Abstract
1. Cells in the A laminae of the dorsal lateral geniculate nucleus receive their primary innervation from either the contralateral (A) or ipsilateral (A1) eye. This paper provides evidence concerning the responses they give to visual stimulation of what is commonly regarded as the ineffective or non-dominant eye. It also examines the contribution of the corticofugal input to these responses. 2. Cells were identified and classified according to their responses to stimulation of the dominant eye receptive field. This was then occluded and the corresponding location in the non-dominant eye field stimulated by a moving bar. Out of fifty-seven cells examined forty-three (75%) gave a response to stimulation of the non-dominant eye. There was no obvious difference between the effects on X and Y cells in these experiments. 3. In most cases (thirty-seven) the response involved an inhibition of the resting discharge level, but three cells gave a mixed excitatory and inhibitory response and three a pure excitatory response. All the responses were weak and only revealed by prolonged periods of averaging (20-100 trials). 4. Ionophoretic application of the GABA antagonist N-methyl-bicuculline (NMB) blocked the visually elicited inhibitory effects and in most cases (twenty-seven out of thirty-two tested) revealed an excitatory response. Out of a further eight cells previously unresponsive to the non-dominant eye, NMB application revealed excitatory responses in three. 5. Increasing background discharge levels and cell excitability by ionophoretic application of either acetylcholine or the excitatory amino acid, quisqualate, did not eliminate inhibitory responses and did not reveal excitatory responses. We suggest that the visually driven non-dominant eye suppression of the