Abstract
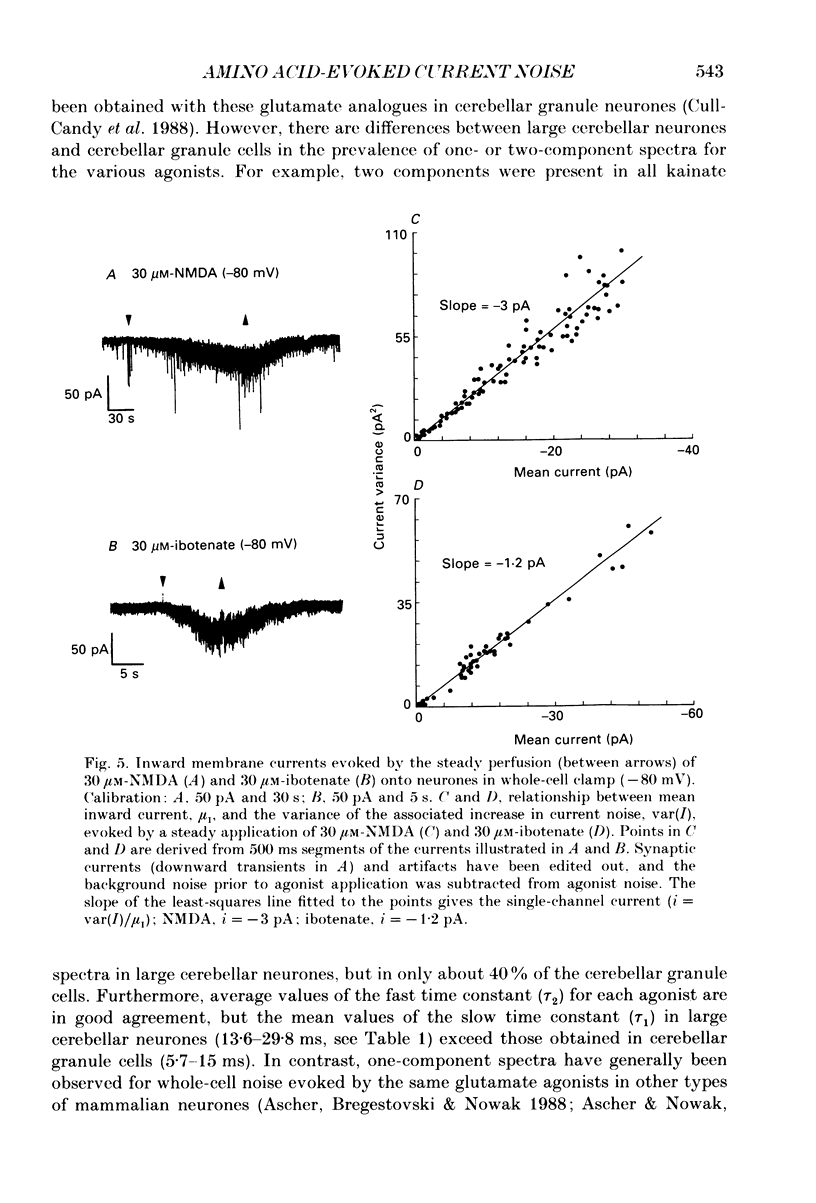
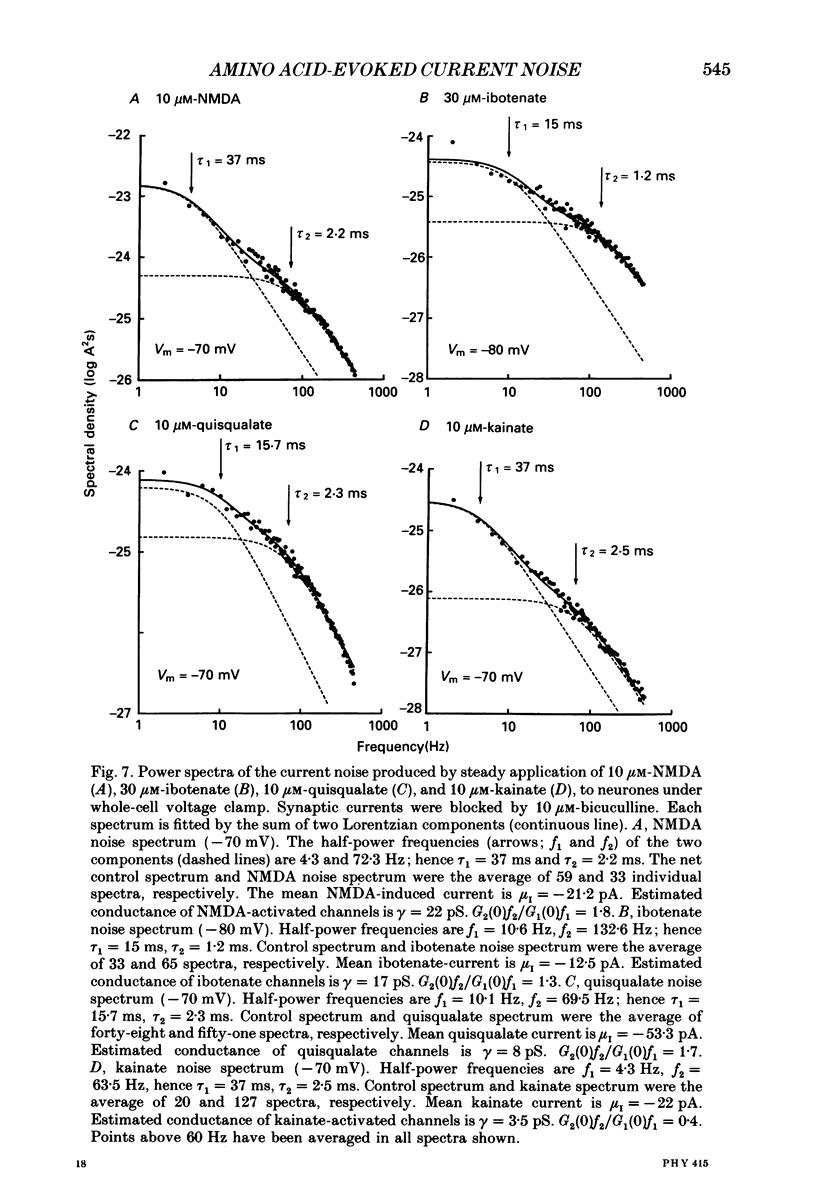
1. Membrane noise and current changes produced by glutamate and related excitatory amino acids have been examined in cultured large cerebellar neurones (including Purkinje cells), with whole-cell patch-clamp methods. The sensitivity of these neurones to the inhibitory amino acids gamma-aminobutyric acid (GABA) and glycine has also been studied. 2. The neurones formed inhibitory synapses in culture, and displayed spontaneous synaptic currents. Reducing the pipette Cl- concentration (i.e. intracellular synaptic currents. Reducing the pipette Cl- concentration (i.e. intracellular concentration) caused a negative shift in their reversal potential, and the currents could be blocked with bicuculline (10 microM), suggesting that they were mediated by GABAA receptors. Spontaneous synaptic activity was also considerably reduced in the presence of 3 microM-tetrodotoxin. 3. Analysis of the increase in whole-cell current noise produced by the application of GABA (3 microM) gave noise spectra that were fitted by two Lorentzian components with slow and fast time constants of 23.6 and 1.9 ms at a membrane potential (Vm) of -110 mV. The mean single-channel conductance estimated from GABA noise was gamma noise = 12 pS. Glycine (10 microM) whole-cell current responses were Cl(-)-mediated and reversibly abolished by 1 microM-strychnine. 4. Bath application of excitatory amino acids gave whole-cell current changes accompanied by an increase in synaptic activity. Postsynaptic responses to the excitatory amino acids were more readily seen after the inhibitory synaptic currents had been abolished by bicuculline. Membrane current changes were obtained in response to the putative transmitters glutamate and aspartate, and the agonists NMDA (N-methyl-D-aspartate), ibotenate, quisqualate and kainate. Their reversal potential was approximately -5 mV. 5. A majority of noise spectra produced by the various glutamate receptor agonists were fitted by two Lorentzian components; the rest were fitted with a single Lorentzian component. The noise time constants were apparently not dependent on the type of glutamate agonist used to activate the receptor channels. Pooling data for all agonists gave a mean time constant for single-component spectra of tau noise = 4.8 +/- 0.3 ms; for two-component spectra the time constants were tau 1 = 22.7 +/- 1.8 ms and tau 2 = 2.2 +/- 0.12 ms (Vm = -110 to -50 mV). It is likely that the two components present in whole-cell noise spectra reflect complex kinetics of glutamate receptor channels. 