and electrolyte absorption by butyrate. J Membr Biol. 1981;62(3):183–193. doi: 10.1007/BF01998164. [DOI] [PubMed] [Google Scholar]
- Petersen O. H., Gallacher D. V. Electrophysiology of pancreatic and salivary acinar cells. Annu Rev Physiol. 1988;50:65–80. doi: 10.1146/annurev.ph.50.030188.000433. [DOI] [PubMed] [Google Scholar]
- Reinhardt R., Bridges R. J., Rummel W., Lindemann B. Properties of an anion-selective channel from rat colonic enterocyte plasma membranes reconstituted into planar phospholipid bilayers. J Membr Biol. 1987;95(1):47–54. doi: 10.1007/BF01869629. [DOI] [PubMed] [Google Scholar]
- Reuss L., Costantin J. L., Bazile J. E. Diphenylamine-2-carboxylate blocks Cl(-)-HCO3- exchange in Necturus gallbladder epithelium. Am J Physiol. 1987 Jul;253(1 Pt 1):C79–C89. doi: 10.1152/ajpcell.1987.253.1.C79. [DOI] [PubMed] [Google Scholar]
- Rink T. J., Tsien R. Y., Pozzan T. Cytoplasmic pH and free Mg2+ in lymphocytes. J Cell Biol. 1982 Oct;95(1):189–196. doi: 10.1083/jcb.95.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos A., Boron W. F. Intracellular pH. Physiol Rev. 1981 Apr;61(2):296–434. doi: 10.1152/physrev.1981.61.2.296. [DOI] [PubMed] [Google Scholar]
- Saito Y., Wright E. M. Regulation of bicarbonate transport across the brush border membrane of the bull-frog choroid plexus. J Physiol. 1984 May;350:327–342. doi: 10.1113/jphysiol.1984.sp015204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva P., Stoff J., Field M., Fine L., Forrest J. N., Epstein F. H. Mechanism of active chloride secretion by shark rectal gland: role of Na-K-ATPase in chloride transport. Am J Physiol. 1977 Oct;233(4):F298–F306. doi: 10.1152/ajprenal.1977.233.4.F298. [DOI] [PubMed] [Google Scholar]
- Swanson C. H., Solomon A. K. Micropuncture analysis of the cellular mechanisms of electrolyte secretion by the in vitro rabbit pancreas. J Gen Physiol. 1975 Jan;65(1):22–45. doi: 10.1085/jgp.65.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wangemann P., Wittner M., Di Stefano A., Englert H. C., Lang H. J., Schlatter E., Greger R. Cl(-)-channel blockers in the thick ascending limb of the loop of Henle. Structure activity relationship. Pflugers Arch. 1986;407 (Suppl 2):S128–S141. doi: 10.1007/BF00584942. [DOI] [PubMed] [Google Scholar]
- Welsh M. J. An apical-membrane chloride channel in human tracheal epithelium. Science. 1986 Jun 27;232(4758):1648–1650. doi: 10.1126/science.2424085. [DOI] [PubMed] [Google Scholar]


