Abstract
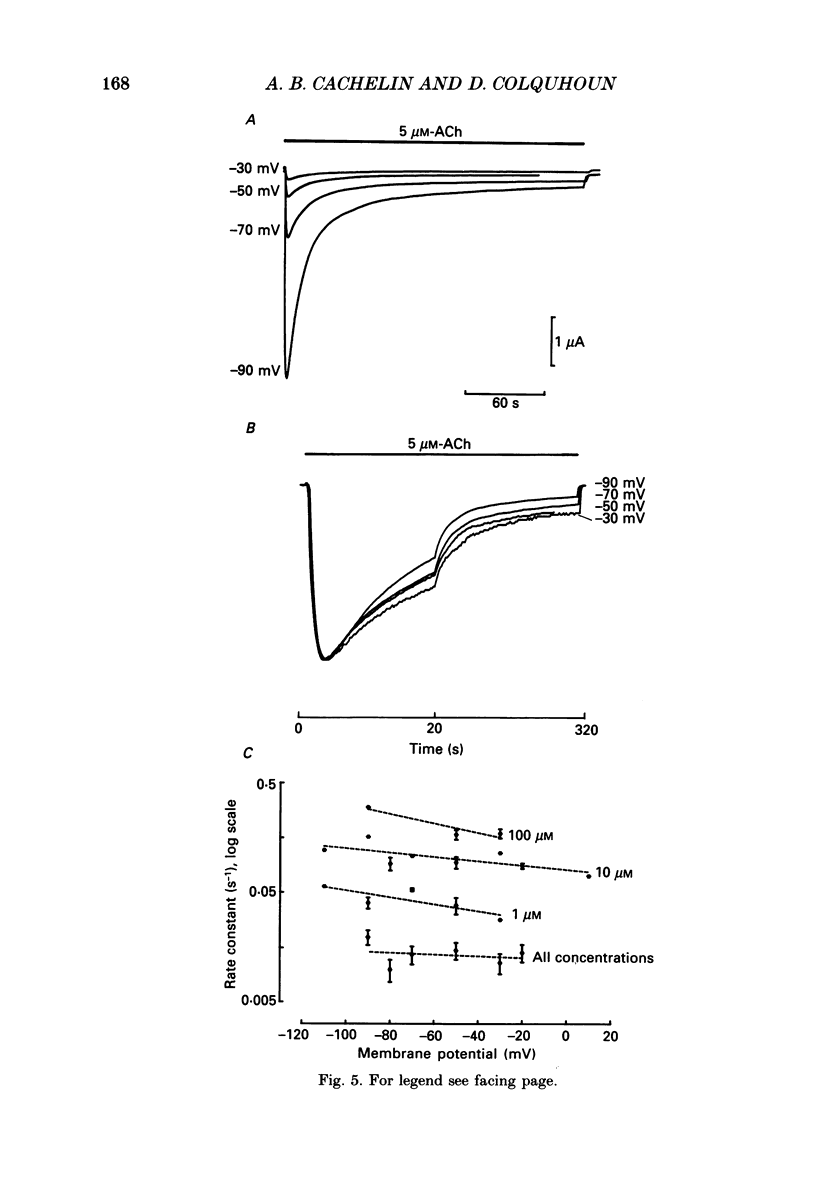
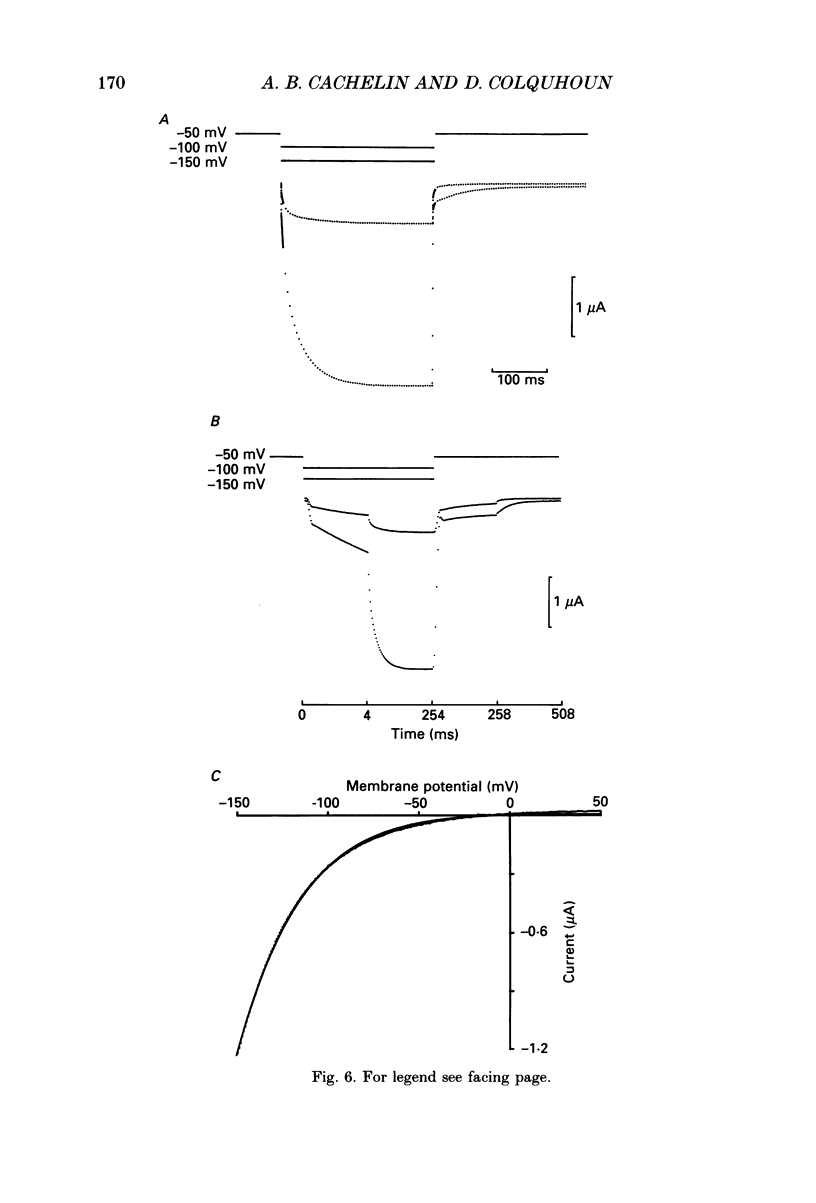
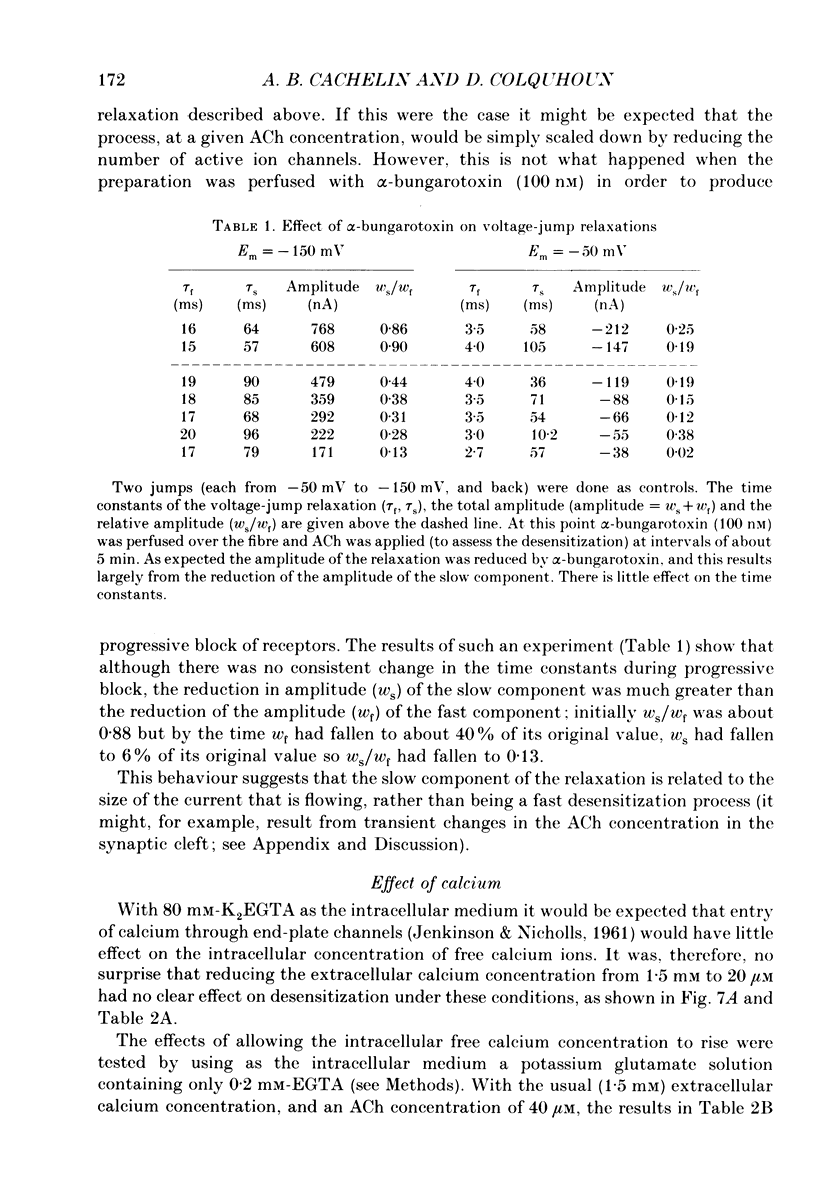
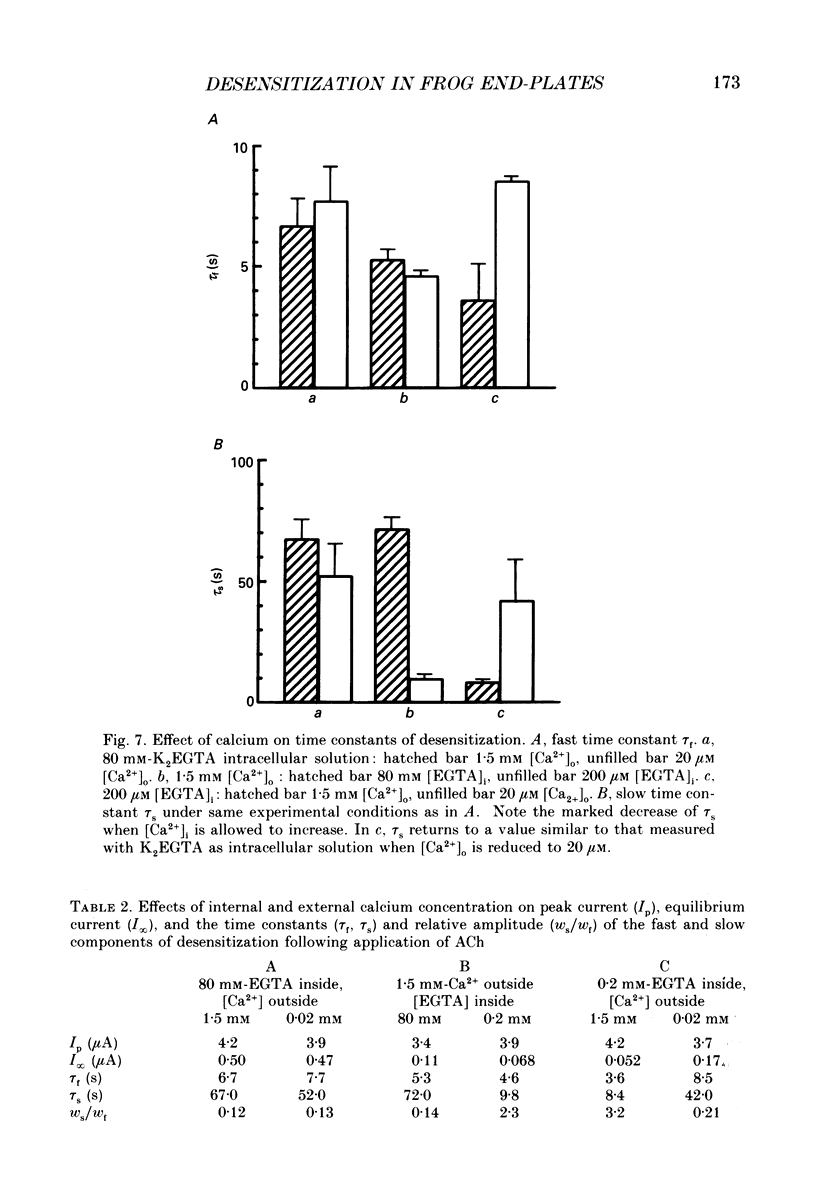
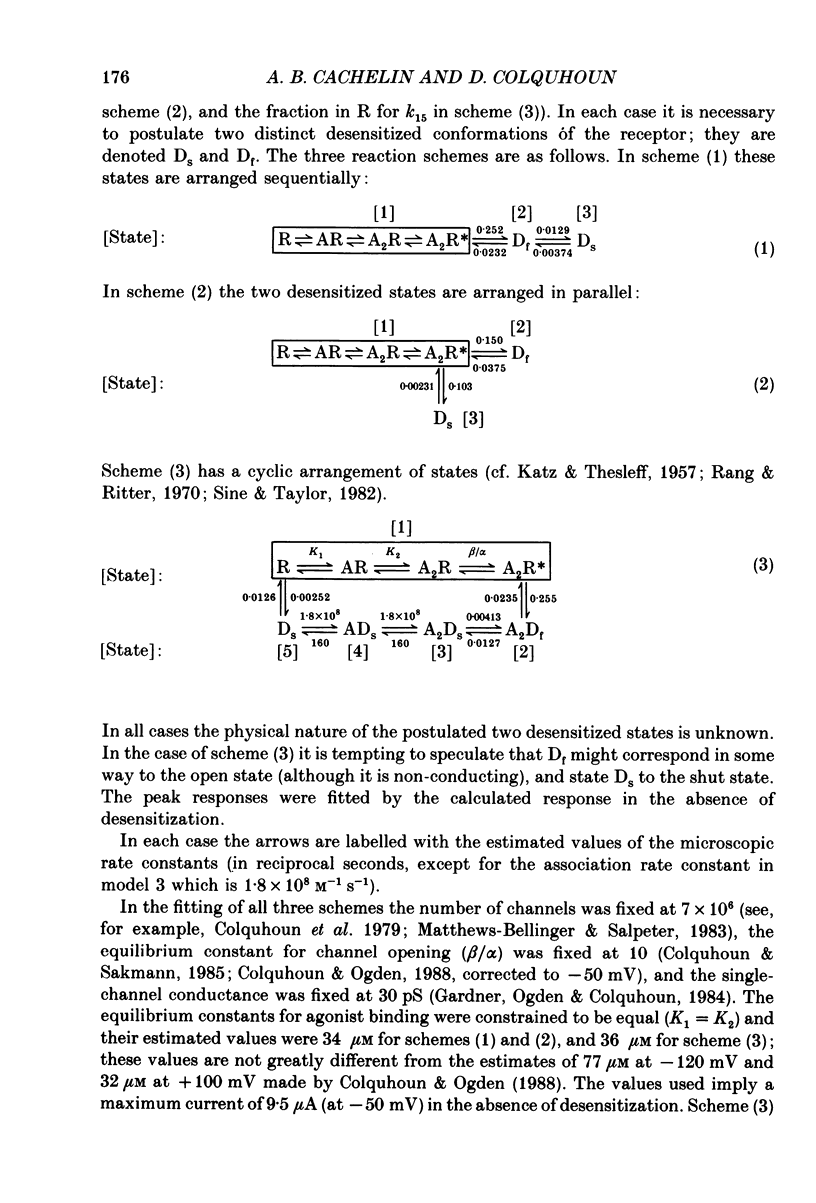
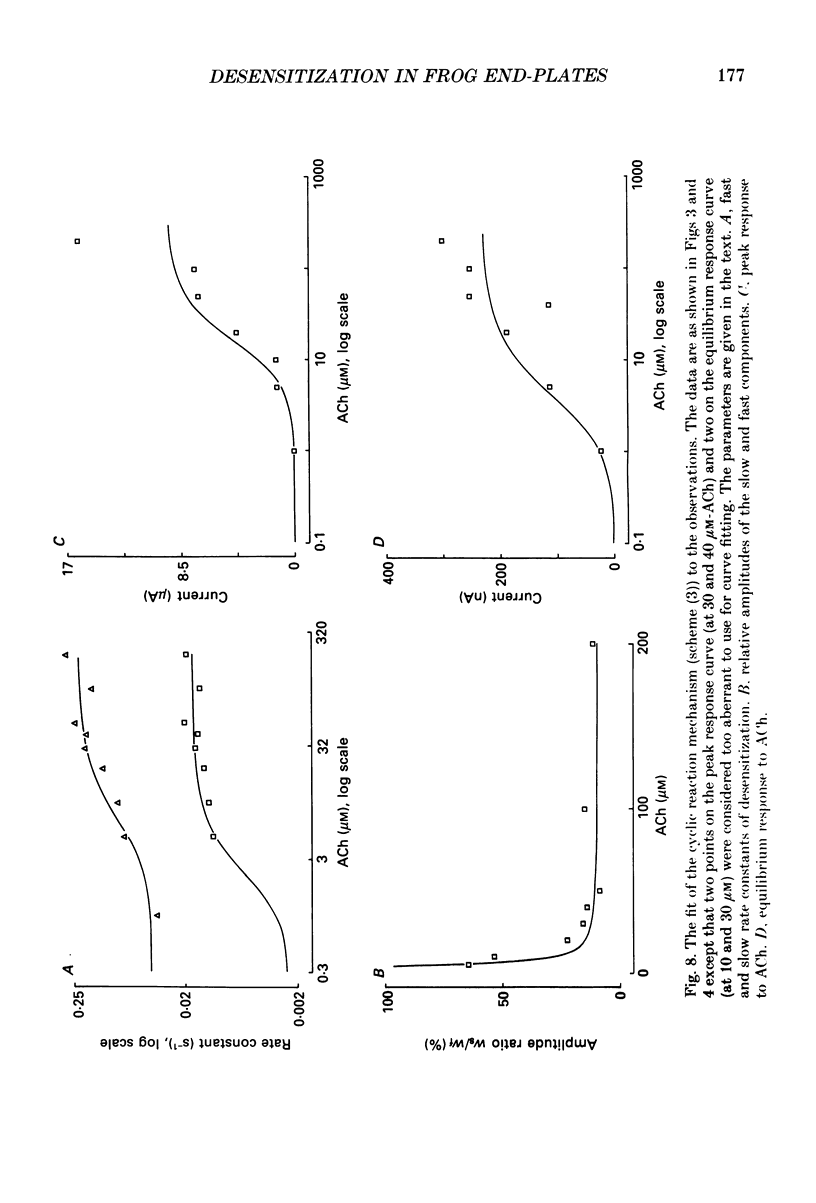
1. Desensitization of the nicotinic acetylcholine receptor of the frog end-plate was investigated in dissociated frog muscle fibres using the Vaseline-gap clamp method so that a wide range of well-defined agonist concentrations