Abstract
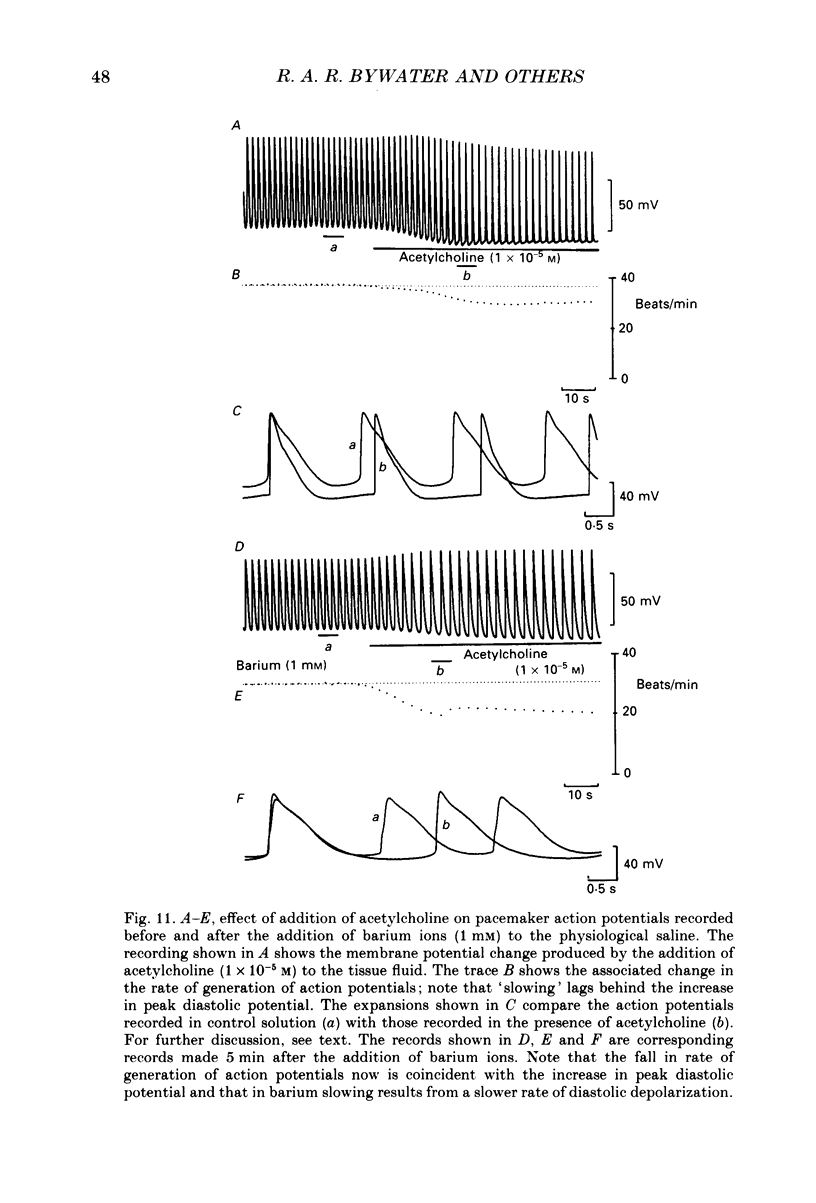
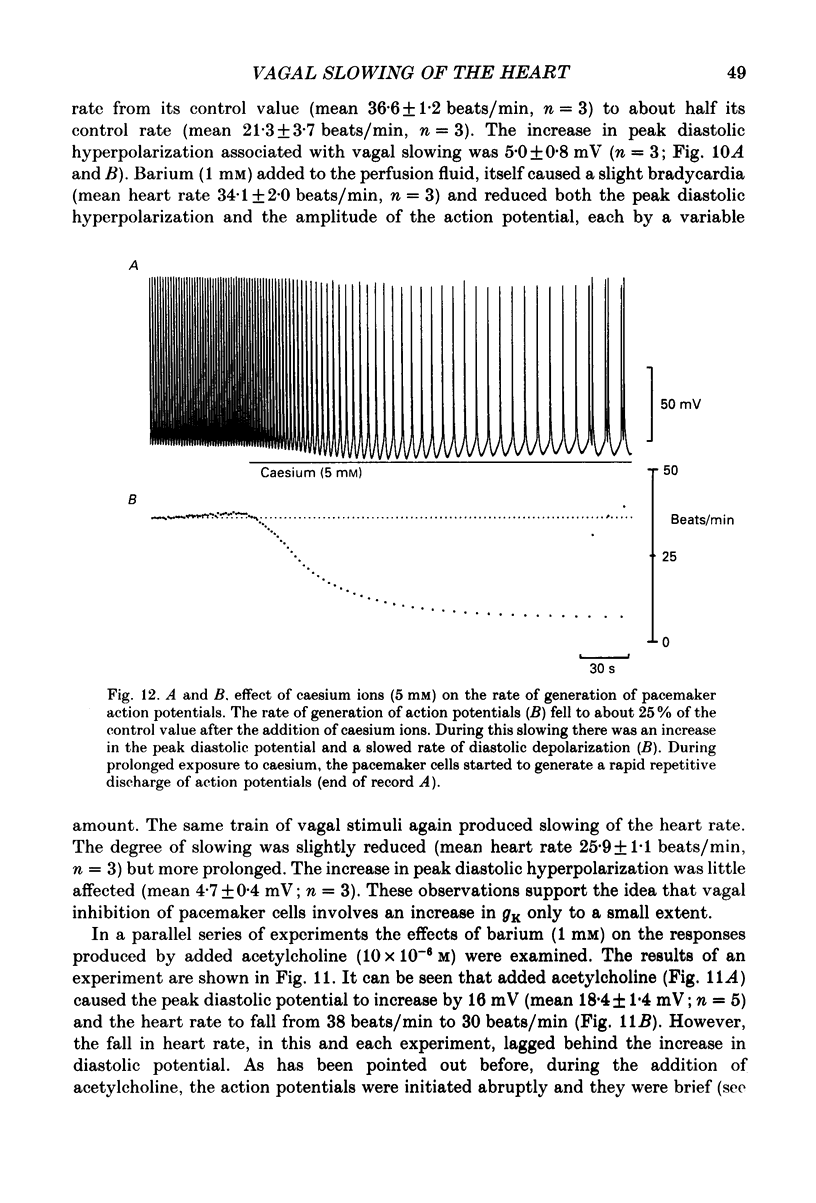
1. The effects of vagal stimulation and applied acetylcholine were compared on the isolated sinus venosus preparation of the toad, Bufo marinus. 2. The effects of applied acetylcholine and of low-frequency, or short bursts of high-frequency vagal stimulation were abolished by hyoscine. 3. When intracellular recordings were made from muscle cells of the sinus venosus, it was found that applied acetylcholine caused bradycardia and a cessation of the heart beat which was associated with membrane hyperpolarization and a reduction in the duration of the action potentials. Much of the effect of acetylcholine can be attributed to it causing an increase in potassium conductance, gK. 4. When slowing was produced by low-frequency vagal stimulation, only a small increase in maximum diastolic potential was detected. During vagal arrest the membrane potential settled to a potential positive of the control maximum diastolic potential. 5. In the presence of barium, much of the bradycardia associated with vagal stimulation persisted. Although the bradycardia produced by added acetylcholine also persisted in the presence of barium, the effects of acetylcholine that could be attributed to an increase in gK were abolished. 6. Addition of caesium ions produced bradycardia with membrane potential changes similar to those seen during vagal stimulation. 7. The results are discussed in relation to the idea that neuronally released acetylcholine reduces inward current flow during diastole. In contrast applied acetylcholine as well as reducing inward current flow during diastole also increases outward current flow by increasing gK.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown H. F. Electrophysiology of the sinoatrial node. Physiol Rev. 1982 Apr;62(2):505–530. doi: 10.1152/physrev.1982.62.2.505. [DOI] [PubMed] [Google Scholar]
- Brown H. F., Giles W., Noble S. J. Membrane currents underlying activity in frog sinus venosus. J Physiol. 1977 Oct;271(3):783–816. doi: 10.1113/jphysiol.1977.sp012026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell G. D., Edwards F. R., Hirst G. D., O'Shea J. E. Effects of vagal stimulation and applied acetylcholine on pacemaker potentials in the guinea-pig heart. J Physiol. 