Abstract
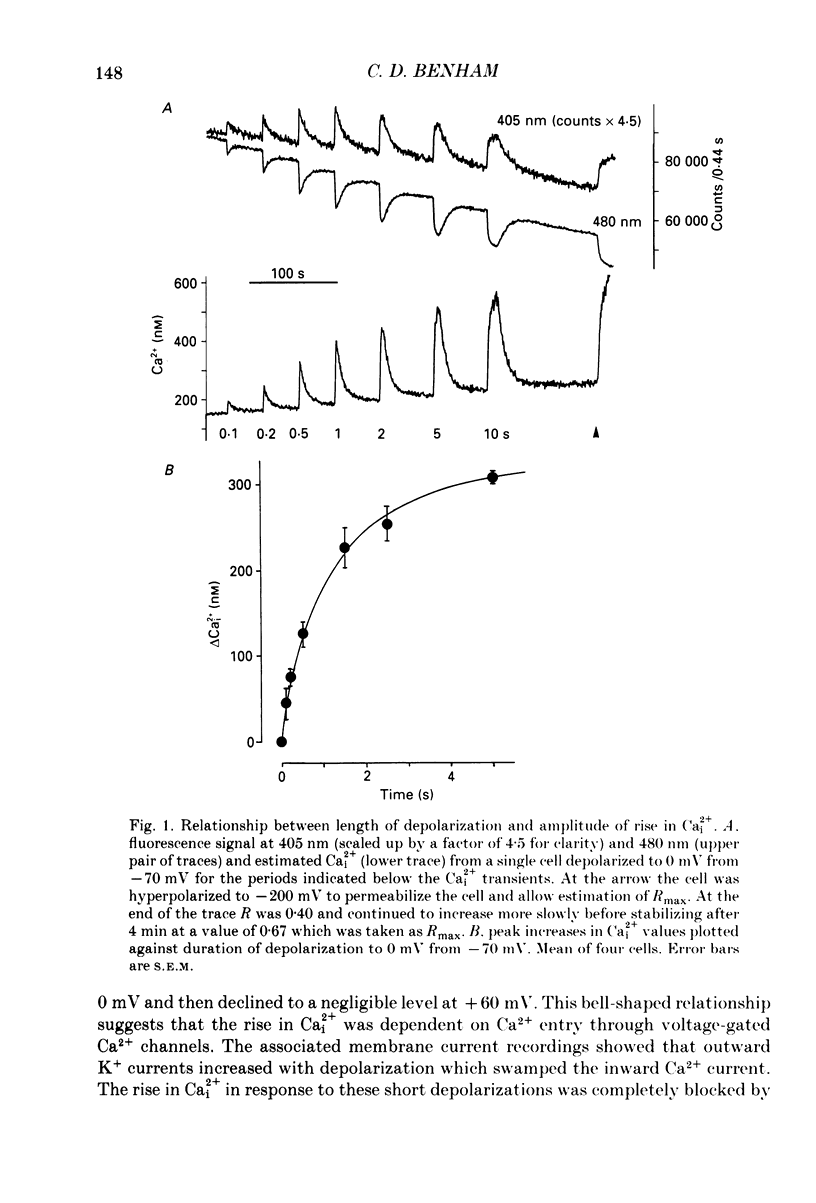
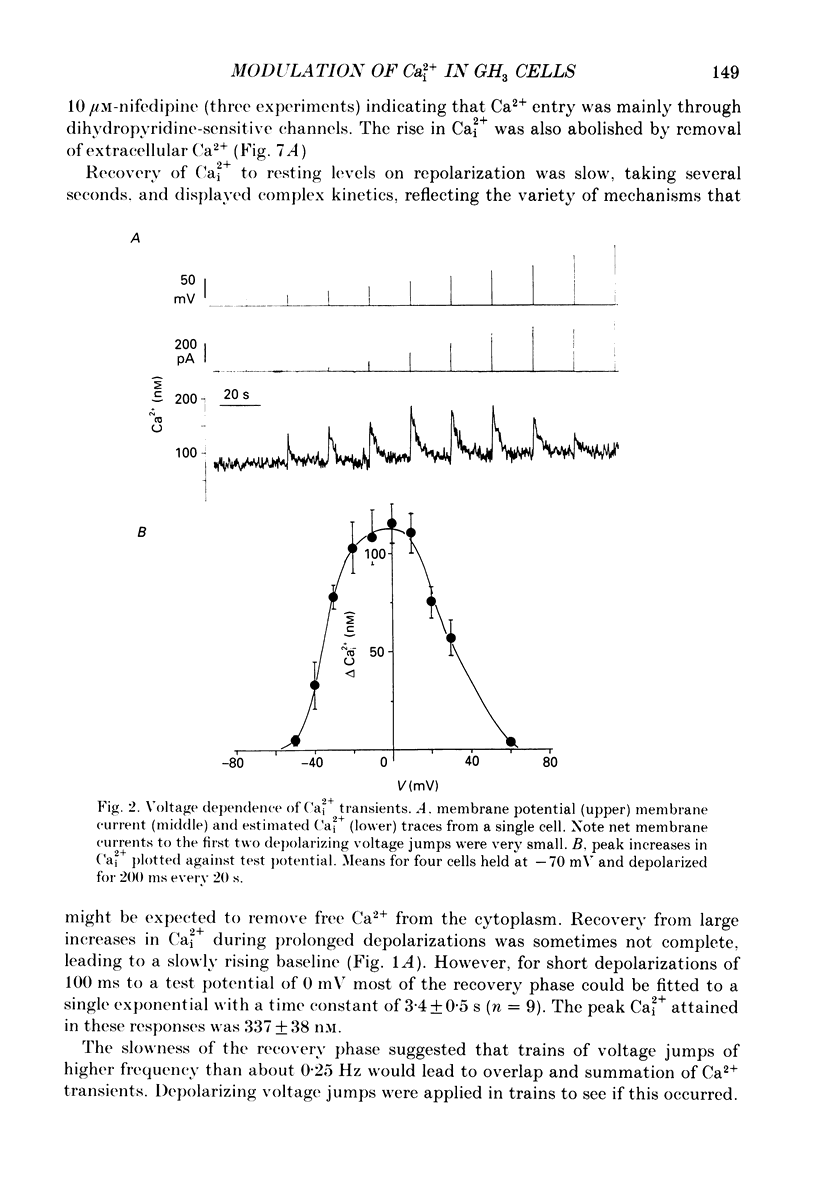
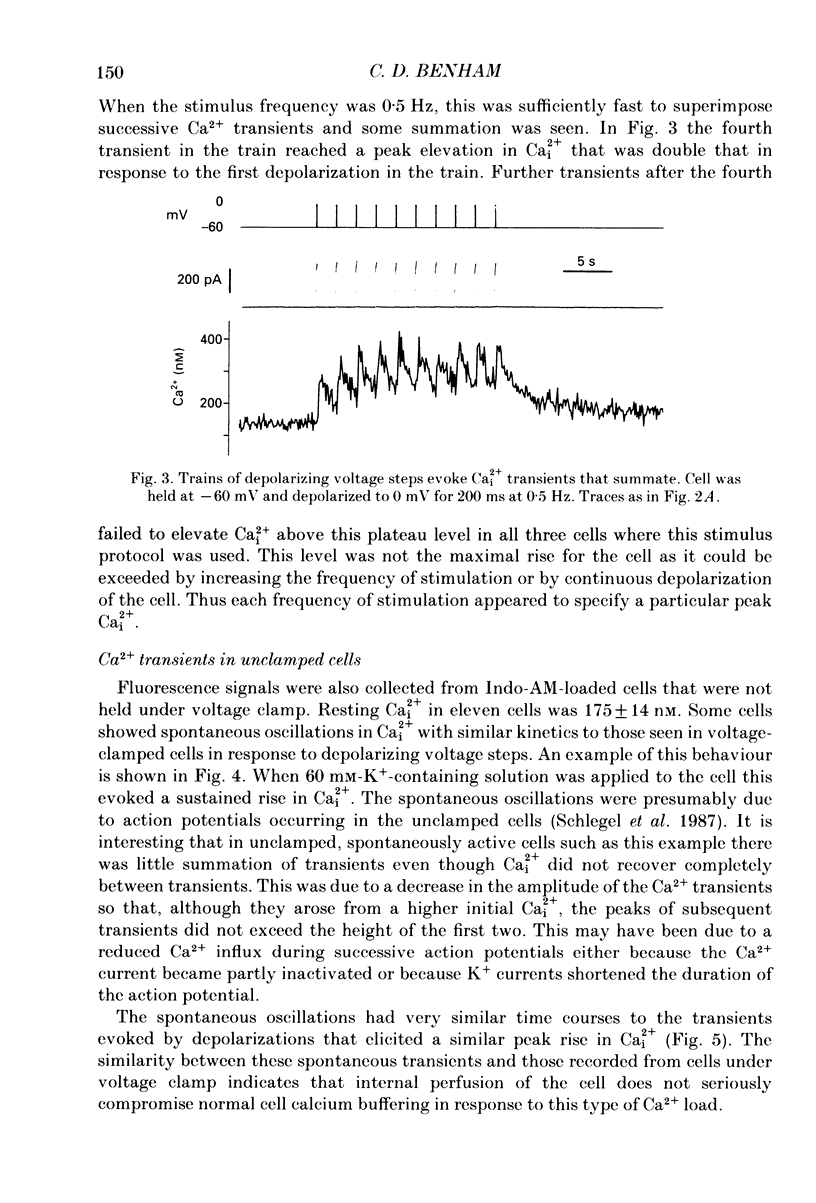
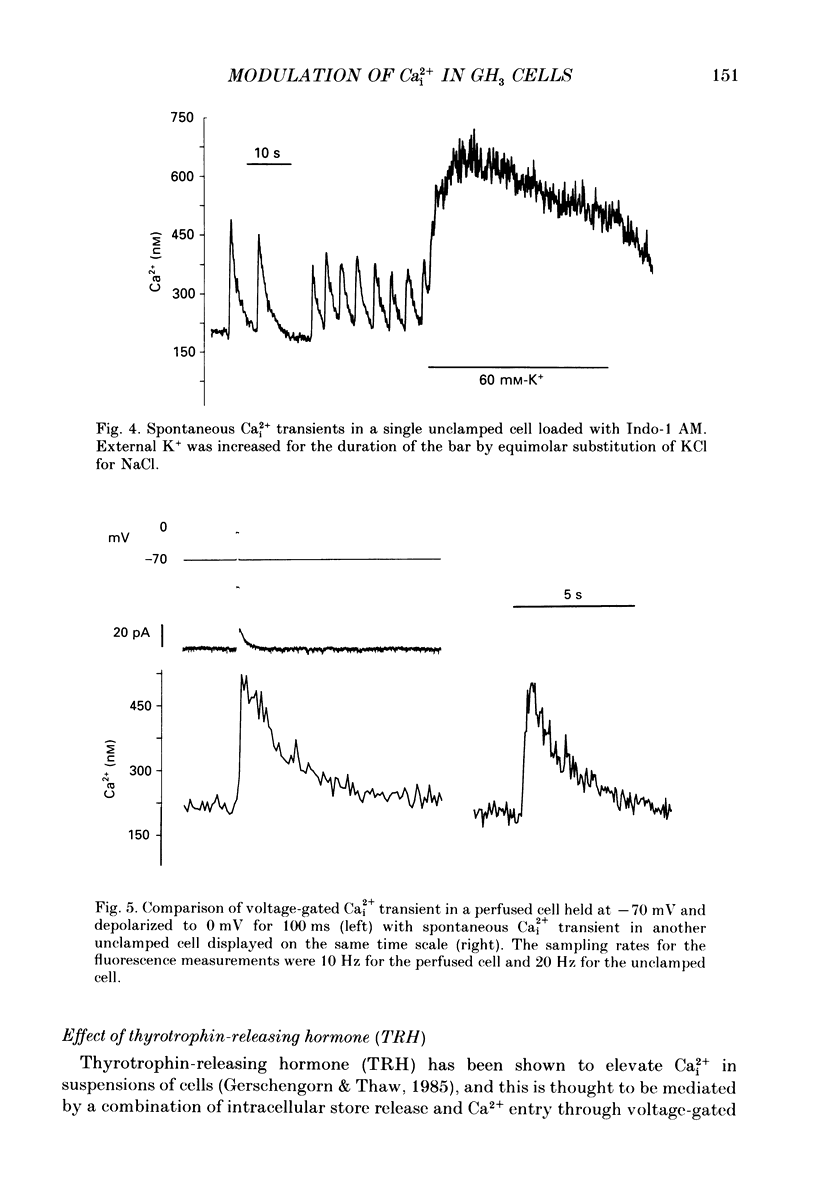
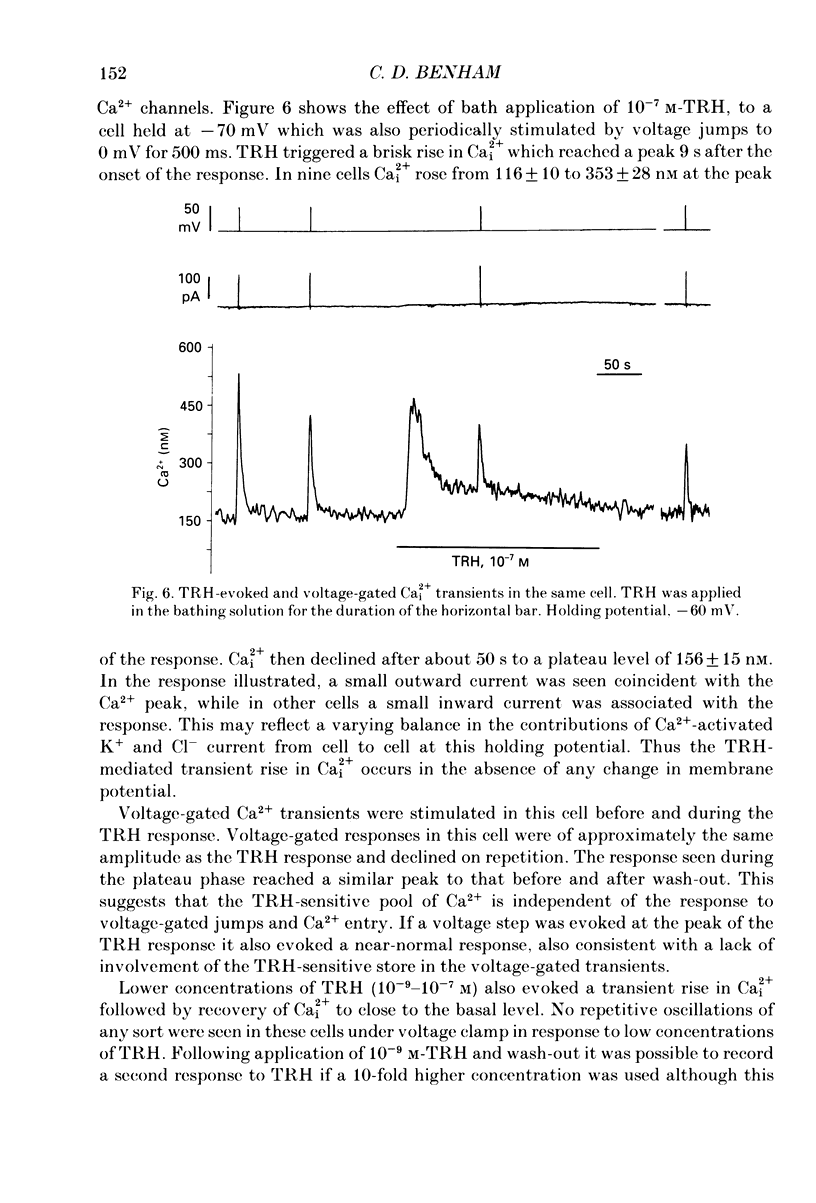
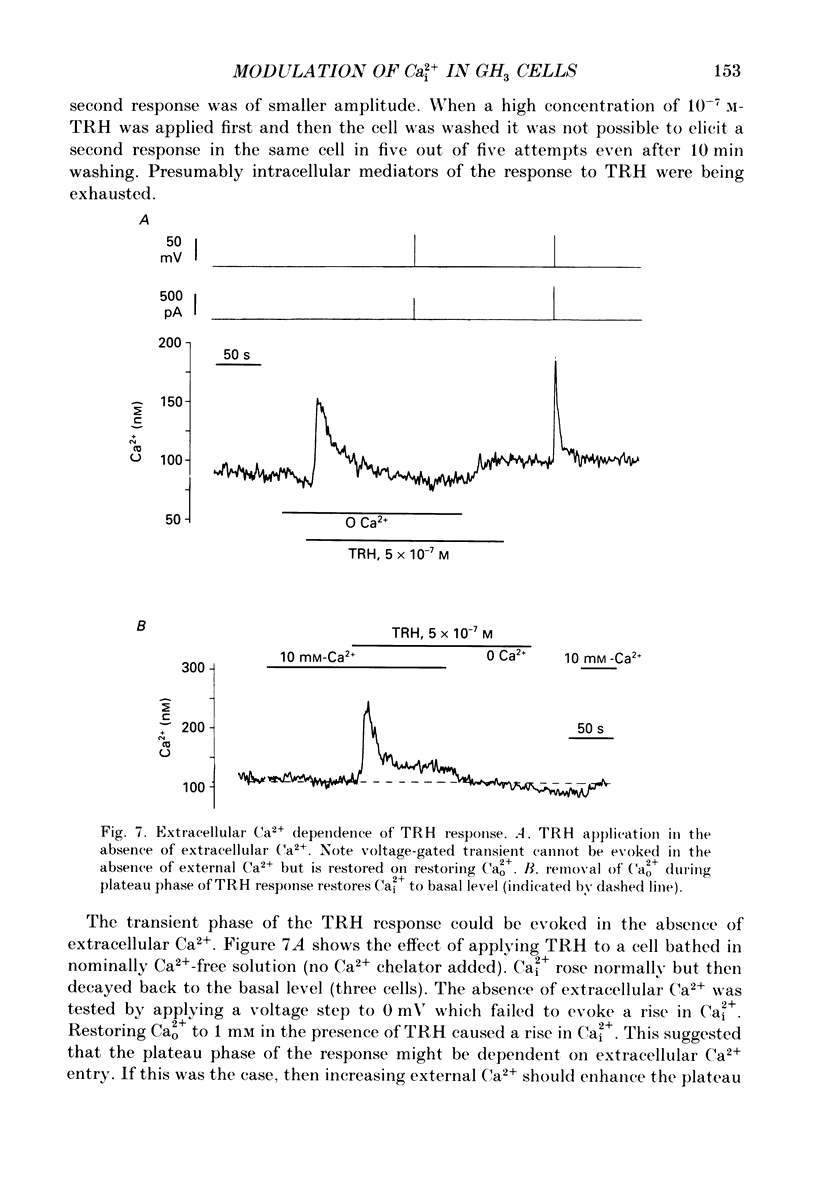
1. Intracellular free calcium (Ca2+i) was estimated in single GH3 cells by dual wavelength emission spectrofluorimetry using the Ca2+ indicator dye Indo-1, while cells were held under voltage clamp using patch clamp techniques. 2. Depolarization of cells evoked a transient rise in Ca2+i that increased with increasing duration of depolarization to a peak at about 10 s. 3. Calcium transients showed a bell-shaped dependence on the amplitude of the depolarizing pulse. They were abolished in the absence of extracellular calcium and by application of 10 microM-nifedipine. 4. Thyrotrophin-releasing hormone (TRH) evoked a transient rise in Ca2+i that was followed by a more sustained period of elevated Ca2+i in some cells. The transient phase of the response but not the sustained phase was seen in the absence of extracellular calcium. 