could be used without having to use alpha-bungarotoxin to reduce currents, and so that the intracellular medium could be controlled. 2. Acetylcholine (ACh) concentrations between 1 and 1000 microM were used, after inactivation of acetylcholinesterase. The intracellular calcium concentration was usually kept near zero by using 80 mM-K2EGTA as the intracellular solution. 3. When using the low intracellular calcium solution, desensitization proceeded as a biphasic process with estimates of fast and slow time constants of about 8 and 80 s at 4 degrees C and 20 microM-ACh (the rates increased with concentration). In contrast, only one (fast) component of desensitization was detected when the intracellular calcium concentration was allowed to increase during ACh application. 4. Despite rapid application of ACh the time to peak response was 0.2 s (with 400 microM-ACh) to 2 s (with 1 microM-ACh); this slow rise was shown to result from diffusion delays. Nevertheless the peak current with 200 microM-ACh corresponded to opening of most of the channels present, so there is probably not much desensitization in the millisecond time range. 5. Both fast and slow time constants for onset of desensitization showed only slight dependence on membrane potential when [Ca2+]i was buffered with 80 mM-K2EGTA. 6. Increasing the intracellular cyclic AMP concentration directly, or indirectly with forskolin and IBMX, had no effect on the time course of desensitization. 7. Intracellular application of submicromolar concentrations of phorbol-12,13-dibutyrate (PDBu) and phorbol-12-myristate-13-acetate (PMA) yielded a small but reproducible reduction of the peak response to ACh. The time course of desensitization was, however, not modified by these substances. 8. The implications of these observations for the mechanism of desensitization, and their relationship to single-channel observations, are discussed.
