Abstract
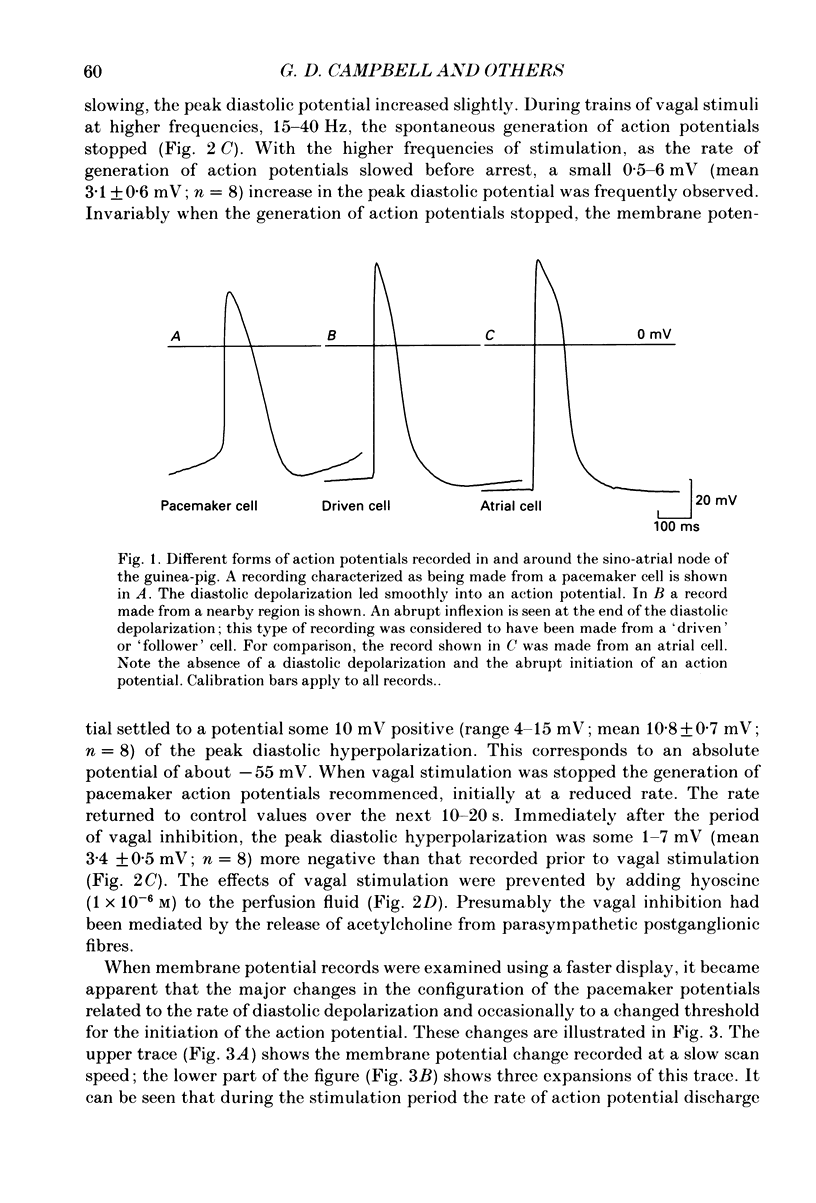
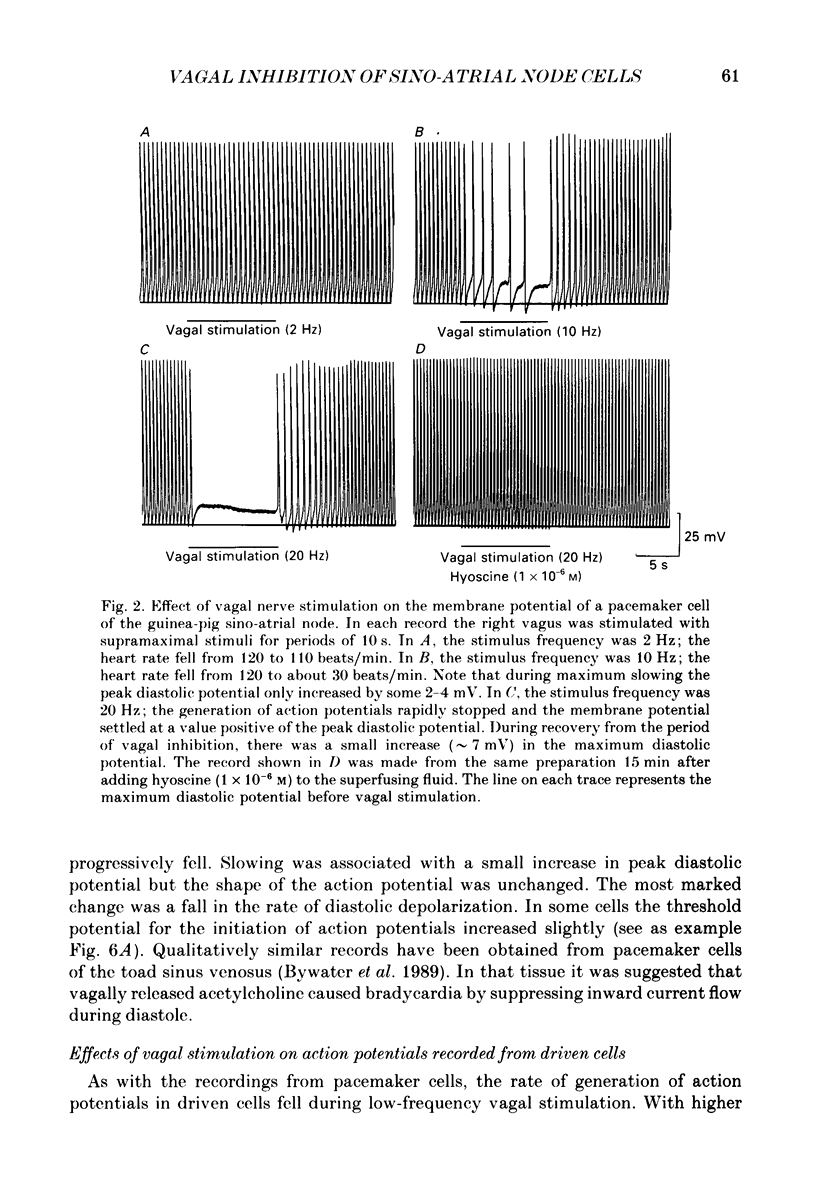
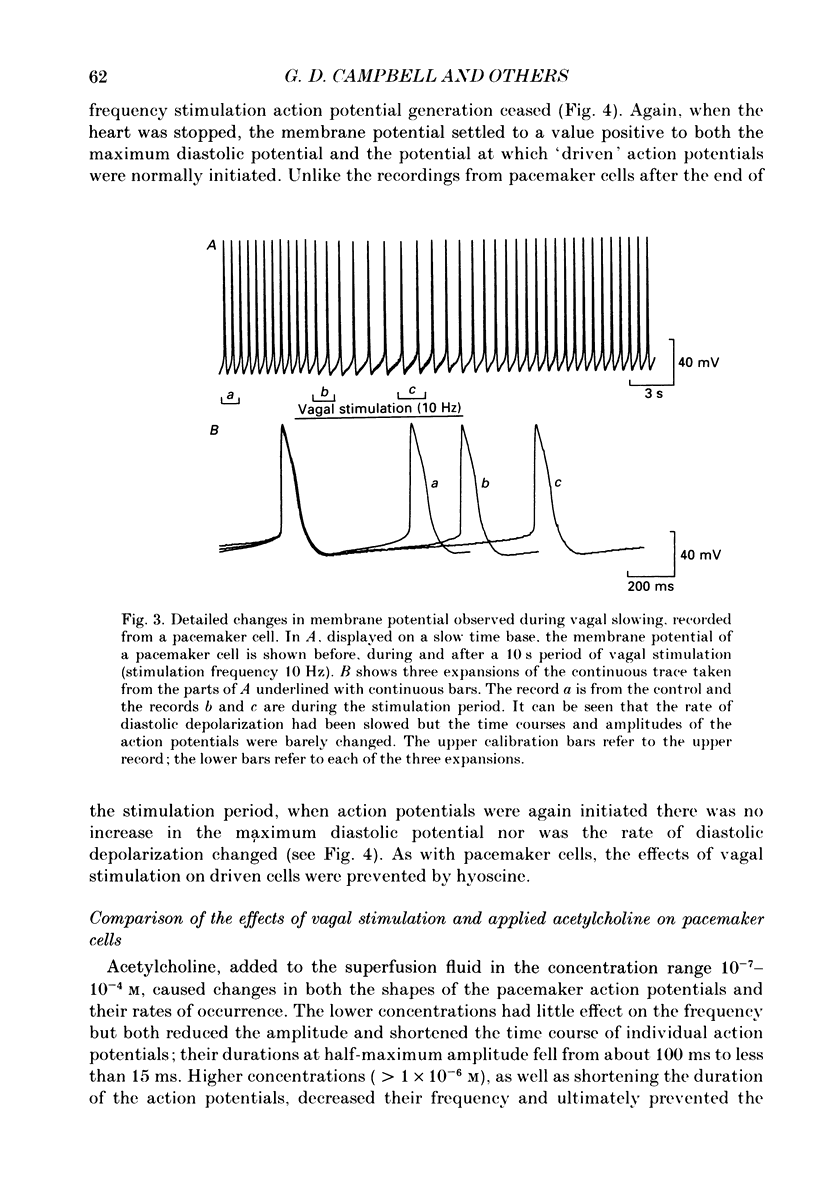
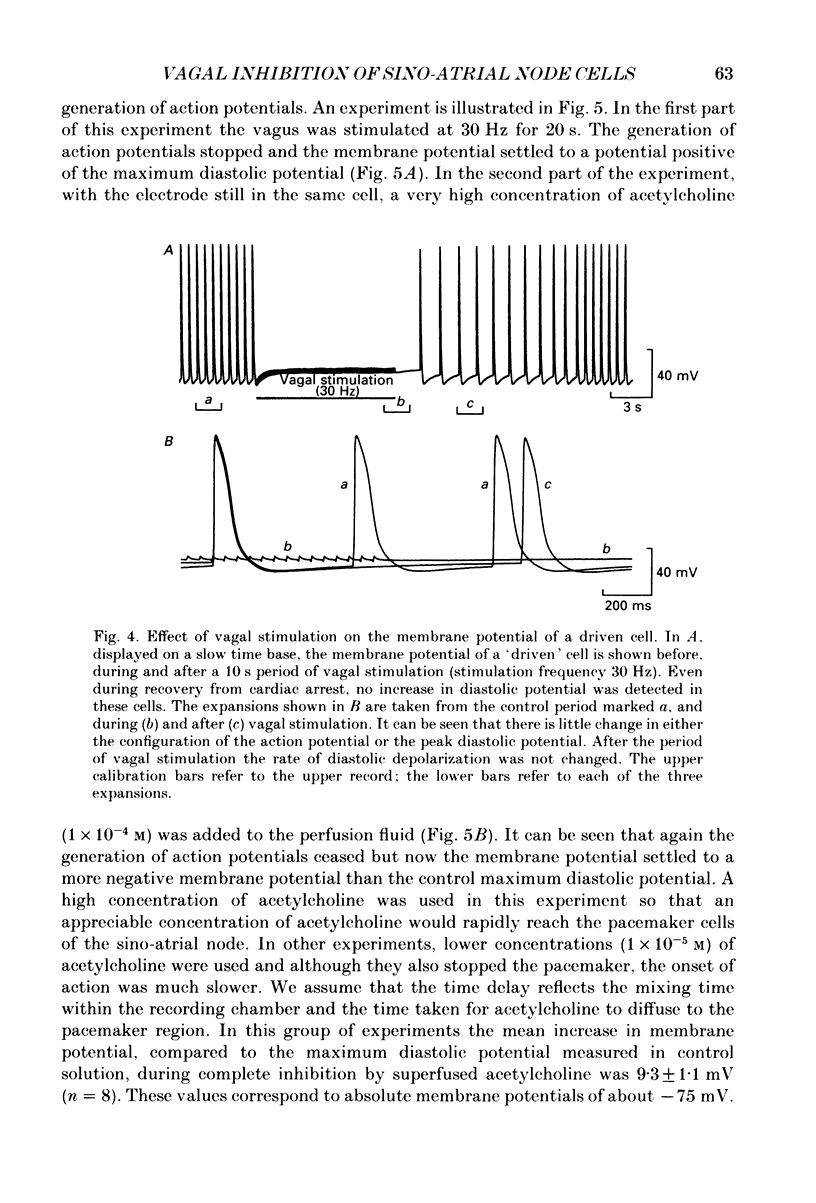
1. Intracellular recordings were made from pacemaker cells lying in the sino-atrial node of guinea-pigs. 2. Low-frequency vagal stimulation slowed the rate of generation of pacemaker action potentials; high-frequency stimulation stopped the generation of action potentials. 3. During vagal stimulation the rate of diastolic depolarization was reduced with the action potential otherwise unchanged: when the heart stopped the membrane potential of pacemaker cells settled to a value positive of the maximum diastolic potential. 4. In contrast, added acetylcholine caused membrane hyperpolarization and shortened the duration of action potentials. 5. The effects of both added acetylcholine and vagally released acetylcholine were abolished by hyoscine. 6. It is suggested that neurally released acetylcholine acts to change the balance between inward and outward current flow during diastole by modifying the properties of existing voltage-dependent channels. In contrast added acetylcholine appears to activate a different set of receptors which increase the potassium conductance of pacemaker cells.
