Abstract
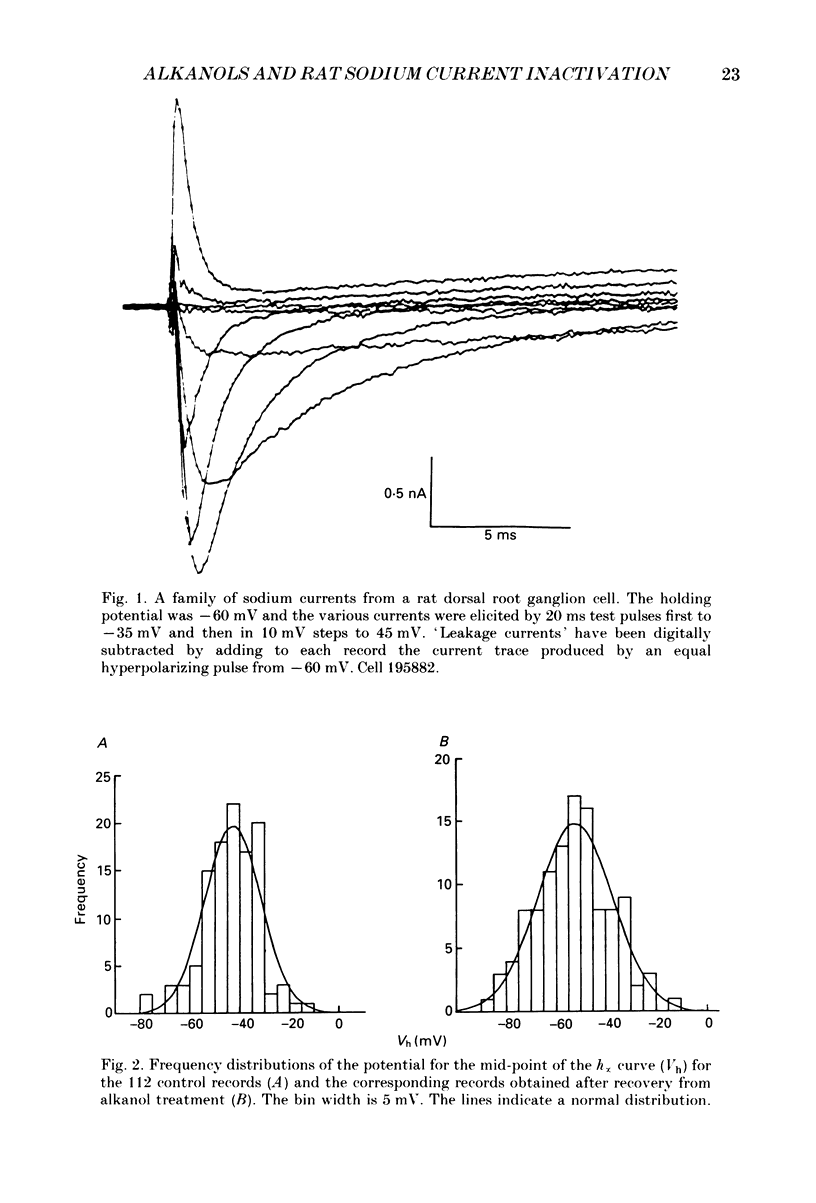
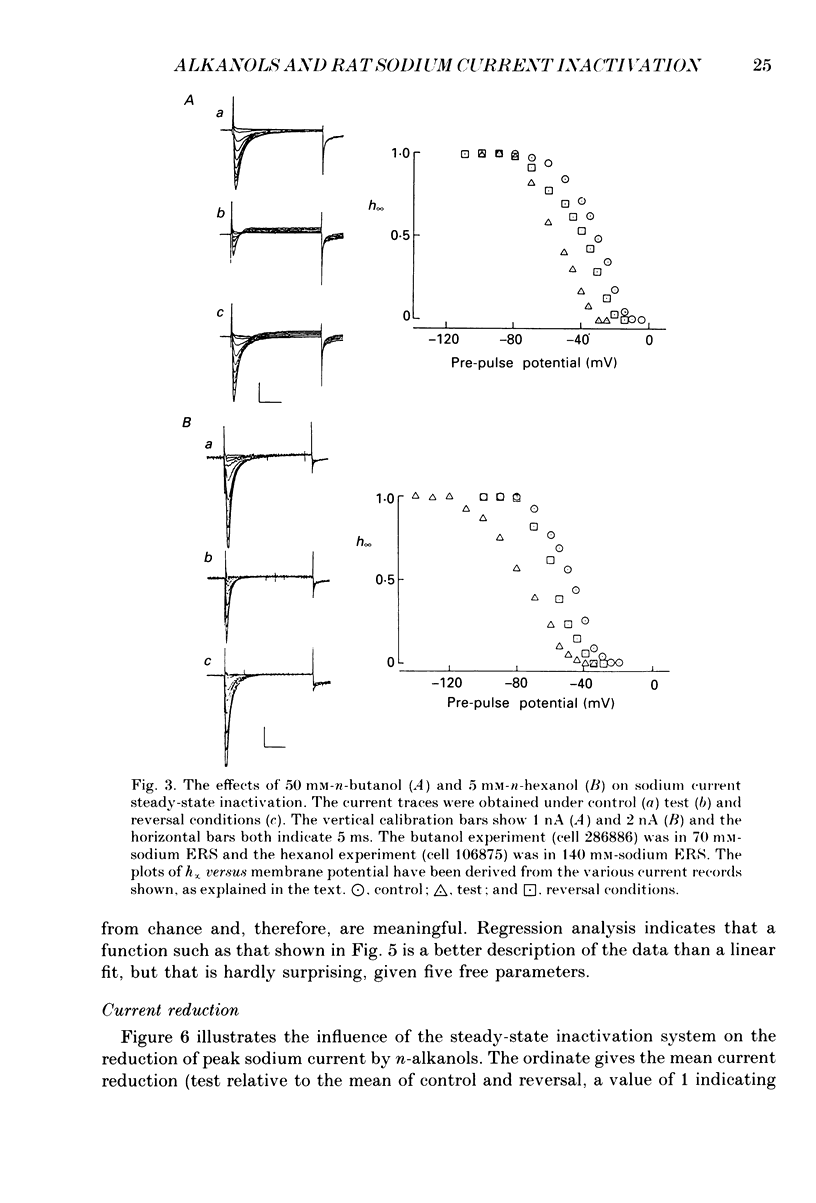
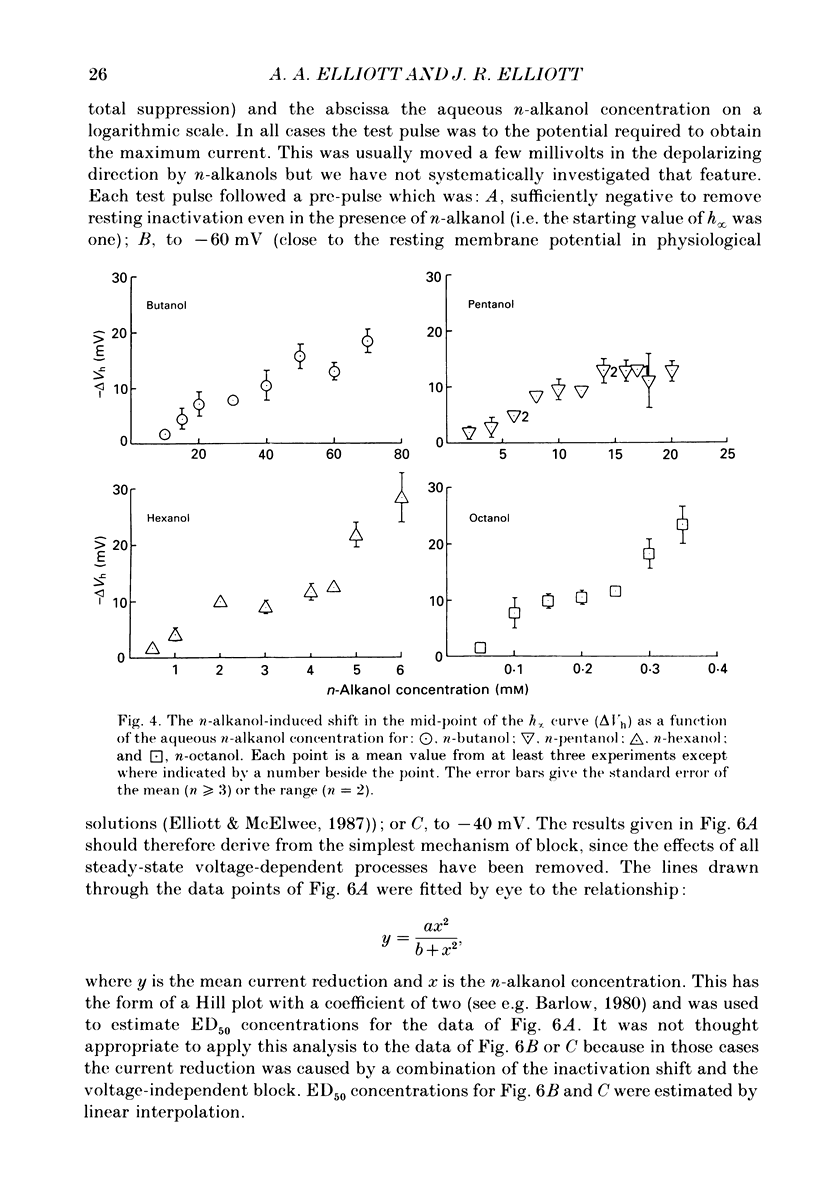
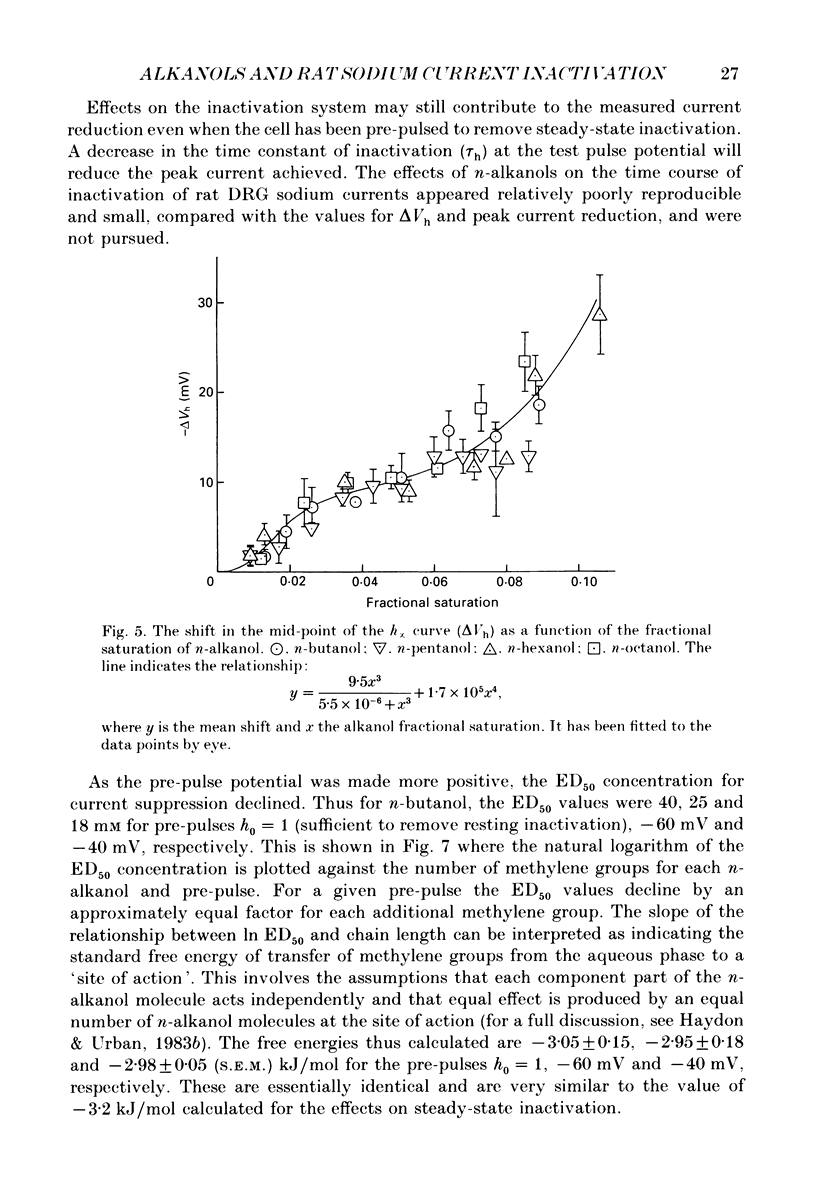
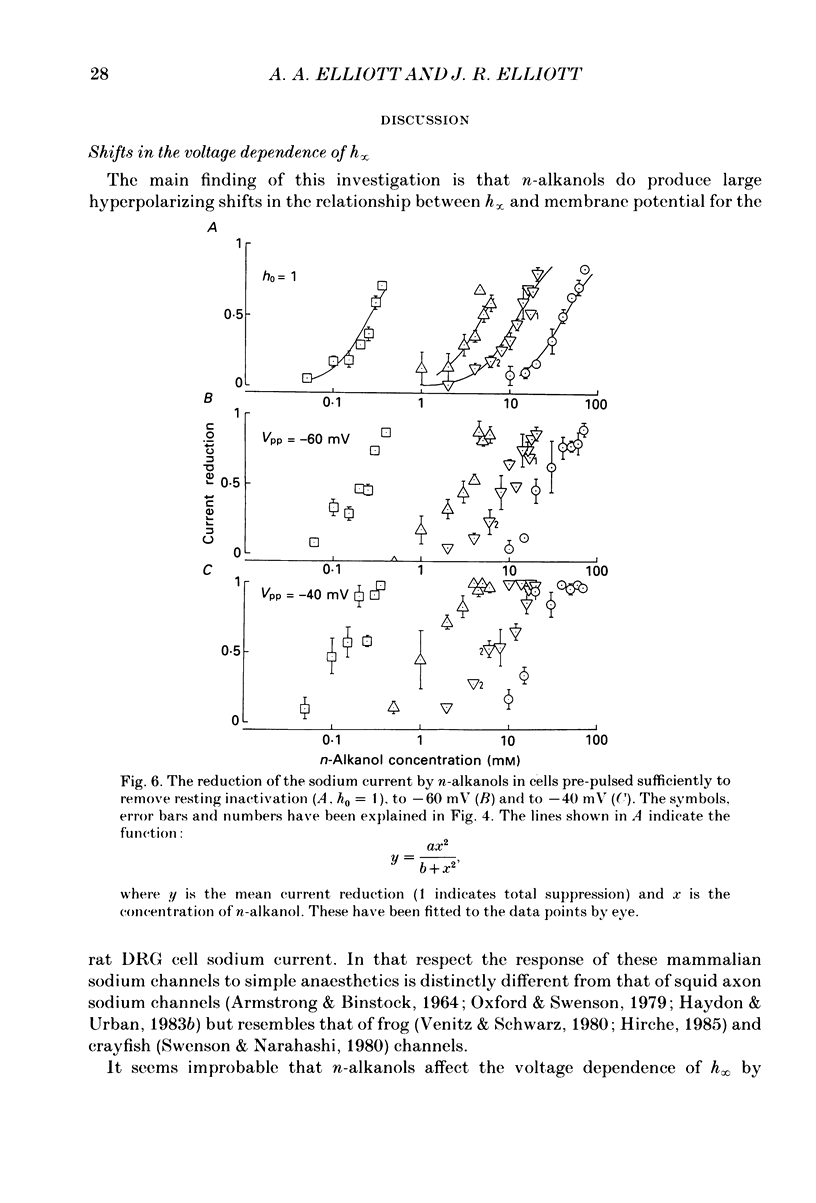
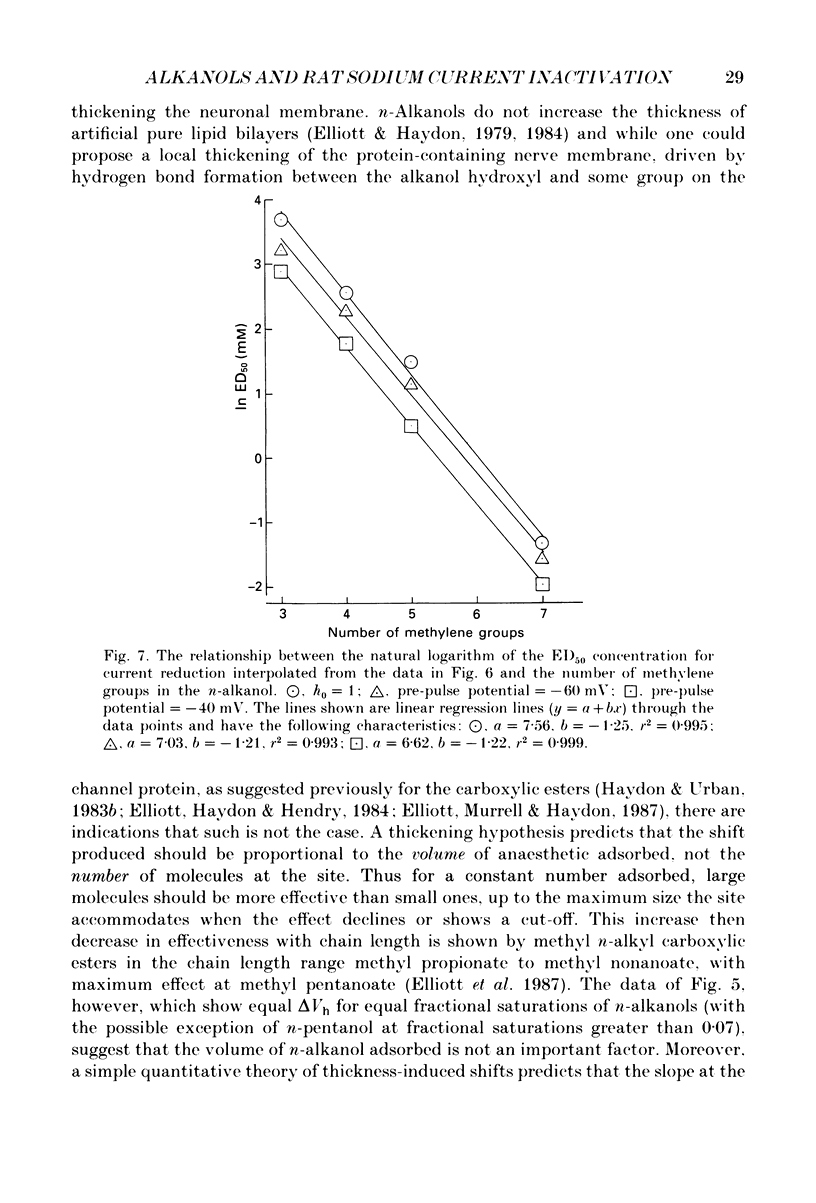
1. The whole-cell patch-clamp technique has been used to investigate the actions of n-butanol, n-pentanol, n-hexanol and n-octanol on the sodium current of cells isolated from the dorsal root ganglia (DRGs) of neonatal rats and maintained in short-term tissue culture. 2. The influence of n-alkanols on the level of steady-state inactivation of the sodium current was investigated by a standard two-pulse protocol. All alkanols increased the level of resting inactivation and this was manifested as a hyperpolarizing shift of the relationship between the steady-state inactivation parameter (h infinity) and membrane potential. The mid-point of the h infinity curve was moved by up to -30 mV. 3. The relationship between the shift in the mid-point of the inactivation curve (delta Vh) and aqueous n-alkanol concentration has been derived for each n-alkanol. These are complex in shape and do not appear consistent with a hypothesis that the increase in inactivation results from 1:1 binding of an alkanol molecule to a single site on the channel protein. 