5. Ca2+i transients evoked by depolarization were not affected by pre-release of internal Ca2+ stores with TRH. 6. The results demonstrate that voltage-gated Ca2+ entry and Ca2+ store release can each elevate cytoplasmic free calcium in GH3 cells and may both be important for stimulus-secretion coupling. Non-voltage-gated Ca2+ entry is not a major source of Ca2+ under these conditions.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almers W., Neher E. The Ca signal from fura-2 loaded mast cells depends strongly on the method of dye-loading. FEBS Lett. 1985 Nov 11;192(1):13–18. doi: 10.1016/0014-5793(85)80033-8. [DOI] [PubMed] [Google Scholar]
- Barcenas-Ruiz L., Wier W. G. Voltage dependence of intracellular [Ca2+]i transients in guinea pig ventricular myocytes. Circ Res. 1987 Jul;61(1):148–154. doi: 10.1161/01.res.61.1.148. [DOI] [PubMed] [Google Scholar]
- Callewaert G., Cleemann L., Morad M. Epinephrine enhances Ca2+ current-regulated Ca2+ release and Ca2+ reuptake in rat ventricular myocytes. Proc Natl Acad Sci U S A. 1988 Mar;85(6):2009–2013. doi: 10.1073/pnas.85.6.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobbold P. H., Rink T. J. Fluorescence and bioluminescence measurement of cytoplasmic free calcium. Biochem J. 1987 Dec 1;248(2):313–328. doi: 10.1042/bj2480313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen C. J., McCarthy R. T. Nimodipine block of calcium channels in rat anterior pituitary cells. J Physiol. 