background discharge involves a GABA-mediated inhibitory input which masks an underlying excitatory input. 6. An excitatory non-dominant eye response could potentially derive from the influence of the corticofugal projection. However, removal of the corticofugal input by aspiration of areas 17 and 18 did not reduce either the excitatory or the inhibitory components of the response. 7. In the absence of corticofugal input all cells tested (fourteen) exhibited a non-dominant eye response and all studied during NMB application (eleven) gave an excitatory response. The primary effect of removing the corticofugal input appeared to involve the loss of a 'damping' influence on the excitatory and inhibitory responses, such that they were more easily revealed. The significance of these findings is discussed.
Full text
PDF



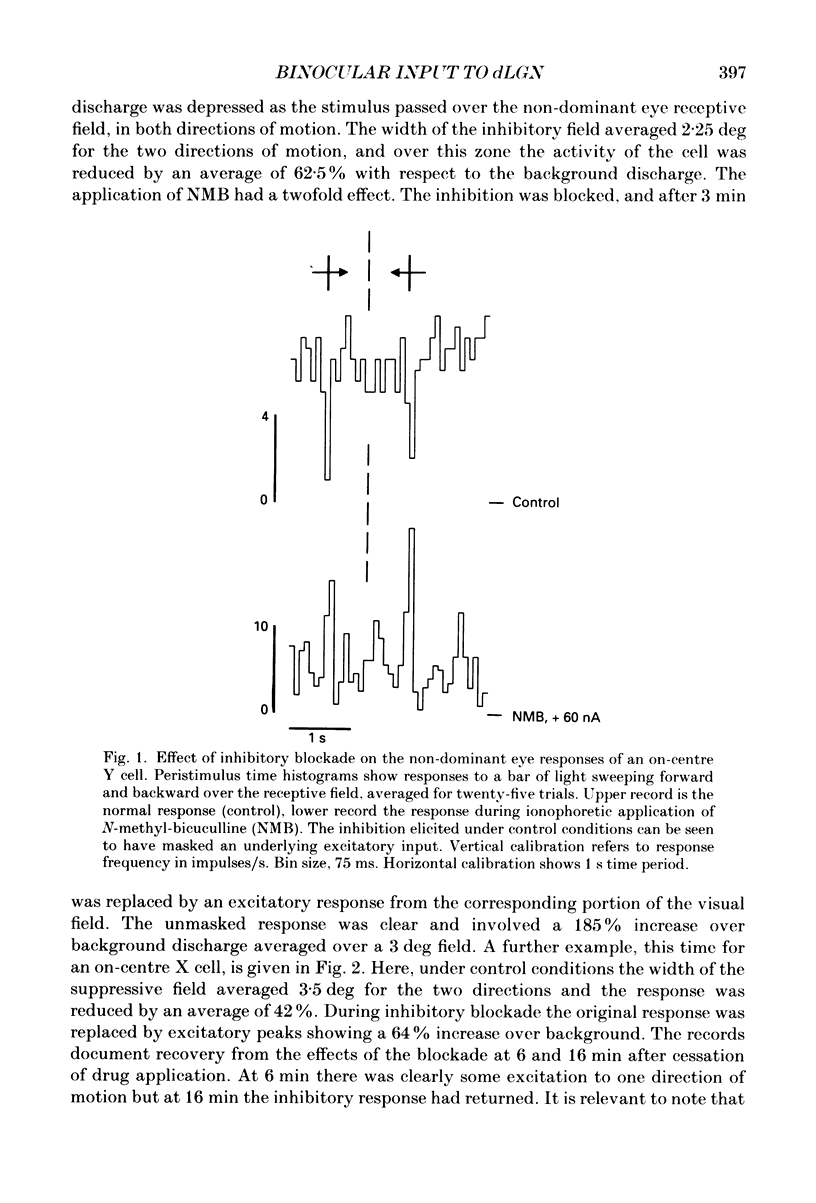
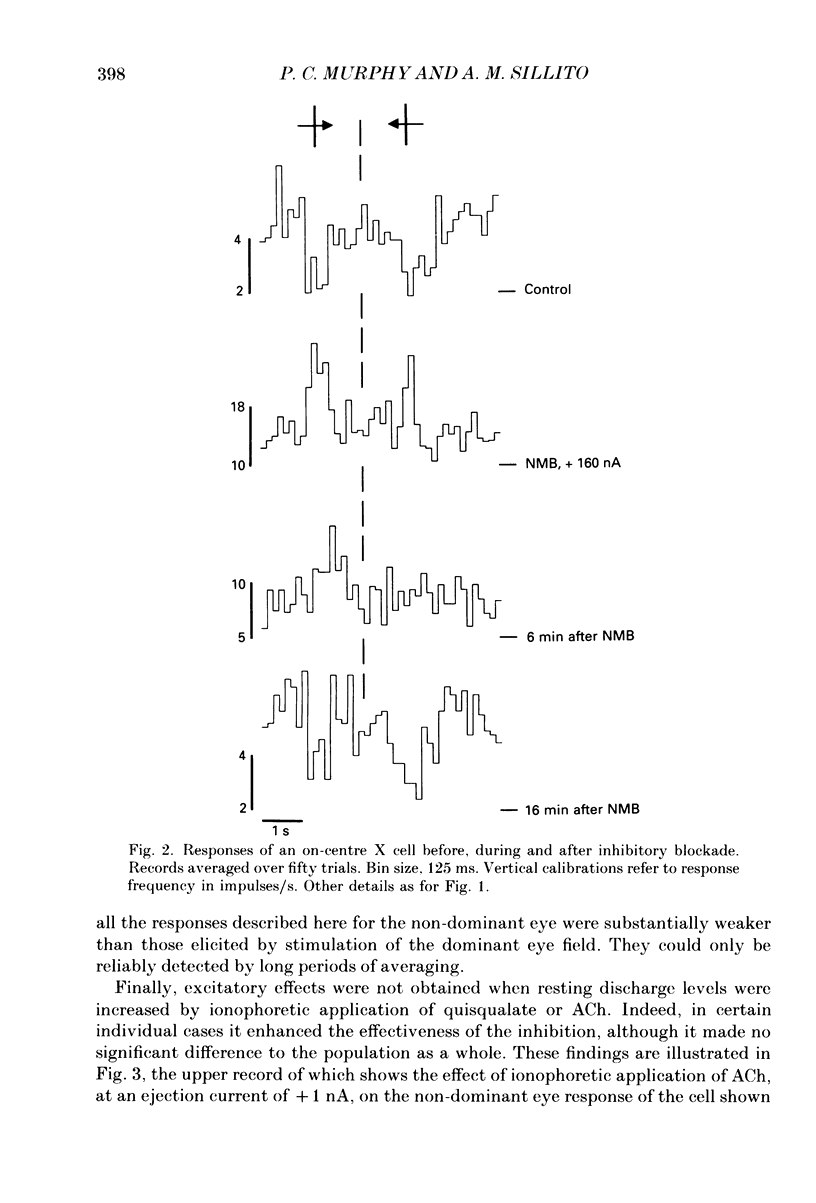
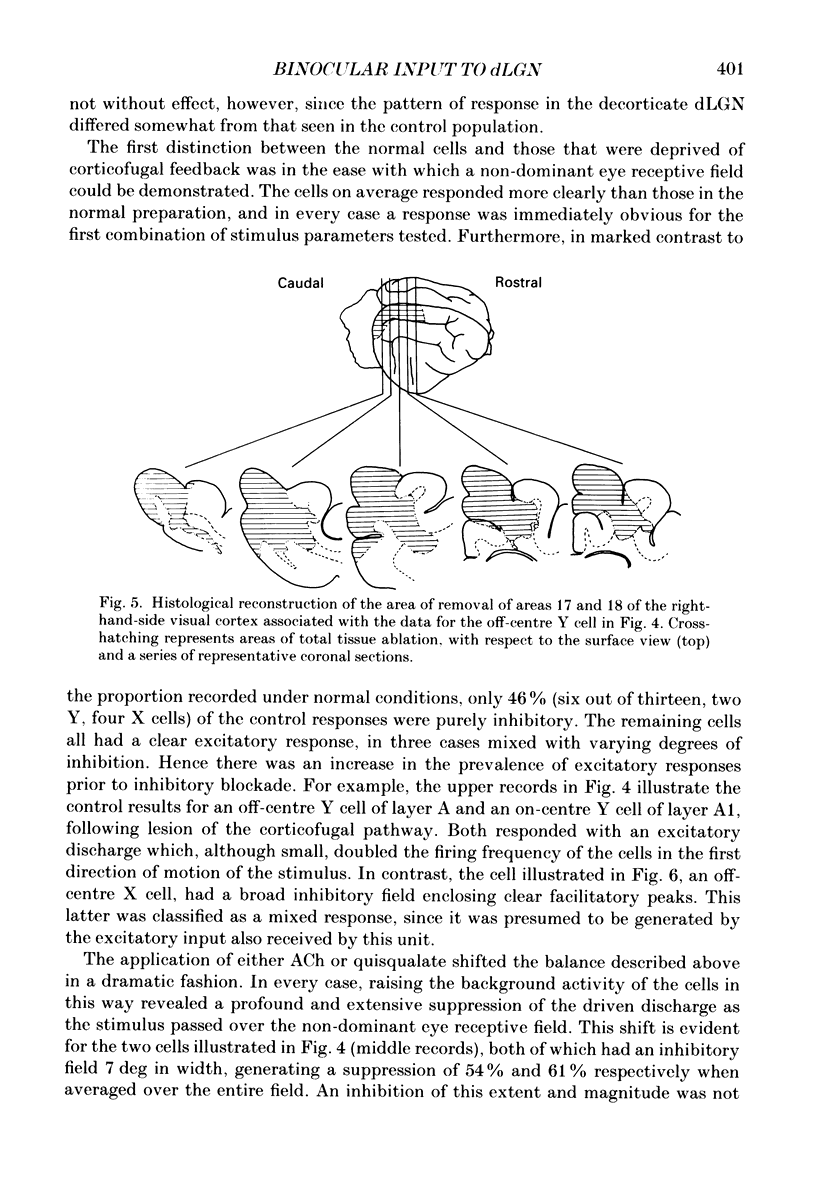
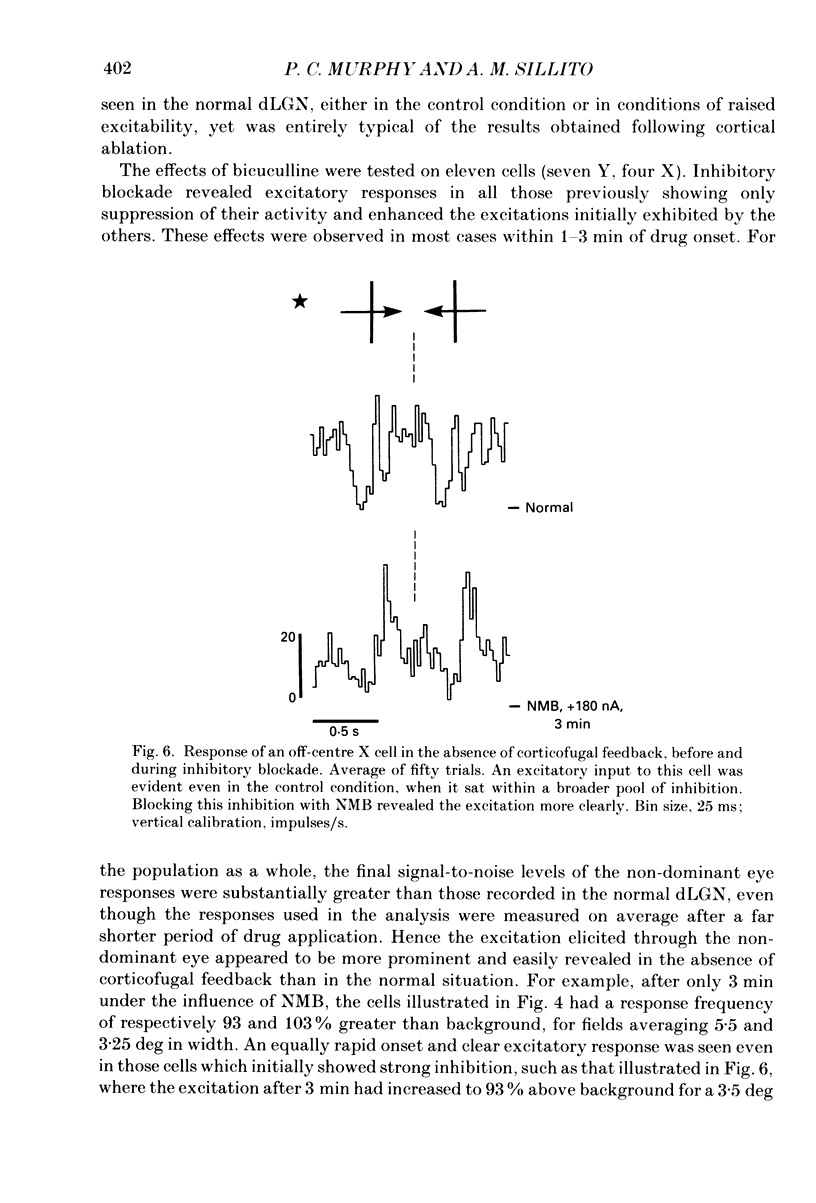
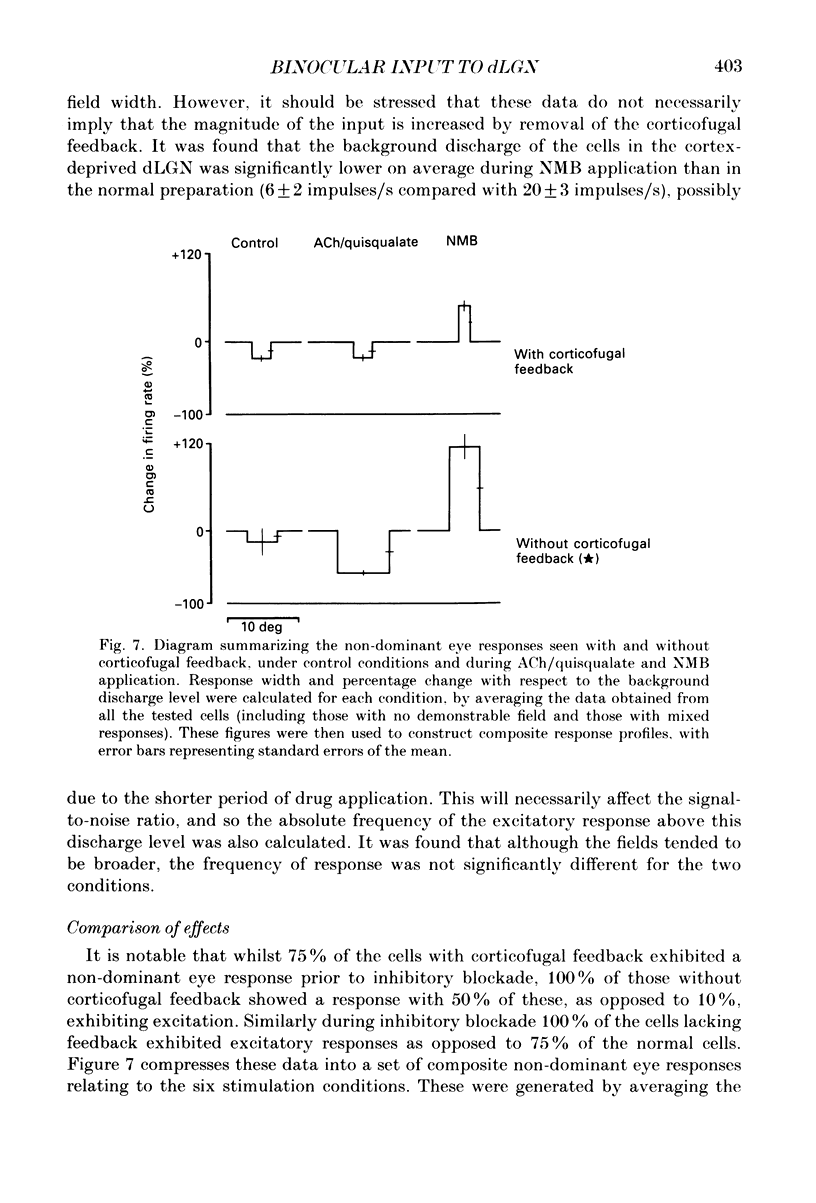





Selected References
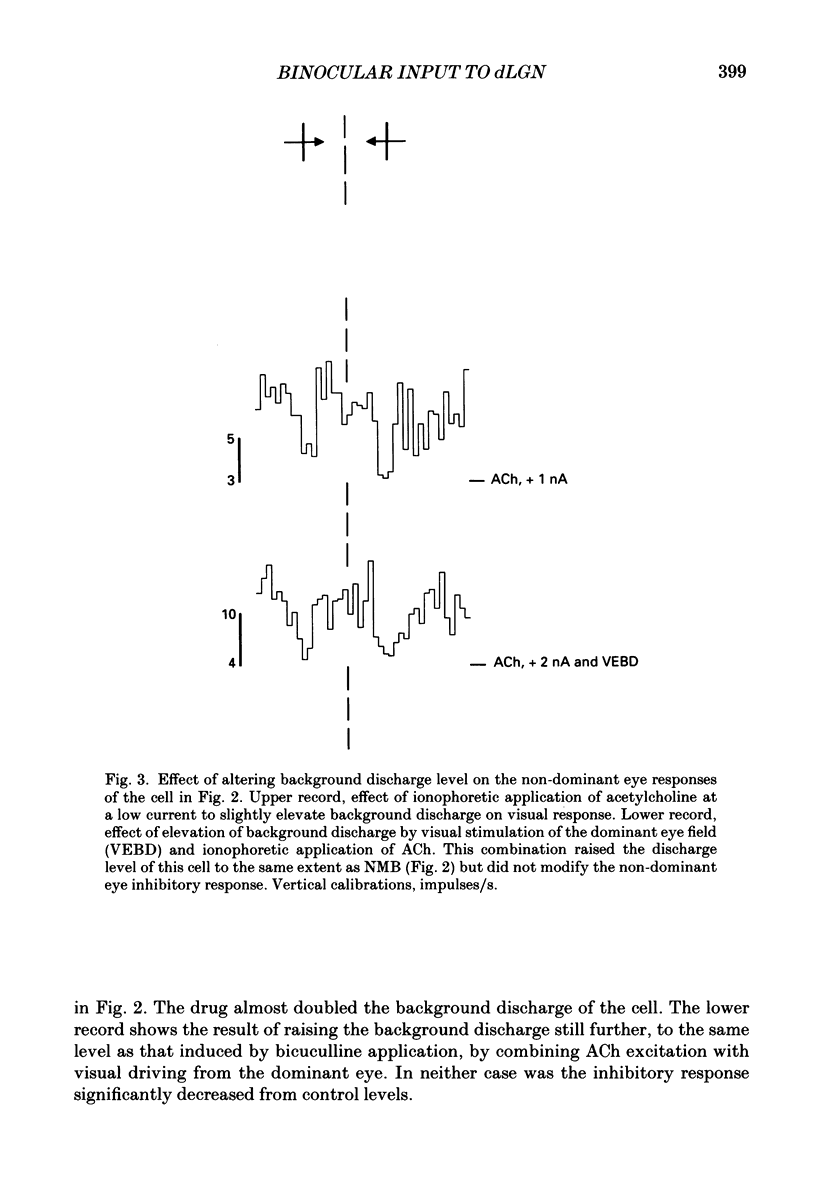
These references are in PubMed. This may not be the complete list of references from this article.