6. The mean single-channel conductance was estimated from whole-cell noise for the various excitatory amino acids.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF


















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. R., Cull-Candy S. G., Miledi R. Glutamate and quisqualate noise in voltage-clamped locust muscle fibres. Nature. 1976 May 13;261(5556):151–153. doi: 10.1038/261151a0. [DOI] [PubMed] [Google Scholar]
- Anderson C. R., Stevens C. F. Voltage clamp analysis of acetylcholine produced end-plate current fluctuations at frog neuromuscular junction. J Physiol. 1973 Dec;235(3):655–691. doi: 10.1113/jphysiol.1973.sp010410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascher P., Bregestovski P., Nowak L. N-methyl-D-aspartate-activated channels of mouse central neurones in magnesium-free solutions. J Physiol. 1988 May;399:207–226. doi: 10.1113/jphysiol.1988.sp017076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascher P., Nowak L. Quisqualate- and kainate-activated channels in mouse central neurones in culture. J Physiol. 1988 May;399:227–245. doi: 10.1113/jphysiol.1988.sp017077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker J. L., McBurney R. N., MacDonald J. F. Fluctuation analysis of neutral amino acid responses in cultured mouse spinal neurones. J Physiol. 1982 Jan;322:365–387. doi: 10.1113/jphysiol.1982.sp014042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank N. K., Seil F. J., Herndon R. M. An ultrastructural study of cortical remodeling in cytosine arabinoside induced granuloprival cerebellum in tissue culture. Neuroscience. 1982 Jun;7(6):1509–1531. doi: 10.1016/0306-4522(82)90261-5. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Dreyer F., Sheridan R. E. The actions of tubocurarine at the frog neuromuscular junction. J Physiol. 