1987 Jun;387:195–225. doi: 10.1113/jphysiol.1987.sp016570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond A. H. Bidirectional control of cytosolic free calcium by thyrotropin-releasing hormone in pituitary cells. 1985 Jun 27-Jul 3Nature. 315(6022):752–755. doi: 10.1038/315752a0. [DOI] [PubMed] [Google Scholar]
- Dubinsky J. M., Oxford G. S. Dual modulation of K channels by thyrotropin-releasing hormone in clonal pituitary cells. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4282–4286. doi: 10.1073/pnas.82.12.4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufy B., Jaken S., Barker J. L. Intracellular Ca2+-dependent protein kinase C activation mimics delayed effects of thyrotropin-releasing hormone on clonal pituitary cell excitability. Endocrinology. 1987 Aug;121(2):793–802. doi: 10.1210/endo-121-2-793. [DOI] [PubMed] [Google Scholar]
- Fernandez J. M., Neher E., Gomperts B. D. Capacitance measurements reveal stepwise fusion events in degranulating mast cells. 1984 Nov 29-Dec 5Nature. 312(5993):453–455. doi: 10.1038/312453a0. [DOI] [PubMed] [Google Scholar]
- Gershengorn M. C. Mechanism of thyrotropin releasing hormone stimulation of pituitary hormone secretion. Annu Rev Physiol. 1986;48:515–526. doi: 10.1146/annurev.ph.48.030186.002503. [DOI] [PubMed] [Google Scholar]
- Gershengorn M. C., Thaw C. Thyrotropin-releasing hormone (TRH) stimulates biphasic elevation of cytoplasmic free calcium in GH3 cells. Further evidence that TRH mobilizes cellular and extracellular Ca2+. Endocrinology. 1985 Feb;116(2):591–596. doi: 10.1210/endo-116-2-591. [DOI] [PubMed] [Google Scholar]
- Gray P. T. Oscillations of free cytosolic calcium evoked by cholinergic and catecholaminergic agonists in rat parotid acinar cells. J Physiol. 1988 Dec;406:35–53. doi: 10.1113/jphysiol.1988.sp017367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hagiwara S., Ohmori H. Studies of calcium channels in rat clonal pituitary cells with patch electrode voltage clamp. J Physiol. 1982 Oct;331:231–252. doi: 10.1113/jphysiol.1982.sp014371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn R., Marty A. Muscarinic activation of ionic currents measured by a new whole-cell recording method. J Gen Physiol. 