Full text
PDF


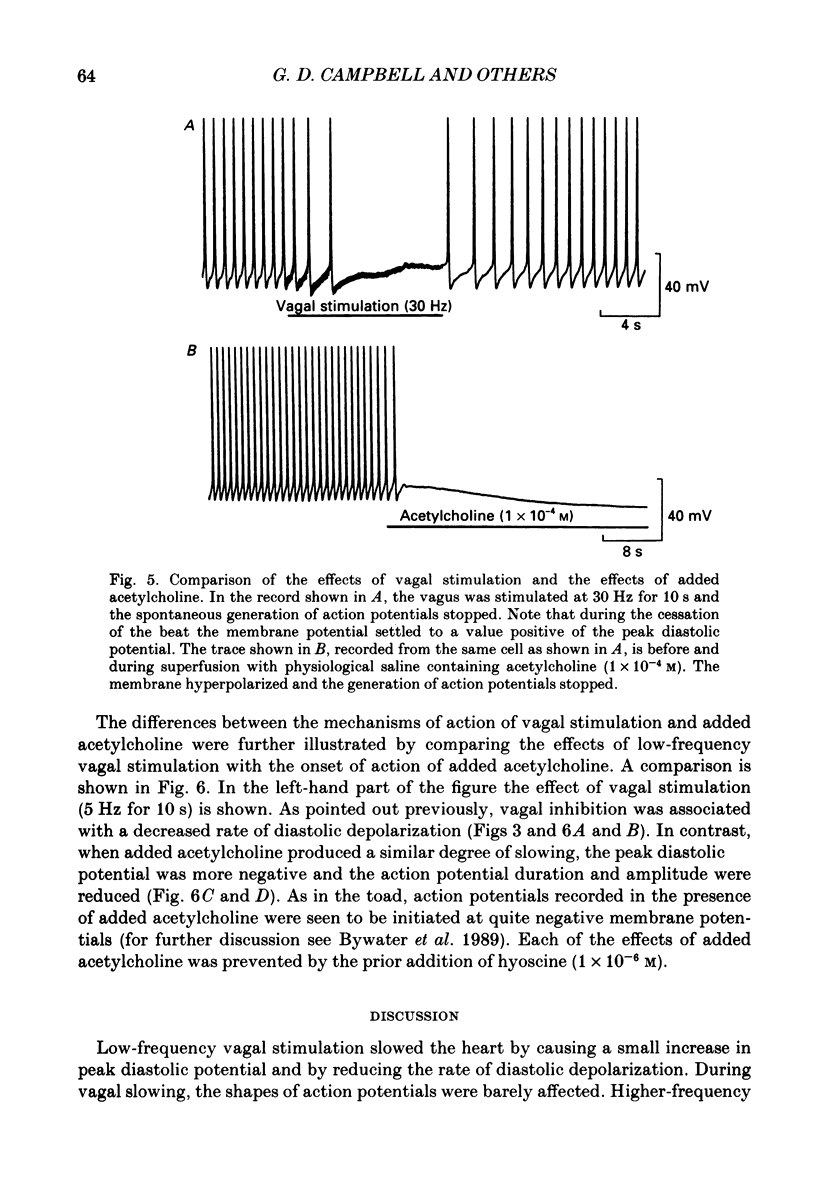
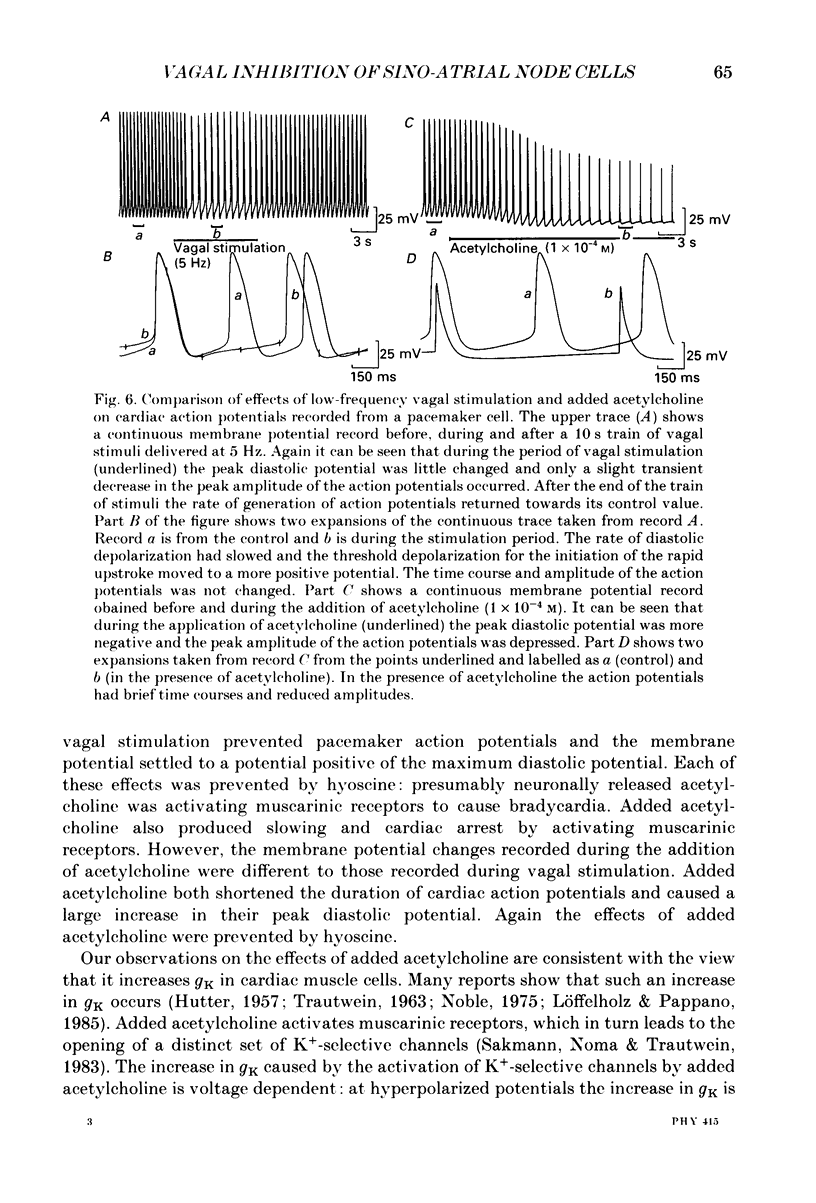



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown H. F. Electrophysiology of the sinoatrial node. Physiol Rev. 1982 Apr;62(2):505–530. doi: 10.1152/physrev.1982.62.2.505. [DOI] [PubMed] [Google Scholar]
- Bywater R. A., Campbell G., Edwards F. R., Hirst G. D., O'Shea J. E. The effects of vagal stimulation and applied acetylcholine on the sinus venosus of the toad. J Physiol. 1989 Aug;415:35–56. doi: 10.1113/jphysiol.1989.sp017710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Production of membrane potential changes in the frog's heart by inhibitory nerve impulses. Nature. 1955 Jun 11;175(4467):1035–1035. doi: 10.1038/1751035a0. [DOI] [PubMed] [Google Scholar]
- DiFrancesco D., Ferroni A., Mazzanti M., Tromba C. Properties of the hyperpolarizing-activated current (if) in cells isolated from the rabbit sino-atrial node. J Physiol. 1986 Aug;377:61–88. doi: 10.1113/jphysiol.1986.sp016177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFrancesco D. The cardiac hyperpolarizing-activated current, if. Origins and developments. Prog Biophys Mol Biol. 1985;46(3):163–183. doi: 10.1016/0079-6107(85)90008-2. [DOI] [PubMed] [Google Scholar]
- DiFrancesco D., Tromba C. Acetylcholine inhibits activation of the cardiac hyperpolarizing-activated current, if. Pflugers Arch. 1987 Sep;410(1-2):139–142. doi: 10.1007/BF00581906. [DOI] [PubMed] [Google Scholar]
- HUTTER O. F. Mode of action of autonomic transmitters on the heart. Br Med Bull. 1957 Sep;13(3):176–180. doi: 10.1093/oxfordjournals.bmb.a069609. [DOI] [PubMed] [Google Scholar]
- HUTTER O. F., TRAUTWEIN W. Effect of vagal stimulation on the sinus venosus of the frog's heart. Nature. 1955 Sep 10;176(4480):512–513. doi: 10.1038/176512a0. [DOI] [PubMed] [Google Scholar]