1979 Aug;293:247–284. doi: 10.1113/jphysiol.1979.sp012888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crepel F., Dhanjal S. S., Sears T. A. Effect of glutamate, aspartate and related derivatives on cerebellar purkinje cell dendrites in the rat: an in vitro study. J Physiol. 1982 Aug;329:297–317. doi: 10.1113/jphysiol.1982.sp014304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cull-Candy S. G., Howe J. R., Ogden D. C. Noise and single channels activated by excitatory amino acids in rat cerebellar granule neurones. J Physiol. 1988 Jun;400:189–222. doi: 10.1113/jphysiol.1988.sp017117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cull-Candy S. G., Miledi R., Parker I. Single glutamate-activated channels recorded from locust muscle fibres with perfused patch-clamp electrodes. J Physiol. 1981 Dec;321:195–210. doi: 10.1113/jphysiol.1981.sp013979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cull-Candy S. G., Ogden D. C. Ion channels activated by L-glutamate and GABA in cultured cerebellar neurons of the rat. Proc R Soc Lond B Biol Sci. 1985 May 22;224(1236):367–373. doi: 10.1098/rspb.1985.0038. [DOI] [PubMed] [Google Scholar]
- Cull-Candy S. G., Usowicz M. M. Glutamate and aspartate activated channels and inhibitory synaptic currents in large cerebellar neurons grown in culture. Brain Res. 1987 Jan 27;402(1):182–187. doi: 10.1016/0006-8993(87)91065-1. [DOI] [PubMed] [Google Scholar]
- Cull-Candy S. G., Usowicz M. M. Multiple-conductance channels activated by excitatory amino acids in cerebellar neurons. Nature. 1987 Feb 5;325(6104):525–528. doi: 10.1038/325525a0. [DOI] [PubMed] [Google Scholar]
- Cull-Candy S. G., Usowicz M. M. On the multiple-conductance single channels activated by excitatory amino acids in large cerebellar neurones of the rat. J Physiol. 1989 Aug;415:555–582. doi: 10.1113/jphysiol.1989.sp017736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis D. R., Johnston G. A. Amino acid transmitters in the mammalian central nervous system. Ergeb Physiol. 1974;69(0):97–188. doi: 10.1007/3-540-06498-2_3. [DOI] [PubMed] [Google Scholar]
- Dupont J. L., Fournier E., Gardette R., Crepel F. Effect of excitatory amino acids on Purkinje cell dendrites in cerebellar slices from normal and staggerer mice. Neuroscience. 1984 Jun;12(2):613–619. doi: 10.1016/0306-4522(84)90076-9. [DOI] [PubMed] [Google Scholar]