1988 Aug;92(2):145–159. doi: 10.1085/jgp.92.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson A. P., Timmerman M. P., Bagshaw C. R., Ashley C. C. The kinetics of calcium binding to fura-2 and indo-1. FEBS Lett. 1987 May 25;216(1):35–39. doi: 10.1016/0014-5793(87)80752-4. [DOI] [PubMed] [Google Scholar]
- Jacob R., Merritt J. E., Hallam T. J., Rink T. J. Repetitive spikes in cytoplasmic calcium evoked by histamine in human endothelial cells. Nature. 1988 Sep 1;335(6185):40–45. doi: 10.1038/335040a0. [DOI] [PubMed] [Google Scholar]
- Kidokoro Y. Spontaneous calcium action potentials in a clonal pituitary cell line and their relationship to prolactin secretion. Nature. 1975 Dec 25;258(5537):741–742. doi: 10.1038/258741a0. [DOI] [PubMed] [Google Scholar]
- Lamb T. D. An inexpensive digital tape recorder suitable for neurophysiological signals. J Neurosci Methods. 1985 Oct;15(1):1–13. doi: 10.1016/0165-0270(85)90057-3. [DOI] [PubMed] [Google Scholar]
- Lindau M., Fernandez J. M. IgE-mediated degranulation of mast cells does not require opening of ion channels. Nature. 1986 Jan 9;319(6049):150–153. doi: 10.1038/319150a0. [DOI] [PubMed] [Google Scholar]