4. The aqueous concentrations used ranged from 70 mM-n-butanol to 0.05 mM-n-octanol. However, equal fractional saturations of n-alkanols produced approximately equal shifts in the h infinity curve, particularly in the range 0.01-0.07 saturated. This implies a hydrophobic site of action, with a standard free energy per methylene group for adsorption to the site from the aqueous phase of ca -3.2 kJ/mol. 5. The increase in resting inactivation was not the sole means by which n-alkanols reduced the sodium current. The current was still reduced in cells pre-pulsed to sufficiently negative potentials to remove steady-state inactivation even in the presence of alkanols. The concentration required to reduce the current by 50% (ED50) has been interpolated for each n-alkanol. From these data it was estimated that the standard free energy per methylene group for adsorption to the site of action was ca -3.1 kJ/mol, similar to that calculated for the effect on inactivation. The concentration dependence of this residual block indicated the involvement of more than one n-alkanol molecule. 6. The n-alkanols increase the level of inactivation of rat DRG cell sodium channels at potentials around the resting membrane potential and this effect contributes to their local anaesthetic action.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARMSTRONG C. M., BINSTOCK L. THE EFFECTS OF SEVERAL ALCOHOLS ON THE PROPERTIES OF THE SQUID GIANT AXON. J Gen Physiol. 1964 Nov;48:265–277. doi: 10.1085/jgp.48.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arhem P., Frankenhaeuser B. Local anesthetics: effects on permeability properties of nodal membrane in myelinated nerve fibres from xenopus. Potential clamp experiments. Acta Physiol Scand. 1974 May;91(1):11–21. doi: 10.1111/j.1748-1716.1974.tb05652.x. [DOI] [PubMed] [Google Scholar]
- Bean B. P., Cohen C. J., Tsien R. W. Lidocaine block of cardiac sodium channels. J Gen Physiol. 1983 May;81(5):613–642. doi: 10.1085/jgp.81.5.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belluzzi O., Sacchi O. A quantitative description of the sodium current in the rat sympathetic neurone. J Physiol. 1986 Nov;380:275–291. doi: 10.1113/jphysiol.1986.sp016285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott J. R., Haydon D. A., Hendry B. M. Anaesthetic action of esters and ketones: evidence for an interaction with the sodium channel protein in squid axons. J Physiol. 