Abstract
1. Single sodium channel currents were recorded from canine ventricular myocytes in cell-attached patches. The relative rates of single-channel activation vs. inactivation as well as the voltage dependence of the rate of open-channel inactivation were studied. 2. Ensemble-averaged sodium currents showed relatively normal activation and inactivation kinetics, although the mid-point of the steady-state inactivation (h infinity) curve was shifted by 20-30 mV in the hyperpolarizing direction. This shift was due to the bath solution, which contained isotonic KCl to depolarize the cell to 0 mV. 3. Steady-state activation showed less of a voltage shift. The threshold for eliciting channel opening was around -70 mV and the mid-point of activation occurred near -50 mV. 4. The decline of the ensemble-averaged sodium current during a maintained depolarization was fitted by a single exponential function characterizing the apparent time constant of inactivation (tau h). The apparent rate of inactivation was voltage dependent, with tau h decreasing e-fold for a 15.4 mV depolarization. 5. The relative contributions of the rates of single-channel activation and inactivation in determining the time course of current decay (tau h) were examined using the approach of Aldrich, Corey & Stevens (1983). Mean channel open time (tau o) showed significant voltage dependence, increasing from 0.5 ms at -70 mV to around 0.8 ms at -40 mV. At -70 mV tau h was much greater than tau o, while at -40 mV the two time constants were similar. 6. The degree to which the kinetics of single-channel activation contribute to tau h was studied using the first latency distribution. The first latency function was fitted by two exponentials. The slow component was voltage dependent, decreasing from 19 ms at -70 mV to 0.5 ms at -40 mV. The fast component (0.1-0.5 ms) was not well resolved. 