- HUTTER O. F., TRAUTWEIN W. Vagal and sympathetic effects on the pacemaker fibers in the sinus venosus of the heart. J Gen Physiol. 1956 May 20;39(5):715–733. doi: 10.1085/jgp.39.5.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara N., Irisawa H., Kameyama M. Contribution of two types of calcium currents to the pacemaker potentials of rabbit sino-atrial node cells. J Physiol. 1988 Jan;395:233–253. doi: 10.1113/jphysiol.1988.sp016916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartzell H. C. Adenosine receptors in frog sinus venosus: slow inhibitory potentials produced by adenine compounds and acetylcholine. J Physiol. 1979 Aug;293:23–49. doi: 10.1113/jphysiol.1979.sp012877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartzell H. C. Distribution of muscarinic acetylcholine receptors and presynaptic nerve terminals in amphibian heart. J Cell Biol. 1980 Jul;86(1):6–20. doi: 10.1083/jcb.86.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins C. B., Vatner S. F., Braunwald E. Parasympathetic control of the heart. Pharmacol Rev. 1973 Mar;25(1):119–155. [PubMed] [Google Scholar]
- Iijima T., Irisawa H., Kameyama M. Membrane currents and their modification by acetylcholine in isolated single atrial cells of the guinea-pig. J Physiol. 1985 Feb;359:485–501. doi: 10.1113/jphysiol.1985.sp015598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löffelholz K., Pappano A. J. The parasympathetic neuroeffector junction of the heart. Pharmacol Rev. 1985 Mar;37(1):1–24. [PubMed] [Google Scholar]
- Momose Y., Giles W., Szabo G. Acetylcholine-induced k current in amphibian atrial cells. Biophys J. 1984 Jan;45(1):20–22. doi: 10.1016/S0006-3495(84)84092-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noma A., Trautwein W. Relaxation of the ACh-induced potassium current in the rabbit sinoatrial node cell. Pflugers Arch. 1978 Nov 30;377(3):193–200. doi: 10.1007/BF00584272. [DOI] [PubMed] [Google Scholar]