Full text
PDF



















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. J., Nonner W., Dwyer T. M., Hille B. Block of endplate channels by permeant cations in frog skeletal muscle. J Gen Physiol. 1981 Dec;78(6):593–615. doi: 10.1085/jgp.78.6.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams P. R. Acetylcholine receptor kinetics. J Membr Biol. 1981 Feb 28;58(3):161–174. doi: 10.1007/BF01870902. [DOI] [PubMed] [Google Scholar]
- Adams P. R. Kinetics of agonist conductance changes during hyperolarization at frog endplates. Br J Pharmacol. 1975 Feb;53(2):308–310. doi: 10.1111/j.1476-5381.1975.tb07364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams P. R. Relaxation experiments using bath-applied suberyldicholine. J Physiol. 1977 Jun;268(2):271–289. doi: 10.1113/jphysiol.1977.sp011857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albuquerque E. X., Deshpande S. S., Aracava Y., Alkondon M., Daly J. W. A possible involvement of cyclic AMP in the expression of desensitization of the nicotinic acetylcholine receptor. A study with forskolin and its analogs. FEBS Lett. 1986 Apr 7;199(1):113–120. doi: 10.1016/0014-5793(86)81235-2. [DOI] [PubMed] [Google Scholar]
- Anwyl R., Narahashi T. Desensitization of the acetylcholine receptor of denervated rat soleus muscle and the effect of calcium. Br J Pharmacol. 1980 May;69(1):91–98. doi: 10.1111/j.1476-5381.1980.tb10886.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd N. D. Two distinct kinetic phases of desensitization of acetylcholine receptors of clonal rat PC12 cells. J Physiol. 1987 Aug;389:45–67. doi: 10.1113/jphysiol.1987.sp016646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett R. S., Dilger J. P., Adams P. R., Lancaster B. A method for the rapid exchange of solutions bathing excised membrane patches. Biophys J. 1986 Nov;50(5):987–992. doi: 10.1016/S0006-3495(86)83539-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chemeris N. K., Kazachenko V. N., Kislov A. N., Kurchikov A. L. Inhibition of acetylcholine responses by intracellular calcium in Lymnaea stagnalis neurones. J Physiol. 1982 Feb;323:1–19. doi: 10.1113/jphysiol.1982.sp014058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesnut T. J. Two-component desensitization at the neuromuscular junction of the frog. J Physiol. 1983 Mar;336:229–241. doi: 10.1113/jphysiol.1983.sp014578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D., Dreyer F., Sheridan R. E. The actions of tubocurarine at the frog neuromuscular junction. J Physiol. 1979 Aug;293:247–284. doi: 10.1113/jphysiol.1979.sp012888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D., Hawkes A. G. On the stochastic properties of bursts of single ion channel openings and of clusters of bursts. Philos Trans R Soc Lond B Biol Sci. 1982 Dec 24;300(1098):1–59. doi: 10.1098/rstb.1982.0156. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Hawkes A. G. Relaxation and fluctuations of membrane currents that flow through drug-operated channels. Proc R Soc Lond B Biol Sci. 1977 Nov 14;199(1135):231–262. doi: 10.1098/rspb.1977.0137. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Ogden D. C. Activation of ion channels in the frog end-plate by high concentrations of acetylcholine. J Physiol. 1988 Jan;395:131–159. doi: 10.1113/jphysiol.1988.sp016912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D., Sakmann B. Fast events in single-channel currents activated by acetylcholine and its analogues at the frog muscle end-plate. J Physiol. 1985 Dec;369:501–557. doi: 10.1113/jphysiol.1985.sp015912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor E. A., Fiekers J. F., Neel D. S., Parsons R. L., Schnitzler R. M. Comparison of cholinergic activation and desensitization at snake twitch and slow muscle fibre end-plates. J Physiol. 1984 Jun;351:657–674. doi: 10.1113/jphysiol.1984.sp015269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R. N., Kaldany R. R., DiPaola M., Karlin A. Time-resolved photolabeling by quinacrine azide of a noncompetitive inhibitor site of the nicotinic acetylcholine receptor in a transient, agonist-induced state. J Biol Chem. 1985 Jun 25;260(12):7186–7193. [PubMed] [Google Scholar]