7. Comparing the first latency distribution with the time course of the ensemble-averaged sodium current at -40 mV showed that activation is nearly complete by the time of peak inward sodium current. However, at -70 mV, activation overlaps significantly with the apparent time course of inactivation of the ensemble-averaged current. 8. Using the methods of Aldrich et al. (1983) we also measured the apparent rate of open-channel closing (a) and open-channel inactivation (b). Both rates were voltage dependent, with a showing an e-fold decrease for an 11 mV depolarization and b showing an e-fold increase for a 30 mV depolarization.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF






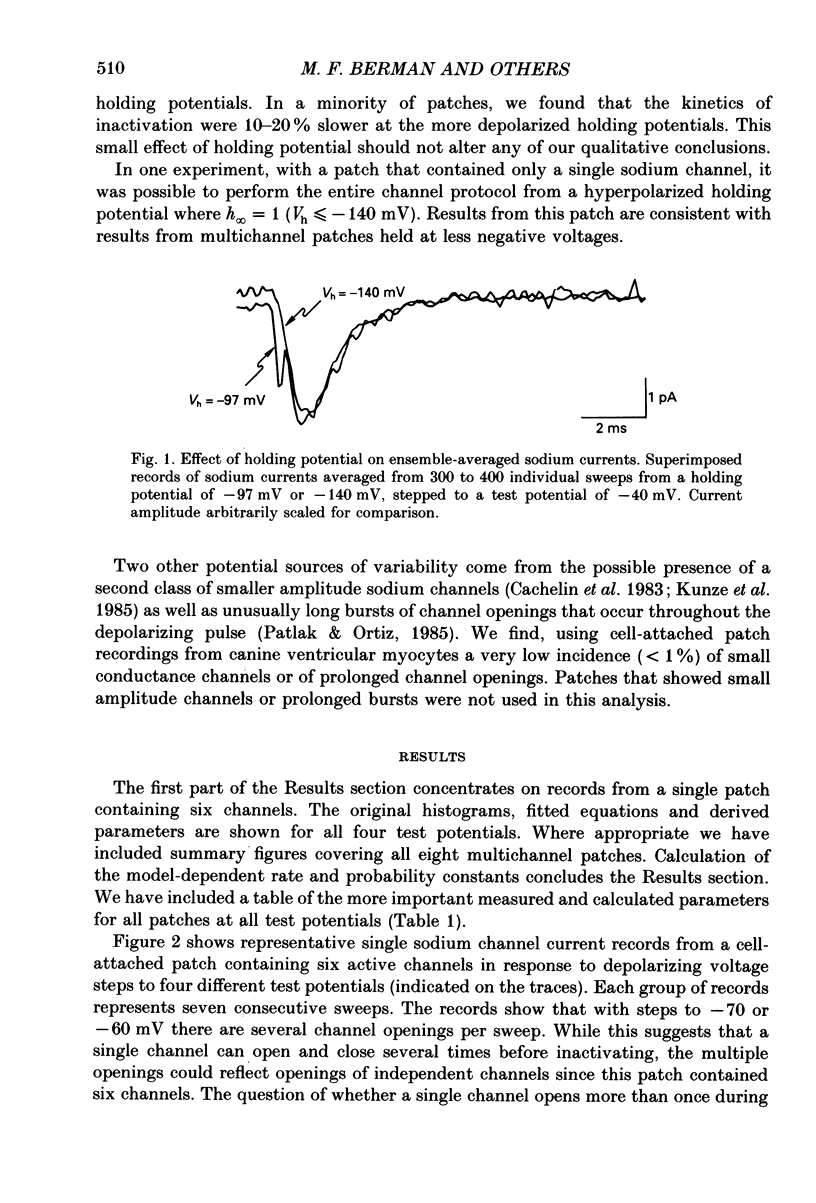
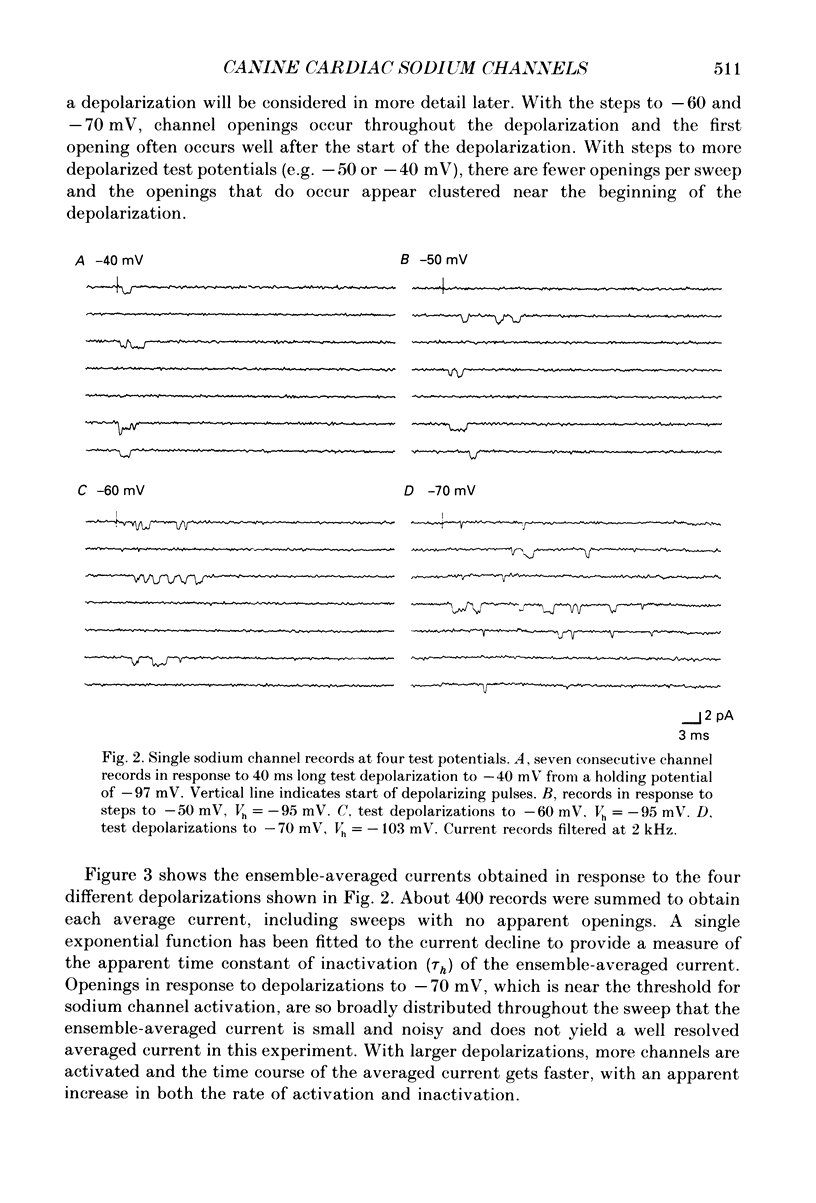
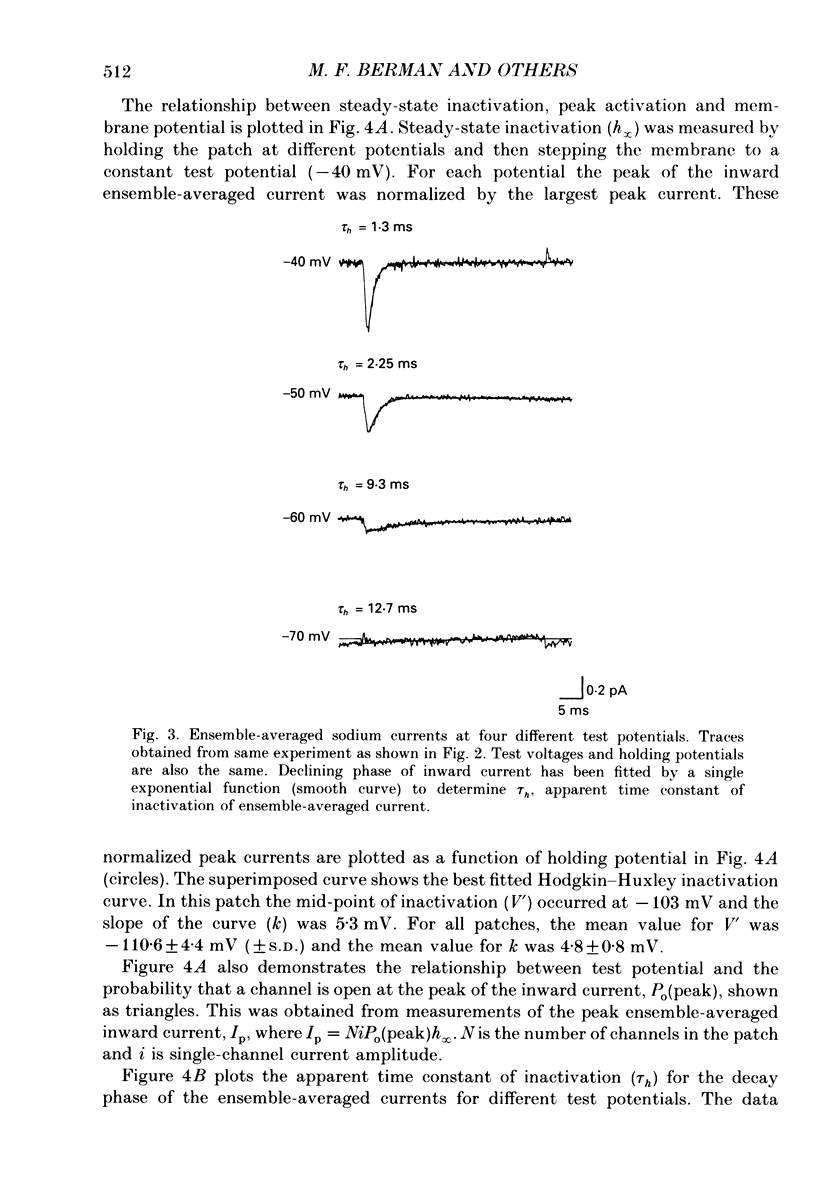
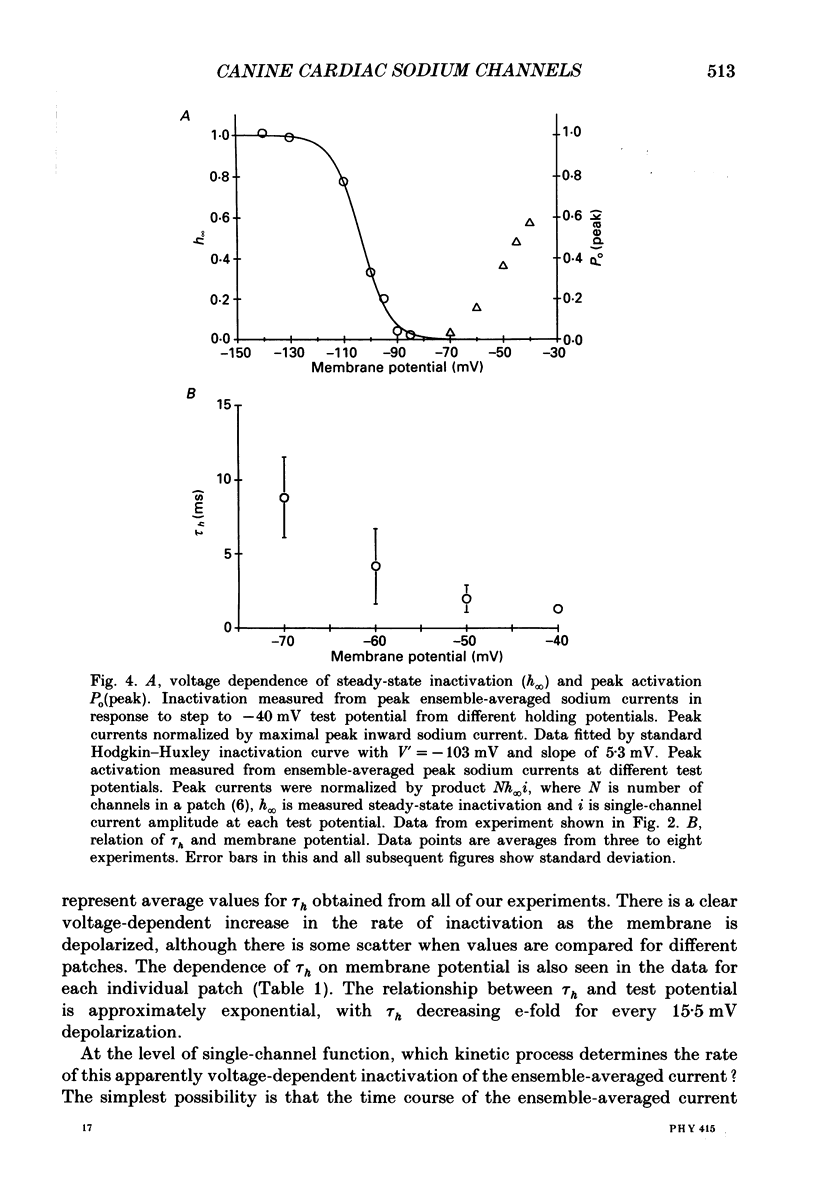
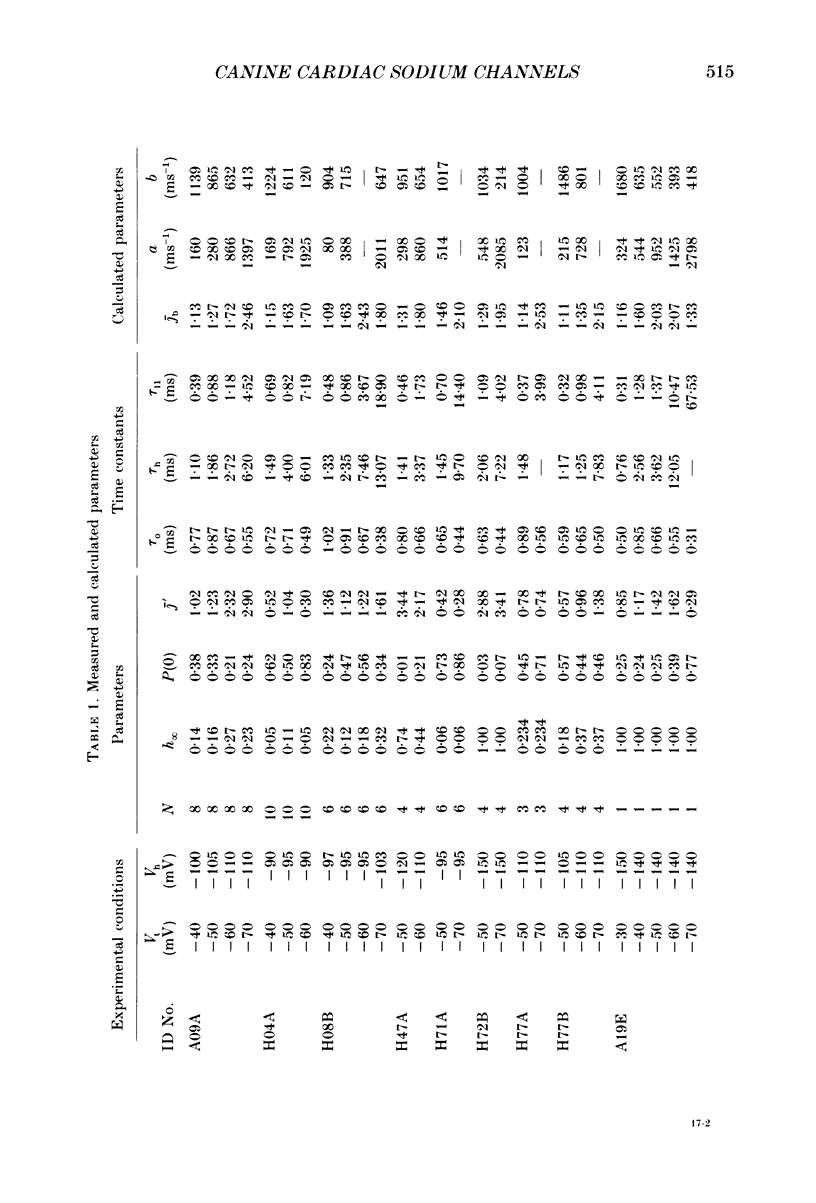










Selected References
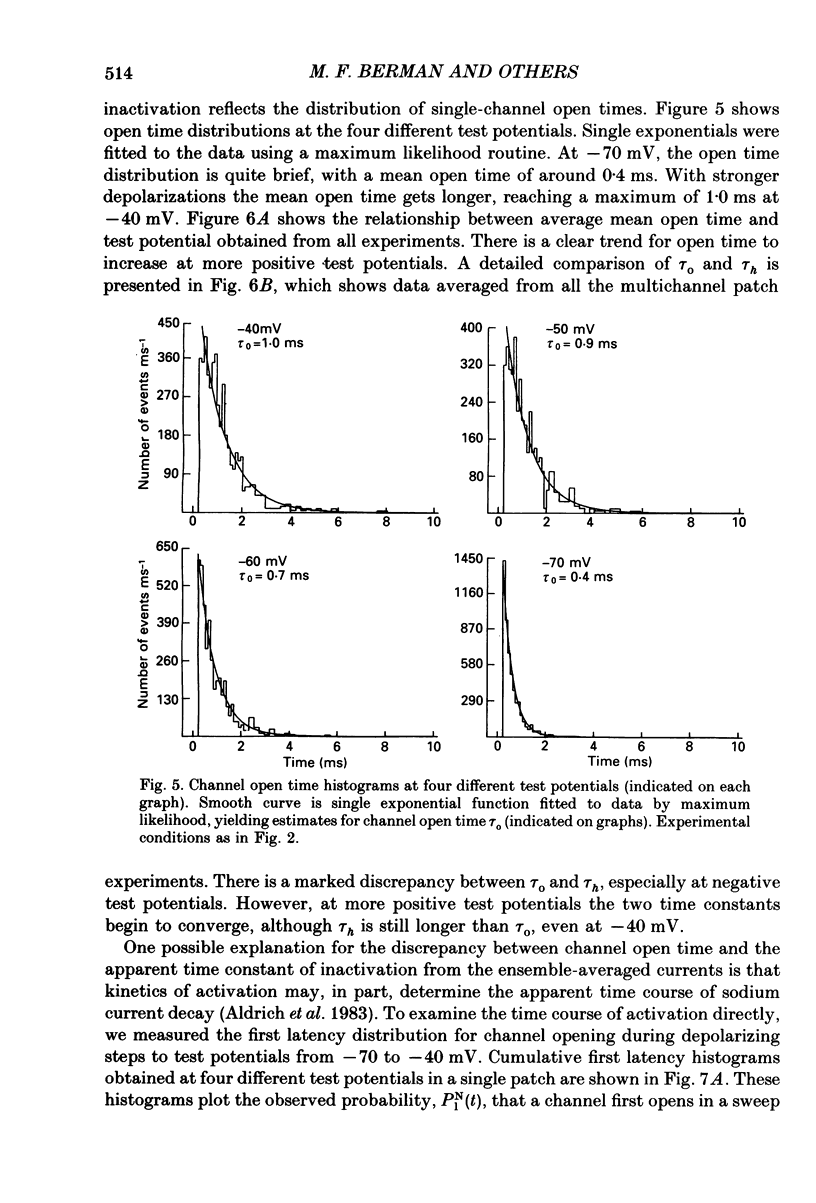
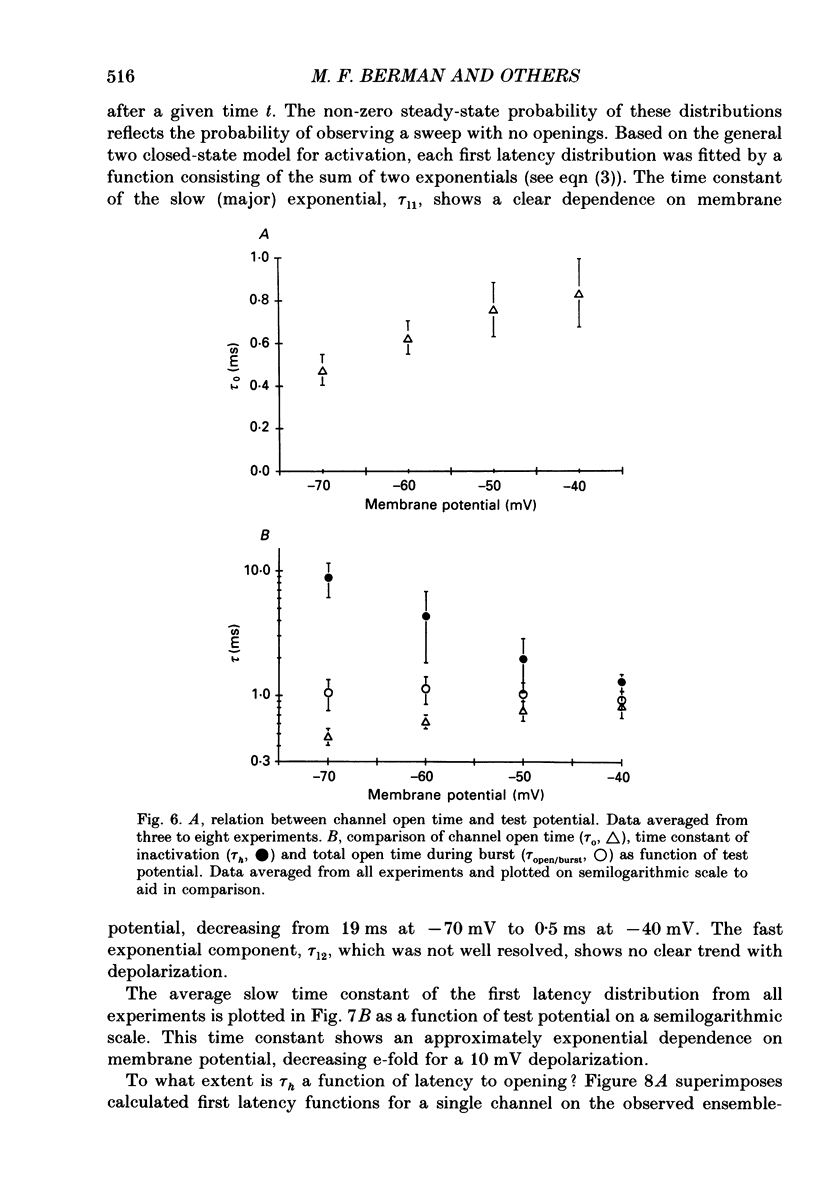
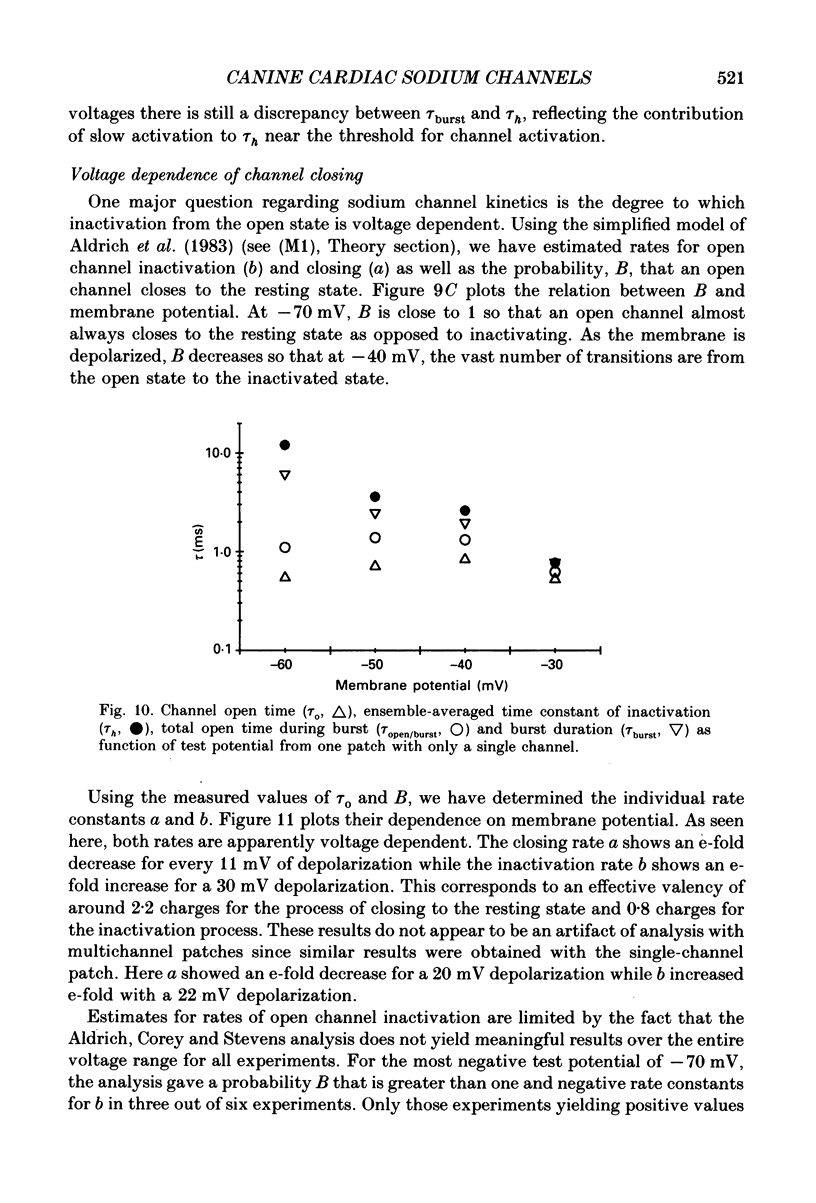
These references are in PubMed. This may not be the complete list of references from this article.