- Opthof T., VanGinneken A. C., Bouman L. N., Jongsma H. J. The intrinsic cycle length in small pieces isolated from the rabbit sinoatrial node. J Mol Cell Cardiol. 1987 Sep;19(9):923–934. doi: 10.1016/s0022-2828(87)80621-1. [DOI] [PubMed] [Google Scholar]
- Opthof T., de Jonge B., Mackaay A. J., Bleeker W. K., Masson-Pevet M., Jongsma H. J., Bouman L. N. Functional and morphological organization of the guinea-pig sinoatrial node compared with the rabbit sinoatrial node. J Mol Cell Cardiol. 1985 Jun;17(6):549–564. doi: 10.1016/s0022-2828(85)80024-9. [DOI] [PubMed] [Google Scholar]
- Sakmann B., Noma A., Trautwein W. Acetylcholine activation of single muscarinic K+ channels in isolated pacemaker cells of the mammalian heart. Nature. 1983 May 19;303(5914):250–253. doi: 10.1038/303250a0. [DOI] [PubMed] [Google Scholar]
- Shibata E. F., Giles W., Pollack G. H. Threshold effects of acetylcholine on primary pacemaker cells of the rabbit sino-atrial node. Proc R Soc Lond B Biol Sci. 1985 Jan 22;223(1232):355–378. doi: 10.1098/rspb.1985.0006. [DOI] [PubMed] [Google Scholar]
- Simmons M. A., Hartzell H. C. A quantitative analysis of the acetylcholine-activated potassium current in single cells from frog atrium. Pflugers Arch. 1987 Aug;409(4-5):454–461. doi: 10.1007/BF00583801. [DOI] [PubMed] [Google Scholar]
- Spear J. F., Kronhaus K. D., Moore E. N., Kline R. P. The effect of brief vagal stimulation on the isolated rabbit sinus node. Circ Res. 1979 Jan;44(1):75–88. doi: 10.1161/01.res.44.1.75. [DOI] [PubMed] [Google Scholar]
- TRAUTWEIN W. Generation and conduction of impulses in the heart as affected by drugs. Pharmacol Rev. 1963 Jun;15:277–332. [PubMed] [Google Scholar]