- Eusebi F., Grassi F., Molinaro M., Zani B. M. Acetylcholine regulation of nicotinic receptor channels through a putative G protein in chick myotubes. J Physiol. 1987 Dec;393:635–645. doi: 10.1113/jphysiol.1987.sp016845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltz A., Trautmann A. Desensitization at the frog neuromuscular junction: a biphasic process. J Physiol. 1982 Jan;322:257–272. doi: 10.1113/jphysiol.1982.sp014036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiekers J. F., Spannbauer P. M., Scubon-Mulieri B., Parsons R. L. Voltage dependence of desensitization. Influence of calcium and activation kinetics. J Gen Physiol. 1980 May;75(5):511–529. doi: 10.1085/jgp.75.5.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner P., Ogden D. C., Colquhoun D. Conductances of single ion channels opened by nicotinic agonists are indistinguishable. Nature. 1984 May 10;309(5964):160–162. doi: 10.1038/309160a0. [DOI] [PubMed] [Google Scholar]
- Heidmann T., Changeux J. P. Time-resolved photolabeling by the noncompetitive blocker chlorpromazine of the acetylcholine receptor in its transiently open and closed ion channel conformations. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1897–1901. doi: 10.1073/pnas.81.6.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B., Campbell D. T. An improved vaseline gap voltage clamp for skeletal muscle fibers. J Gen Physiol. 1976 Mar;67(3):265–293. doi: 10.1085/jgp.67.3.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huganir R. L., Delcour A. H., Greengard P., Hess G. P. Phosphorylation of the nicotinic acetylcholine receptor regulates its rate of desensitization. Nature. 1986 Jun 19;321(6072):774–776. doi: 10.1038/321774a0. [DOI] [PubMed] [Google Scholar]
- Huganir R. L., Greengard P. cAMP-dependent protein kinase phosphorylates the nicotinic acetylcholine receptor. Proc Natl Acad Sci U S A. 1983 Feb;80(4):1130–1134. doi: 10.1073/pnas.80.4.1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häggblad J., Eriksson H., Hedlund B., Heilbronn E. Forskolin blocks carbachol-mediated ion-permeability of chick myotube nicotinic receptors and inhibits binding of 3H-phencyclidine to Torpedo microsac nicotinic receptors. Naunyn Schmiedebergs Arch Pharmacol. 1987 Oct;336(4):381–386. doi: 10.1007/BF00164869. [DOI] [PubMed] [Google Scholar]
- Irving M., Maylie J., Sizto N. L., Chandler W. K. Intrinsic optical and passive electrical properties of cut frog twitch fibers. J Gen Physiol. 1987 Jan;89(1):1–40. doi: 10.1085/jgp.89.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JENKINSON D. H., NICHOLLS J. G. Contractures and permeability changes produced by acetylcholine in depolarized denervated muscle. J Physiol. 1961 Nov;159:111–127. doi: 10.1113/jphysiol.1961.sp006796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATZ B., THESLEFF S. A study of the desensitization produced by acetylcholine at the motor end-plate. J Physiol. 1957 Aug 29;138(1):63–80. doi: 10.1113/jphysiol.1957.sp005838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kienker P. Equivalence of aggregated Markov models of ion-channel gating. Proc R Soc Lond B Biol Sci. 1989 Apr 22;236(1284):269–309. doi: 10.1098/rspb.1989.0024. [DOI] [PubMed] [Google Scholar]
- Lester H. A., Changeux J. P., Sheridan R. E. Conductance increases produced by bath application of cholinergic agonists to Electrophorus electroplaques. J Gen Physiol. 1975 Jun;65(6):797–816. doi: 10.1085/jgp.65.6.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magazanik L. G., Vyskocil F. Dependence of acetylcholine desensitization on the membrane potential of frog muscle fibre and on the ionic changes in the medium. J Physiol. 1970 Oct;210(3):507–518. doi: 10.1113/jphysiol.1970.sp009223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manthey A. A. Further studies of the effect of calcium on the time course of action of carbamylcholine at the neuromuscular junction. J Gen Physiol. 1970 Sep;56(3):407–419. doi: 10.1085/jgp.56.3.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manthey A. A. The effect of calcium on the desensitization of membrane receptors at the neuromuscular junction. J Gen Physiol. 1966 May;49(5):963–976. doi: 10.1085/jgp.49.5.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews-Bellinger J. A., Salpeter M. M. Fine structural distribution of acetylcholine receptors at developing mouse neuromuscular junctions. J Neurosci. 1983 Mar;3(3):644–657. doi: 10.1523/JNEUROSCI.03-03-00644.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh E. M., McGee R., Jr Direct anesthetic-like effects of forskolin on the nicotinic acetylcholine receptors of PC12 cells. J Biol Chem. 1986 Mar 5;261(7):3103–3106. [PubMed] [Google Scholar]