1984 Sep;354:407–418. doi: 10.1113/jphysiol.1984.sp015384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott J. R., Haydon D. A., Hendry B. M., Needham D. Inactivation of the sodium current in squid giant axons by hydrocarbons. Biophys J. 1985 Oct;48(4):617–622. doi: 10.1016/S0006-3495(85)83817-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott J. R., Haydon D. A., Hendry B. M. The asymmetrical effects of some ionized n-octyl derivatives on the sodium current of the giant axon of Loligo forbesi. J Physiol. 1984 May;350:429–445. doi: 10.1113/jphysiol.1984.sp015210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott J. R., Murrell R. D., Haydon D. A. Local anesthetic action of carboxylic esters: evidence for the significance of molecular volume and for the number of sites involved. J Membr Biol. 1987;95(2):143–149. doi: 10.1007/BF01869159. [DOI] [PubMed] [Google Scholar]
- Fernandez J. M., Fox A. P., Krasne S. Membrane patches and whole-cell membranes: a comparison of electrical properties in rat clonal pituitary (GH3) cells. J Physiol. 1984 Nov;356:565–585. doi: 10.1113/jphysiol.1984.sp015483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forda S., Kelly J. S. The possible modulation of the development of rat dorsal root ganglion cells by the presence of 5-HT-containing neurones of the brainstem in dissociated cell culture. Brain Res. 1985 Sep;354(1):55–65. doi: 10.1016/0165-3806(85)90068-9. [DOI] [PubMed] [Google Scholar]