- Marchetti C., Brown A. M. Protein kinase activator 1-oleoyl-2-acetyl-sn-glycerol inhibits two types of calcium currents in GH3 cells. Am J Physiol. 1988 Jan;254(1 Pt 1):C206–C210. doi: 10.1152/ajpcell.1988.254.1.C206. [DOI] [PubMed] [Google Scholar]
- Mason W. T., Waring D. W. Patch clamp recordings of single ion channel activation by gonadotrophin-releasing hormone in ovine pituitary gonadotrophs. Neuroendocrinology. 1986;43(2):205–219. doi: 10.1159/000124529. [DOI] [PubMed] [Google Scholar]
- Ozawa S. Biphasic effect of thyrotropin-releasing hormone on membrane K+ permeability in rat clonal pituitary cells. Brain Res. 1981 Mar 23;209(1):240–244. doi: 10.1016/0006-8993(81)91188-4. [DOI] [PubMed] [Google Scholar]
- Ozawa S., Sand O. Electrophysiology of excitable endocrine cells. Physiol Rev. 1986 Oct;66(4):887–952. doi: 10.1152/physrev.1986.66.4.887. [DOI] [PubMed] [Google Scholar]
- Penner R., Matthews G., Neher E. Regulation of calcium influx by second messengers in rat mast cells. Nature. 1988 Aug 11;334(6182):499–504. doi: 10.1038/334499a0. [DOI] [PubMed] [Google Scholar]