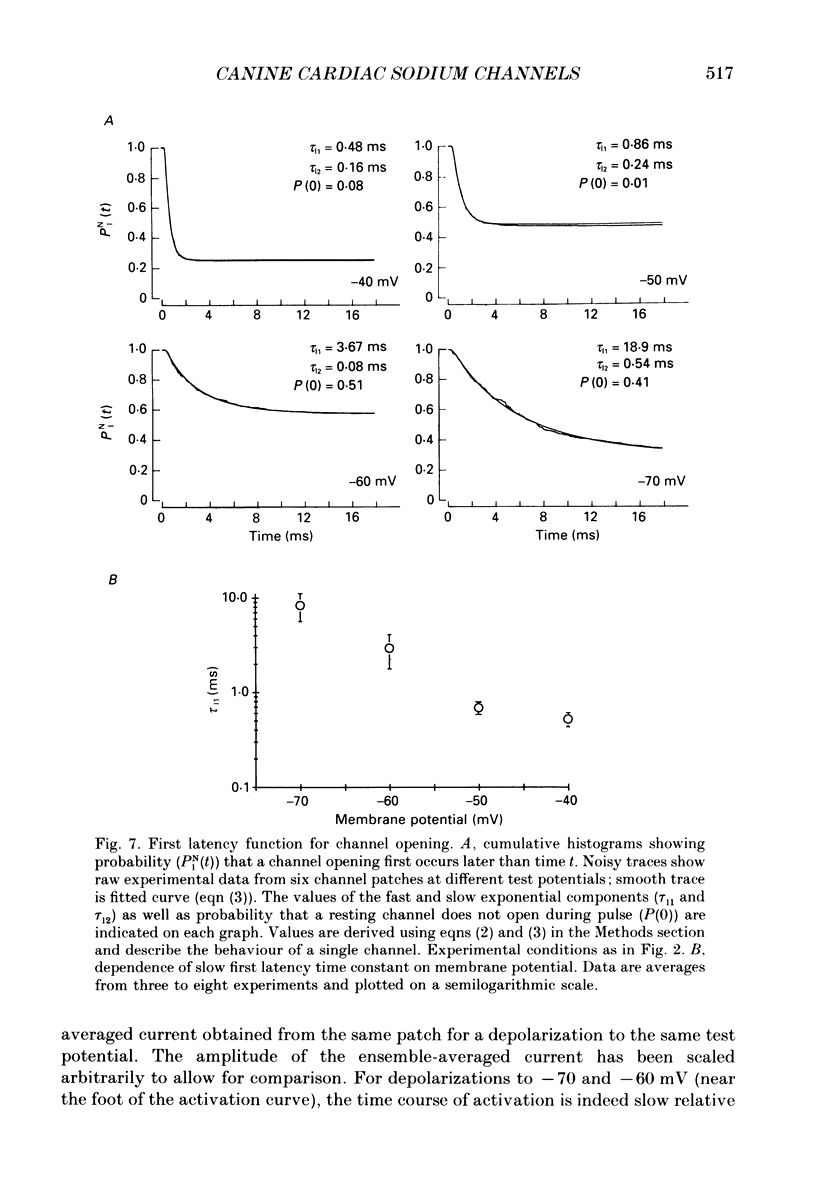
- Aldrich R. W., Corey D. P., Stevens C. F. A reinterpretation of mammalian sodium channel gating based on single channel recording. Nature. 1983 Dec 1;306(5942):436–441. doi: 10.1038/306436a0. [DOI] [PubMed] [Google Scholar]
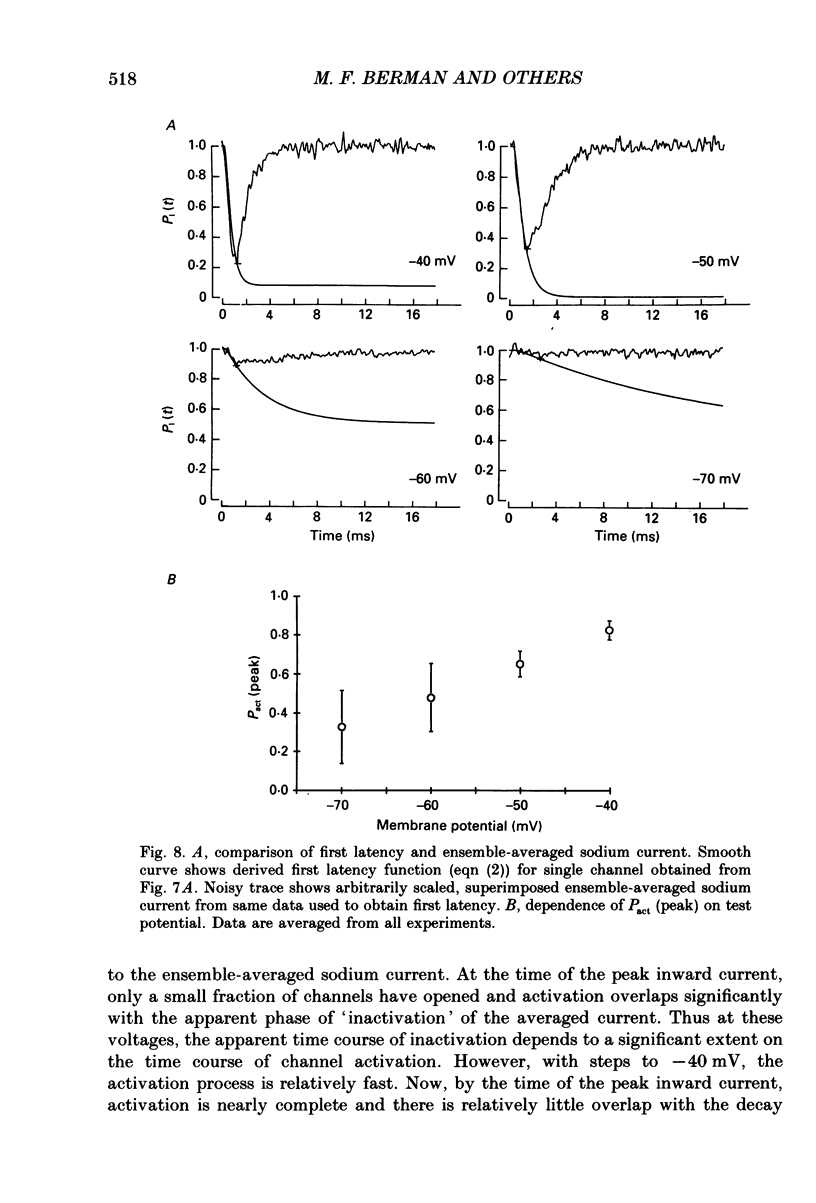
- Aldrich R. W., Stevens C. F. Voltage-dependent gating of single sodium channels from mammalian neuroblastoma cells. J Neurosci. 1987 Feb;7(2):418–431. doi: 10.1523/JNEUROSCI.07-02-00418.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
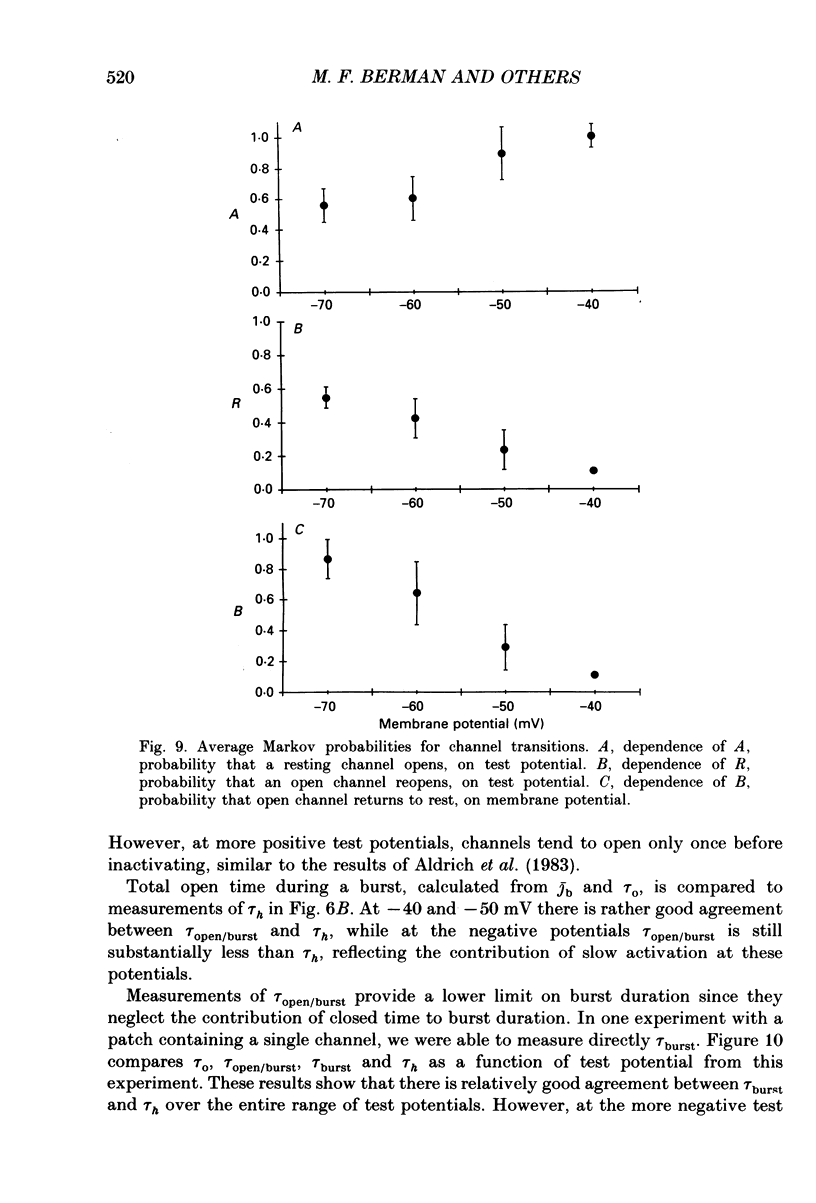
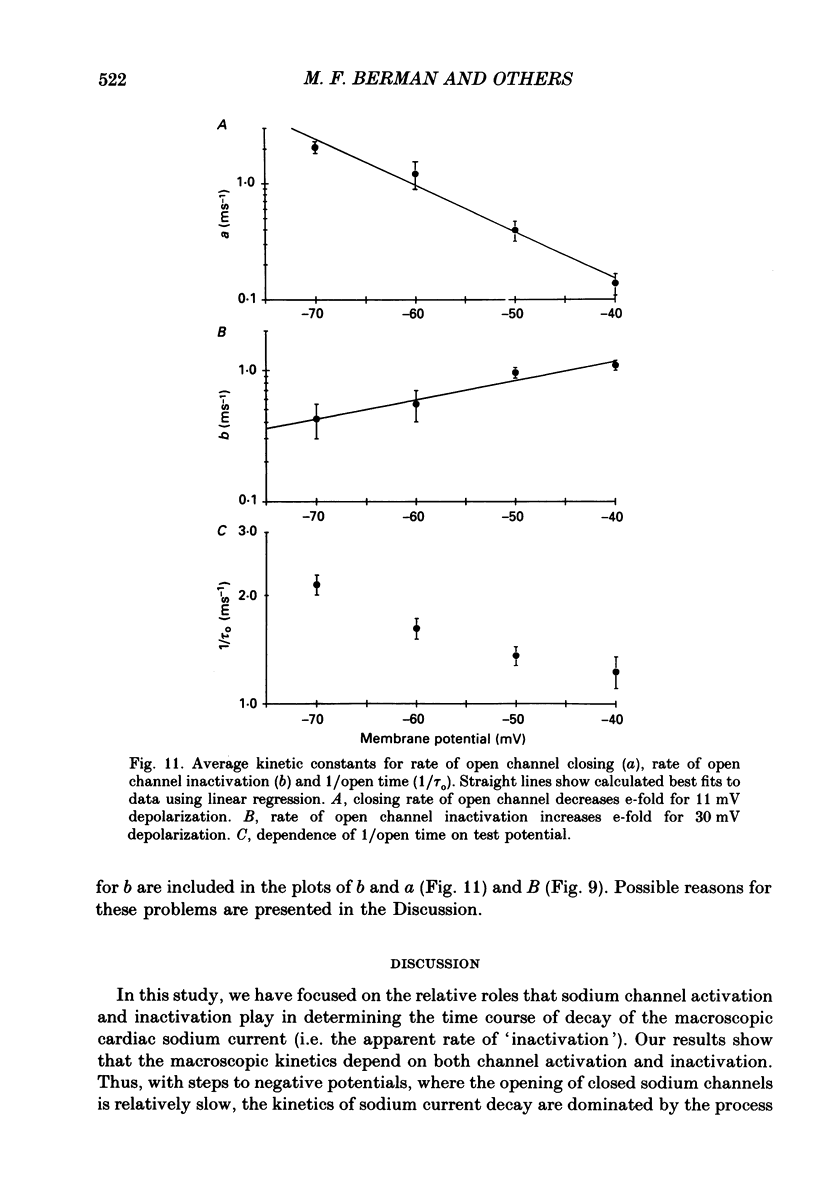
- Armstrong C. M., Bezanilla F. Inactivation of the sodium channel. II. Gating current experiments. J Gen Physiol. 1977 Nov;70(5):567–590. doi: 10.1085/jgp.70.5.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezanilla F., Armstrong C. M. Inactivation of the sodium channel. I. Sodium current experiments. J Gen Physiol. 1977 Nov;70(5):549–566. doi: 10.1085/jgp.70.5.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cachelin A. B., De Peyer J. E., Kokubun S., Reuter H. Sodium channels in cultured cardiac cells. J Physiol. 1983 Jul;340:389–401. doi: 10.1113/jphysiol.1983.sp014768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colatsky T. J. Voltage clamp measurements of sodium channel properties in rabbit cardiac Purkinje fibres. J Physiol. 1980 Aug;305:215–234. doi: 10.1113/jphysiol.1980.sp013359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebihara L., Johnson E. A. Fast sodium current in cardiac muscle. A quantitative description. Biophys J. 1980 Nov;32(2):779–790. doi: 10.1016/S0006-3495(80)85016-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenwick E. M., Marty A., Neher E. Sodium and calcium channels in bovine chromaffin cells. J Physiol. 1982 Oct;331:599–635. doi: 10.1113/jphysiol.1982.sp014394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Follmer C. H., ten Eick R. E., Yeh J. Z. Sodium current kinetics in cat atrial myocytes. J Physiol. 