- Goldman L., Schauf C. L. Inactivation of the sodium current in Myxicola giant axons. Evidence for coupling to the activation process. J Gen Physiol. 1972 Jun;59(6):659–675. doi: 10.1085/jgp.59.6.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonoi T., Hille B. Gating of Na channels. Inactivation modifiers discriminate among models. J Gen Physiol. 1987 Feb;89(2):253–274. doi: 10.1085/jgp.89.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. The dual effect of membrane potential on sodium conductance in the giant axon of Loligo. J Physiol. 1952 Apr;116(4):497–506. doi: 10.1113/jphysiol.1952.sp004719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haydon D. A., Hendry B. M., Levinson S. R., Requena J. Anaesthesia by the n-alkanes. A comparative study of nerve impulse blockage and the properties of black lipid bilayer membranes. Biochim Biophys Acta. 1977 Oct 3;470(1):17–34. doi: 10.1016/0005-2736(77)90058-x. [DOI] [PubMed] [Google Scholar]
- Haydon D. A., Kimura J. E. Some effects of n-pentane on the sodium and potassium currents of the squid giant axon. J Physiol. 1981 Mar;312:57–70. doi: 10.1113/jphysiol.1981.sp013615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haydon D. A., Urban B. W. The action of alcohols and other non-ionic surface active substances on the sodium current of the squid giant axon. J Physiol. 1983 Aug;341:411–427. doi: 10.1113/jphysiol.1983.sp014813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haydon D. A., Urban B. W. The action of hydrocarbons and carbon tetrachloride on the sodium current of the squid giant axon. J Physiol. 1983 May;338:435–450. doi: 10.1113/jphysiol.1983.sp014682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. Local anesthetics: hydrophilic and hydrophobic pathways for the drug-receptor reaction. J Gen Physiol. 1977 Apr;69(4):497–515. doi: 10.1085/jgp.69.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda S. R., Schofield G. G., Weight F. F. Na+ and Ca2+ currents of acutely isolated adult rat nodose ganglion cells. J Neurophysiol. 1986 Mar;55(3):527–539. doi: 10.1152/jn.1986.55.3.527. [DOI] [PubMed] [Google Scholar]
- Kostyuk P. G., Veselovsky N. S., Tsyndrenko A. Y. Ionic currents in the somatic membrane of rat dorsal root ganglion neurons-I. Sodium currents. Neuroscience. 1981;6(12):2423–2430. doi: 10.1016/0306-4522(81)90088-9. [DOI] [PubMed] [Google Scholar]