- Rebecchi M. J., Gershengorn M. C. Thyroliberin stimulates rapid hydrolysis of phosphatidylinositol 4,5-bisphosphate by a phosphodiesterase in rat mammotropic pituitary cells. Evidence for an early Ca2+-independent action. Biochem J. 1983 Nov 15;216(2):287–294. doi: 10.1042/bj2160287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlegel W., Winiger B. P., Mollard P., Vacher P., Wuarin F., Zahnd G. R., Wollheim C. B., Dufy B. Oscillations of cytosolic Ca2+ in pituitary cells due to action potentials. Nature. 1987 Oct 22;329(6141):719–721. doi: 10.1038/329719a0. [DOI] [PubMed] [Google Scholar]
- Thorner M. O., Hackett J. T., Murad F., MacLeod R. M. Calcium rather than cyclic AMP as the physiological intracellular regulator of prolactin release. Neuroendocrinology. 1980 Dec;31(6):390–402. doi: 10.1159/000123109. [DOI] [PubMed] [Google Scholar]
- Woods N. M., Cuthbertson K. S., Cobbold P. H. Repetitive transient rises in cytoplasmic free calcium in hormone-stimulated hepatocytes. Nature. 1986 Feb 13;319(6054):600–602. doi: 10.1038/319600a0. [DOI] [PubMed] [Google Scholar]
- Yakel J. L., Trussell L. O., Jackson M. B. Three serotonin responses in cultured mouse hippocampal and striatal neurons. J Neurosci. 1988 Apr;8(4):1273–1285. doi: 10.1523/JNEUROSCI.08-04-01273.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]