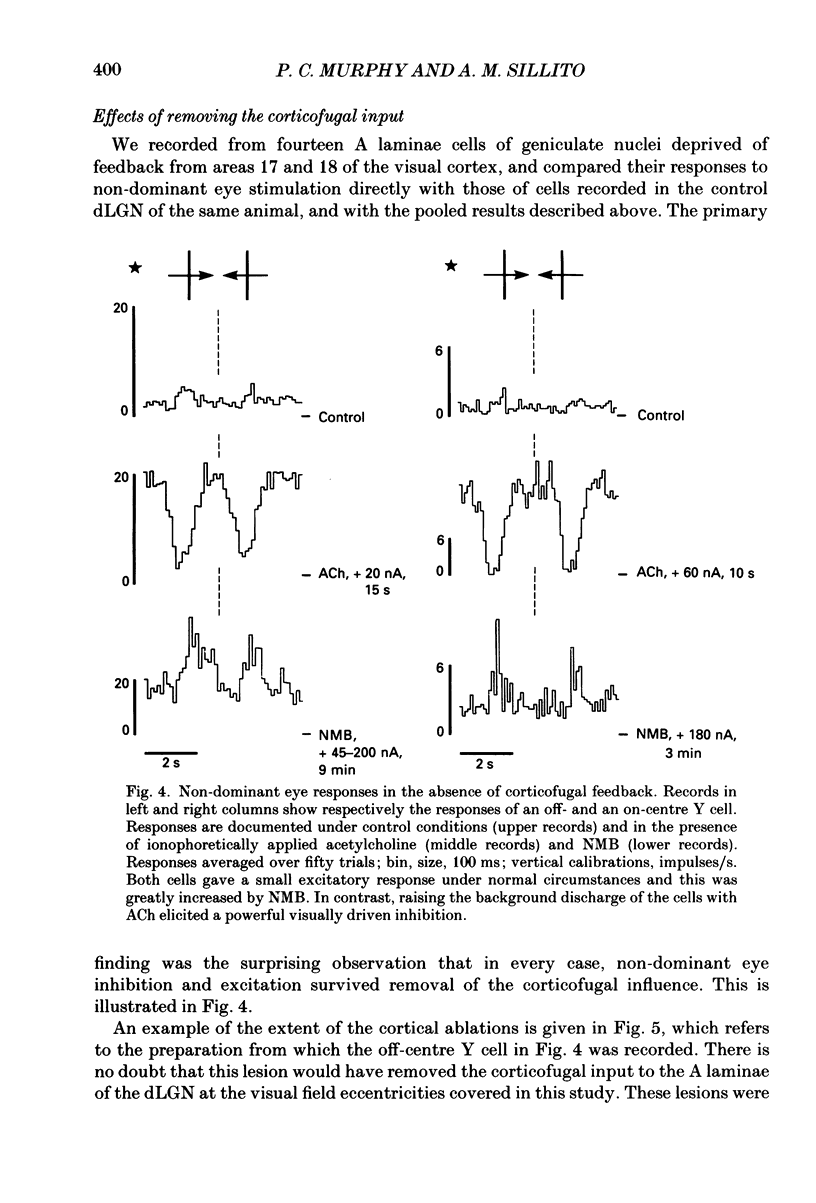
- Ahlsén G., Lindström S., Lo F. S. Interaction between inhibitory pathways to principal cells in the lateral geniculate nucleus of the cat. Exp Brain Res. 1985;58(1):134–143. doi: 10.1007/BF00238961. [DOI] [PubMed] [Google Scholar]
- Bloomfield S. A., Hamos J. E., Sherman S. M. Passive cable properties and morphological correlates of neurones in the lateral geniculate nucleus of the cat. J Physiol. 1987 Feb;383:653–692. doi: 10.1113/jphysiol.1987.sp016435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowling D. B., Michael C. R. Terminal patterns of single, physiologically characterized optic tract fibers in the cat's lateral geniculate nucleus. J Neurosci. 1984 Jan;4(1):198–216. doi: 10.1523/JNEUROSCI.04-01-00198.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyapati J., Henry G. Corticofugal axons in the lateral geniculate nucleus of the cat. Exp Brain Res. 1984;53(2):335–340. doi: 10.1007/BF00238163. [DOI] [PubMed] [Google Scholar]
- Derrington A. M., Fuchs A. F. Spatial and temporal properties of X and Y cells in the cat lateral geniculate nucleus. J Physiol. 1979 Aug;293:347–364. doi: 10.1113/jphysiol.1979.sp012893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enroth-Cugell C., Robson J. G. The contrast sensitivity of retinal ganglion cells of the cat. J Physiol. 1966 Dec;187(3):517–552. doi: 10.1113/jphysiol.1966.sp008107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Famiglietti E. V., Jr, Peters A. The synaptic glomerulus and the intrinsic neuron in the dorsal lateral geniculate nucleus of the cat. J Comp Neurol. 1972 Mar;144(3):285–334. doi: 10.1002/cne.901440304. [DOI] [PubMed] [Google Scholar]
- Friedlander M. J., Lin C. S., Stanford L. R., Sherman S. M. Morphology of functionally identified neurons in lateral geniculate nucleus of the cat. J Neurophysiol. 1981 Jul;46(1):80–129. doi: 10.1152/jn.1981.46.1.80. [DOI] [PubMed] [Google Scholar]
- Gilbert C. D. Laminar differences in receptive field properties of cells in cat primary visual cortex. J Physiol. 1977 Jun;268(2):391–421. doi: 10.1113/jphysiol.1977.sp011863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graybiel A. M., Berson D. M. Autoradiographic evidence for a projection from the pretectal nucleus of the optic tract to the dorsal lateral geniculate complex in the cat. Brain Res. 1980 Aug 11;195(1):1–12. doi: 10.1016/0006-8993(80)90861-6. [DOI] [PubMed] [Google Scholar]
- Guillery R. W. Patterns of fiber degeneration in the dorsal lateral geniculate nucleus of the cat following lesions in the visual cortex. J Comp Neurol. 1967 Jul;130(3):197–221. doi: 10.1002/cne.901300303. [DOI] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. Integrative action in the cat's lateral geniculate body. J Physiol. 1961 Feb;155:385–398. doi: 10.1113/jphysiol.1961.sp006635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamos J. E., Van Horn S. C., Raczkowski D., Sherman S. M. Synaptic circuits involving an individual retinogeniculate axon in the cat. J Comp Neurol. 1987 May 8;259(2):165–192. doi: 10.1002/cne.902590202. [DOI] [PubMed] [Google Scholar]