1987 Mar;384:169–197. doi: 10.1113/jphysiol.1987.sp016449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fozzard H. A., Hanck D. A., Makielski J. C., Scanley B. E., Sheets M. F. Sodium channels in cardiac Purkinje cells. Experientia. 1987 Dec 1;43(11-12):1162–1168. doi: 10.1007/BF01945516. [DOI] [PubMed] [Google Scholar]
- Fozzard H. A., January C. T., Makielski J. C. New studies of the excitatory sodium currents in heart muscle. Circ Res. 1985 Apr;56(4):475–485. doi: 10.1161/01.res.56.4.475. [DOI] [PubMed] [Google Scholar]
- Gonoi T., Hille B. Gating of Na channels. Inactivation modifiers discriminate among models. J Gen Physiol. 1987 Feb;89(2):253–274. doi: 10.1085/jgp.89.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant A. O., Starmer C. F. Mechanisms of closure of cardiac sodium channels in rabbit ventricular myocytes: single-channel analysis. Circ Res. 1987 Jun;60(6):897–913. doi: 10.1161/01.res.60.6.897. [DOI] [PubMed] [Google Scholar]
- Grant A. O., Starmer C. F., Strauss H. C. Unitary sodium channels in isolated cardiac myocytes of rabbit. Circ Res. 1983 Dec;53(6):823–829. doi: 10.1161/01.res.53.6.823. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewett K., Legato M. J., Danilo P., Jr, Robinson R. B. Isolated myocytes from adult canine left ventricle: Ca2+ tolerance, electrophysiology, and ultrastructure. Am J Physiol. 1983 Nov;245(5 Pt 1):H830–H839. doi: 10.1152/ajpheart.1983.245.5.H830. [DOI] [PubMed] [Google Scholar]
- Horn R., Vandenberg C. A., Lange K. Statistical analysis of single sodium channels. Effects of N-bromoacetamide. Biophys J. 1984 Jan;45(1):323–335. doi: 10.1016/S0006-3495(84)84158-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn R., Vandenberg C. A. Statistical properties of single sodium channels. J Gen Physiol. 1984 Oct;84(4):505–534. doi: 10.1085/jgp.84.4.