1989 Aug;415:57–68. doi: 10.1113/jphysiol.1989.sp017711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell G., Gibbins I. L., Morris J. L., Furness J. B., Costa M., Oliver J. R., Beardsley A. M., Murphy R. Somatostatin is contained in and released from cholinergic nerves in the heart of the toad Bufo marinus. Neuroscience. 1982;7(9):2013–2023. doi: 10.1016/0306-4522(82)90116-6. [DOI] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. On the localization of acetylcholine receptors. J Physiol. 1955 Apr 28;128(1):157–181. doi: 10.1113/jphysiol.1955.sp005297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Production of membrane potential changes in the frog's heart by inhibitory nerve impulses. Nature. 1955 Jun 11;175(4467):1035–1035. doi: 10.1038/1751035a0. [DOI] [PubMed] [Google Scholar]
- Dennis M. J., Harris A. J., Kuffler S. W. Synaptic transmission and its duplication by focally applied acetylcholine in parasympathetic neurons in the heart of the frog. Proc R Soc Lond B Biol Sci. 1971 Apr 27;177(1049):509–539. doi: 10.1098/rspb.1971.0045. [DOI] [PubMed] [Google Scholar]
- DiFrancesco D., Ferroni A., Mazzanti M., Tromba C. Properties of the hyperpolarizing-activated current (if) in cells isolated from the rabbit sino-atrial node. J Physiol. 1986 Aug;377:61–88. doi: 10.1113/jphysiol.1986.sp016177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFrancesco D. The cardiac hyperpolarizing-activated current, if. Origins and developments. Prog Biophys Mol Biol. 1985;46(3):163–183. doi: 10.1016/0079-6107(85)90008-2. [DOI] [PubMed] [Google Scholar]
- DiFrancesco D., Tromba C. Acetylcholine inhibits activation of the cardiac hyperpolarizing-activated current, if. Pflugers Arch. 1987 Sep;410(1-2):139–142. doi: 10.1007/BF00581906. [DOI] [PubMed] [Google Scholar]
- Egan T. M., Noble S. J. Acetylcholine and the mammalian 'slow inward' current: a computer investigation. Proc R Soc Lond B Biol Sci. 1987 Apr 22;230(1260):315–337. doi: 10.1098/rspb.1987.0022. [DOI] [PubMed] [Google Scholar]
- Fischmeister R., Hartzell H. C. Mechanism of action of acetylcholine on calcium current in single cells from frog ventricle. J Physiol. 1986 Jul;376:183–202. doi: 10.1113/jphysiol.1986.sp016148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giles W., Noble S. J. Changes in membrane currents in bullfrog atrium produced by acetylcholine. J Physiol. 1976 Sep;261(1):103–123. doi: 10.1113/jphysiol.1976.sp011550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giles W., Tsien R. W. Effects of acetylcholine on membrane currents in frog artrial muscle. J Physiol. 1975 Mar;246(2):64P–66P. [PubMed] [Google Scholar]