- Harvey A. R. A physiological analysis of subcortical and commissural projections of areas 17 and 18 of the cat. J Physiol. 1980 May;302:507–534. doi: 10.1113/jphysiol.1980.sp013258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ide L. S. The fine structure of the perigeniculate nucleus in the cat. J Comp Neurol. 1982 Oct 1;210(4):317–334. doi: 10.1002/cne.902100402. [DOI] [PubMed] [Google Scholar]
- Kawamura S., Sprague J. M., Niimi K. Corticofugal projections from the visual cortices to the thalamus, pretectum and superior colliculus in the cat. J Comp Neurol. 1974 Dec 1;158(3):339–362. doi: 10.1002/cne.901580308. [DOI] [PubMed] [Google Scholar]
- Kemp J. A., Sillito A. M. The nature of the excitatory transmitter mediating X and Y cell inputs to the cat dorsal lateral geniculate nucleus. J Physiol. 1982 Feb;323:377–391. doi: 10.1113/jphysiol.1982.sp014078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindström S. Synaptic organization of inhibitory pathways to principal cells in the lateral geniculate nucleus of the cat. Brain Res. 1982 Feb 25;234(2):447–453. doi: 10.1016/0006-8993(82)90885-x. [DOI] [PubMed] [Google Scholar]
- McCormick D. A., Pape H. C. Acetylcholine inhibits identified interneurons in the cat lateral geniculate nucleus. Nature. 1988 Jul 21;334(6179):246–248. doi: 10.1038/334246a0. [DOI] [PubMed] [Google Scholar]
- Montero V. M., Singer W. Ultrastructural identification of somata and neural processes immunoreactive to antibodies against glutamic acid decarboxylase (GAD) in the dorsal lateral geniculate nucleus of the cat. Exp Brain Res. 1985;59(1):151–165. doi: 10.1007/BF00237675. [DOI] [PubMed] [Google Scholar]
- Montero V. M., Singer W. Ultrastructure and synaptic relations of neural elements containing glutamic acid decarboxylase (GAD) in the perigeniculate nucleus of the cat. A light and electron microscopic immunocytochemical study. Exp Brain Res. 1984;56(1):115–125. doi: 10.1007/BF00237447. [DOI] [PubMed] [Google Scholar]
- Murphy P. C., Sillito A. M. Corticofugal feedback influences the generation of length tuning in the visual pathway. Nature. 1987 Oct 22;329(6141):727–729. doi: 10.1038/329727a0. [DOI] [PubMed] [Google Scholar]
- Pape H. C., Eysel U. T. Binocular interactions in the lateral geniculate nucleus of the cat: GABAergic inhibition reduced by dominant afferent activity. Exp Brain Res. 1986;61(2):265–271. doi: 10.1007/BF00239516. [DOI] [PubMed] [Google Scholar]
- Rapisardi S. C., Miles T. P. Synaptology of retinal terminals in the dorsal lateral geniculate nucleus of the cat. J Comp Neurol. 1984 Mar 10;223(4):515–534. doi: 10.1002/cne.902230405. [DOI] [PubMed] [Google Scholar]
- Robson J. A. The morphology of corticofugal axons to the dorsal lateral geniculate nucleus in the cat. J Comp Neurol. 1983 May 1;216(1):89–103. doi: 10.1002/cne.902160108. [DOI] [PubMed] [Google Scholar]