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunze D. L., Lacerda A. E., Wilson D. L., Brown A. M. Cardiac Na currents and the inactivating, reopening, and waiting properties of single cardiac Na channels. J Gen Physiol. 1985 Nov;86(5):691–719. doi: 10.1085/jgp.86.5.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makielski J. C., Sheets M. F., Hanck D. A., January C. T., Fozzard H. A. Sodium current in voltage clamped internally perfused canine cardiac Purkinje cells. Biophys J. 1987 Jul;52(1):1–11. doi: 10.1016/S0006-3495(87)83182-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilius B., Benndorf K., Markwardt F. Modified gating behaviour of aconitine treated single sodium channels from adult cardiac myocytes. Pflugers Arch. 1986 Dec;407(6):691–693. doi: 10.1007/BF00582653. [DOI] [PubMed] [Google Scholar]
- Noda M., Ikeda T., Kayano T., Suzuki H., Takeshima H., Kurasaki M., Takahashi H., Numa S. Existence of distinct sodium channel messenger RNAs in rat brain. Nature. 1986 Mar 13;320(6058):188–192. doi: 10.1038/320188a0. [DOI] [PubMed] [Google Scholar]
- Patlak J. B., Ortiz M. Slow currents through single sodium channels of the adult rat heart. J Gen Physiol. 1985 Jul;86(1):89–104. doi: 10.1085/jgp.86.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng G. N., Robinson R. B., Hoffman B. F. Passive properties and membrane currents of canine ventricular myocytes. J Gen Physiol. 1987 Nov;90(5):671–701. doi: 10.1085/jgp.90.5.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg C. A., Horn R. Inactivation viewed through single sodium channels. J Gen Physiol. 1984 Oct;84(4):535–564. doi: 10.1085/jgp.84.4.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatani A., Kirsch G. E., Possani L. D., Brown A. M. Effects of New World scorpion toxins on single-channel and whole cell cardiac sodium currents. Am J Physiol. 1988 Mar;254(3 Pt 2):H443–H451. doi: 10.1152/ajpheart.1988.254.3.H443. [DOI] [PubMed] [Google Scholar]