- Meeder T., Ulbricht W. Action of benzocaine on sodium channels of frog nodes of Ranvier treated with chloramine-T. Pflugers Arch. 1987 Jul;409(3):265–273. doi: 10.1007/BF00583475. [DOI] [PubMed] [Google Scholar]
- Oxford G. S., Swenson R. P. n-Alkanols potentiate sodium channel inactivation in squid giant axons. Biophys J. 1979 Jun;26(3):585–590. doi: 10.1016/S0006-3495(79)85273-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen M., Pierau F. K., Weyrich M. The influence of capsaicin on membrane currents in dorsal root ganglion neurones of guinea-pig and chicken. Pflugers Arch. 1987 Aug;409(4-5):403–410. doi: 10.1007/BF00583794. [DOI] [PubMed] [Google Scholar]
- Rodríguez N., Villegas R., Requena J. The interaction of homologous series of alkanols with sodium channels in nerve membrane vesicles. J Membr Biol. 1988 Sep;104(2):139–146. doi: 10.1007/BF01870926. [DOI] [PubMed] [Google Scholar]
- Schofield G. G., Ikeda S. R. Sodium and calcium currents of acutely isolated adult rat superior cervical ganglion neurons. Pflugers Arch. 1988 May;411(5):481–490. doi: 10.1007/BF00582368. [DOI] [PubMed] [Google Scholar]
- Swenson R. P., Narahashi T. Block of sodium conductance by n-octanol in crayfish giant axons. Biochim Biophys Acta. 1980 Dec 12;603(2):228–236. doi: 10.1016/0005-2736(80)90369-7. [DOI] [PubMed] [Google Scholar]