- Sanderson K. J., Bishop P. O., Darian-Smith I. The properties of the binocular receptive fields of lateral geniculate neurons. Exp Brain Res. 1971;13(2):178–207. doi: 10.1007/BF00234085. [DOI] [PubMed] [Google Scholar]
- Schmielau F., Singer W. The role of visual cortex for binocular interactions in the cat lateral geniculate nucleus. Brain Res. 1977 Jan 21;120(2):354–361. doi: 10.1016/0006-8993(77)90914-3. [DOI] [PubMed] [Google Scholar]
- Sillito A. M., Kemp J. A. The influence of GABAergic inhibitory processes on the receptive field structure of X and Y cells in cat dorsal lateral geniculate nucleus (dLGN). Brain Res. 1983 Oct 24;277(1):63–77. doi: 10.1016/0006-8993(83)90908-3. [DOI] [PubMed] [Google Scholar]
- Sur M., Sherman S. M. Retinogeniculate terminations in cats: morphological differences between X and Y cell axons. Science. 1982 Oct 22;218(4570):389–389. doi: 10.1126/science.7123239. [DOI] [PubMed] [Google Scholar]
- Suzuki H., Kato E. Binocular interaction at cat's lateral geniculate body. J Neurophysiol. 1966 Sep;29(5):909–920. doi: 10.1152/jn.1966.29.5.909. [DOI] [PubMed] [Google Scholar]
- Tsumoto T., Suda K. Three groups of cortico-geniculate neurons and their distribution in binocular and monocular segments of cat striate cortex. J Comp Neurol. 1980 Sep 1;193(1):223–236. doi: 10.1002/cne.901930115. [DOI] [PubMed] [Google Scholar]
- Tusa R. J., Palmer L. A., Rosenquist A. C. The retinotopic organization of area 17 (striate cortex) in the cat. J Comp Neurol. 1978 Jan 15;177(2):213–235. doi: 10.1002/cne.901770204. [DOI] [PubMed] [Google Scholar]
- Tusa R. J., Rosenquist A. C., Palmer L. A. Retinotopic organization of areas 18 and 19 in the cat. J Comp Neurol. 1979 Jun 15;185(4):657–678. doi: 10.1002/cne.901850405. [DOI] [PubMed] [Google Scholar]
- Updyke B. V. Projections from visual areas of the middle suprasylvian sulcus onto the lateral posterior complex and adjacent thalamic nuclei in cat. J Comp Neurol. 1981 Oct 1;201(4):477–506. doi: 10.1002/cne.902010403. [DOI] [PubMed] [Google Scholar]
- Updyke B. V. The patterns of projection of cortical areas 17, 18, and 19 onto the laminae of the dorsal lateral geniculate nucleus in the cat. J Comp Neurol. 1975 Oct 15;163(4):377–395. doi: 10.1002/cne.901630402. [DOI] [PubMed] [Google Scholar]
- Varela F. J., Singer W. Neuronal dynamics in the visual corticothalamic pathway revealed through binocular rivalry. Exp Brain Res. 1987;66(1):10–20. doi: 10.1007/BF00236196. [DOI] [PubMed] [Google Scholar]
- Wilson J. R., Friedlander M. J., Sherman S. M. Fine structural morphology of identified X- and Y-cells in the cat's lateral geniculate nucleus. Proc R Soc Lond B Biol Sci. 1984 Jun 22;221(1225):411–436. doi: 10.1098/rspb.1984.0042. [DOI] [PubMed] [Google Scholar]
- Xue J. T., Ramoa A. S., Carney T., Freeman R. D. Binocular interaction in the dorsal lateral geniculate nucleus of the cat. Exp Brain Res. 1987;68(2):305–310. doi: 10.1007/BF00248796. [DOI] [PubMed] [Google Scholar]