- Miledi R. Intracellular calcium and desensitization of acetylcholine receptors. Proc R Soc Lond B Biol Sci. 1980 Sep 26;209(1176):447–452. doi: 10.1098/rspb.1980.0106. [DOI] [PubMed] [Google Scholar]
- Miles K., Anthony D. T., Rubin L. L., Greengard P., Huganir R. L. Regulation of nicotinic acetylcholine receptor phosphorylation in rat myotubes by forskolin and cAMP. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6591–6595. doi: 10.1073/pnas.84.18.6591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E., Sakmann B. Voltage-dependence of drug-induced conductance in frog neuromuscular junction. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2140–2144. doi: 10.1073/pnas.72.6.2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson N., Anholt R., Lindstrom J., Montal M. Reconstitution of purified acetylcholine receptors with functional ion channels in planar lipid bilayers. Proc Natl Acad Sci U S A. 1980 May;77(5):3057–3061. doi: 10.1073/pnas.77.5.3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallotta B. S., Webb G. D. The effects of external Ca++ and Mg++ on the voltage sensitivity of desensitization in Electrophorus electroplaques. J Gen Physiol. 1980 Jun;75(6):693–708. doi: 10.1085/jgp.75.6.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popot J. L., Cartaud J., Changeux J. P. Reconstitution of a functional acetylcholine receptor. Incorporation into artificial lipid vesicles and pharmacology of the agonist-controlled permeability changes. Eur J Biochem. 1981 Aug;118(2):203–214. [PubMed] [Google Scholar]
- Rang H. P., Ritter J. M. On the mechanism of desensitization at cholinergic receptors. Mol Pharmacol. 1970 Jul;6(4):357–382. [PubMed] [Google Scholar]
- Safran A., Sagi-Eisenberg R., Neumann D., Fuchs S. Phosphorylation of the acetylcholine receptor by protein kinase C and identification of the phosphorylation site within the receptor delta subunit. J Biol Chem. 1987 Aug 5;262(22):10506–10510. [PubMed] [Google Scholar]
- Sakmann B., Patlak J., Neher E. Single acetylcholine-activated channels show burst-kinetics in presence of desensitizing concentrations of agonist. Nature. 1980 Jul 3;286(5768):71–73. doi: 10.1038/286071a0. [DOI] [PubMed] [Google Scholar]
- Sakmann B., Trube G. Voltage-dependent inactivation of inward-rectifying single-channel currents in the guinea-pig heart cell membrane. J Physiol. 1984 Feb;347:659–683. doi: 10.1113/jphysiol.1984.sp015089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler H., Quast U. Functional acetylcholine receptor from Torpedo marmorata in planar membranes. Proc Natl Acad Sci U S A. 1980 May;77(5):3052–3056. doi: 10.1073/pnas.77.5.3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scubon-Mulieri B., Parsons R. L. Desensitization onset and recovery at the potassium-depolarized frog neuromuscular junction are voltage sensitive. J Gen Physiol. 1978 Mar;71(3):285–299. doi: 10.1085/jgp.71.3.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sine S. M., Taylor P. Local anesthetics and histrionicotoxin are allosteric inhibitors of the acetylcholine receptor. Studies of clonal muscle cells. J Biol Chem. 1982 Jul 25;257(14):8106–8104. [PubMed] [Google Scholar]
- Slater N. T., Hall A. F., Carpenter D. O. Kinetic properties of cholinergic desensitization in Aplysia neurons. Proc R Soc Lond B Biol Sci. 1984 Nov 22;223(1230):63–78. doi: 10.1098/rspb.1984.0083. [DOI] [PubMed] [Google Scholar]
- Smith M. M., Merlie J. P., Lawrence J. C., Jr Regulation of phosphorylation of nicotinic acetylcholine receptors in mouse BC3H1 myocytes. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6601–6605. doi: 10.1073/pnas.84.18.6601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumikawa K., Miledi R. Change in desensitization of cat muscle acetylcholine receptor caused by coexpression of Torpedo acetylcholine receptor subunits in Xenopus oocytes. Proc Natl Acad Sci U S A. 1989 Jan;86(1):367–371. doi: 10.1073/pnas.86.1.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagoner P. K., Pallotta B. S. Modulation of acetylcholine receptor desensitization by forskolin is independent of cAMP. Science. 1988 Jun 17;240(4859):1655–1657. doi: 10.1126/science.2454507. [DOI] [PubMed] [Google Scholar]
- Yee G. H., Huganir R. L. Determination of the sites of cAMP-dependent phosphorylation on the nicotinic acetylcholine receptor. J Biol Chem. 1987 Dec 5;262(34):16748–16753. [PubMed] [Google Scholar]