- Godfraind T., Miller R., Wibo M. Calcium antagonism and calcium entry blockade. Pharmacol Rev. 1986 Dec;38(4):321–416. [PubMed] [Google Scholar]
- HUTTER O. F., TRAUTWEIN W. Effect of vagal stimulation on the sinus venosus of the frog's heart. Nature. 1955 Sep 10;176(4480):512–513. doi: 10.1038/176512a0. [DOI] [PubMed] [Google Scholar]
- HUTTER O. F., TRAUTWEIN W. Vagal and sympathetic effects on the pacemaker fibers in the sinus venosus of the heart. J Gen Physiol. 1956 May 20;39(5):715–733. doi: 10.1085/jgp.39.5.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara N., Irisawa H., Kameyama M. Contribution of two types of calcium currents to the pacemaker potentials of rabbit sino-atrial node cells. J Physiol. 1988 Jan;395:233–253. doi: 10.1113/jphysiol.1988.sp016916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara N., Irisawa H. Modulation by intracellular Ca2+ of the hyperpolarization-activated inward current in rabbit single sino-atrial node cells. J Physiol. 1989 Feb;409:121–141. doi: 10.1113/jphysiol.1989.sp017488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartzell H. C. Adenosine receptors in frog sinus venosus: slow inhibitory potentials produced by adenine compounds and acetylcholine. J Physiol. 1979 Aug;293:23–49. doi: 10.1113/jphysiol.1979.sp012877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartzell H. C. Distribution of muscarinic acetylcholine receptors and presynaptic nerve terminals in amphibian heart. J Cell Biol. 1980 Jul;86(1):6–20. doi: 10.1083/jcb.86.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hino N., Ochi R. Effect of acetylcholine on membrane currents in guinea-pig papillary muscle. J Physiol. 1980 Oct;307:183–197. doi: 10.1113/jphysiol.1980.sp013430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst G. D., Holman M. E., Spence I. Two types of neurones in the myenteric plexus of duodenum in the guinea-pig. J Physiol. 1974 Jan;236(2):303–326. doi: 10.1113/jphysiol.1974.sp010436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst G. D., Neild T. O. Localization of specialized noradrenaline receptors at neuromuscular junctions on arterioles of the guinea-pig. J Physiol. 1981;313:343–350. doi: 10.1113/jphysiol.1981.sp013669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iijima T., Irisawa H., Kameyama M. Membrane currents and their modification by acetylcholine in isolated single atrial cells of the guinea-pig. J Physiol. 1985 Feb;359:485–501. doi: 10.1113/jphysiol.1985.sp015598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luff S. E., McLachlan E. M., Hirst G. D. An ultrastructural analysis of the sympathetic neuromuscular junctions on arterioles of the submucosa of the guinea pig ileum. J Comp Neurol. 1987 Mar 22;257(4):578–594. doi: 10.1002/cne.902570407. [DOI] [PubMed] [Google Scholar]
- Momose Y., Giles W., Szabo G. Acetylcholine-induced k current in amphibian atrial cells. Biophys J. 1984 Jan;45(1):20–22. doi: 10.1016/S0006-3495(84)84092-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble D. The surprising heart: a review of recent progress in cardiac electrophysiology. J Physiol. 1984 Aug;353:1–50. doi: 10.1113/jphysiol.1984.sp015320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noma A., Trautwein W. Relaxation of the ACh-induced potassium current in the rabbit sinoatrial node cell. Pflugers Arch. 1978 Nov 30;377(3):193–200. doi: 10.1007/BF00584272. [DOI] [PubMed] [Google Scholar]
- Sakmann B., Noma A., Trautwein W. Acetylcholine activation of single muscarinic K+ channels in isolated pacemaker cells of the mammalian heart. Nature. 1983 May 19;303(5914):250–253. doi: 10.1038/303250a0. [DOI] [PubMed] [Google Scholar]
- Shibata E. F., Giles W., Pollack G. H. Threshold effects of acetylcholine on primary pacemaker cells of the rabbit sino-atrial node. Proc R Soc Lond B Biol Sci. 1985 Jan 22;223(1232):355–378. doi: 10.1098/rspb.1985.0006. [DOI] [PubMed] [Google Scholar]
- Simmons M. A., Hartzell H. C. A quantitative analysis of the acetylcholine-activated potassium current in single cells from frog atrium. Pflugers Arch. 1987 Aug;409(4-5):454–461. doi: 10.1007/BF00583801. [DOI] [PubMed] [Google Scholar]
- Spear J. F., Kronhaus K. D., Moore E. N., Kline R. P. The effect of brief vagal stimulation on the isolated rabbit sinus node. Circ Res. 1979 Jan;44(1):75–88. doi: 10.1161/01.res.44.1.75. [DOI] [PubMed] [Google Scholar]
- TRAUTWEIN W. Generation and conduction of impulses in the heart as affected by drugs. Pharmacol Rev. 1963 Jun;15:277–332. [PubMed] [Google Scholar]
- Ten Eick R., Nawrath H., McDonald T. F., Trautwein W. On the mechanism of the negative inotropic effect of acetylcholine. Pflugers Arch. 1976 Feb 24;361(3):207–213. doi: 10.1007/BF00587284. [DOI] [PubMed] [Google Scholar]
- Yanagihara K., Irisawa H. Inward current activated during hyperpolarization in the rabbit sinoatrial node cell. Pflugers Arch. 1980 May;385(1):11–19. doi: 10.1007/BF00583909. [DOI] [PubMed] [Google Scholar]