- Dupont J. L., Gardette R., Crepel F. Postnatal development of the chemosensitivity of rat cerebellar Purkinje cells to excitatory amino acids. An in vitro study. Brain Res. 1987 Jul;431(1):59–68. doi: 10.1016/0165-3806(87)90195-7. [DOI] [PubMed] [Google Scholar]
- Gallo V., Ciotti M. T., Coletti A., Aloisi F., Levi G. Selective release of glutamate from cerebellar granule cells differentiating in culture. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7919–7923. doi: 10.1073/pnas.79.24.7919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardette R., Crepel F. Chemoresponsiveness of intracellular nuclei neurones to L-aspartate, L-glutamate and related derivatives in rat cerebellar slices maintained in vitro. Neuroscience. 1986 May;18(1):93–103. doi: 10.1016/0306-4522(86)90181-8. [DOI] [PubMed] [Google Scholar]
- Gardner P., Ogden D. C., Colquhoun D. Conductances of single ion channels opened by nicotinic agonists are indistinguishable. Nature. 1984 May 10;309(5964):160–162. doi: 10.1038/309160a0. [DOI] [PubMed] [Google Scholar]
- Garthwaite G., Yamini B., Jr, Garthwaite J. Selective loss of Purkinje and granule cell responsiveness to N-methyl-D-aspartate in rat cerebellum during development. Brain Res. 1987 Dec 1;433(2):288–292. doi: 10.1016/0165-3806(87)90034-4. [DOI] [PubMed] [Google Scholar]
- Gruol D. L. Cultured cerebellar neurons: endogenous and exogenous components of Purkinje cell activity and membrane response to putative transmitters. Brain Res. 1983 Mar 21;263(2):223–241. doi: 10.1016/0006-8993(83)90315-3. [DOI] [PubMed] [Google Scholar]
- Gruol D. L., Franklin C. L. Morphological and physiological differentiation of Purkinje neurons in cultures of rat cerebellum. J Neurosci. 1987 May;7(5):1271–1293. doi: 10.1523/JNEUROSCI.07-05-01271.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hendelman W. J., Jande S. S., Lawson D. E. Calcium-binding protein immunocytochemistry in organotypic cultures of cerebellum. Brain Res Bull. 1984 Jul;13(1):181–184. doi: 10.1016/0361-9230(84)90019-4. [DOI] [PubMed] [Google Scholar]
- Howe J. R., Colquhoun D., Cull-Candy S. G. On the kinetics of large-conductance glutamate-receptor ion channels in rat cerebellar granule neurons. Proc R Soc Lond B Biol Sci. 1988 May 23;233(1273):407–422. doi: 10.1098/rspb.1988.0030. [DOI] [PubMed] [Google Scholar]
- Ishida A. T., Neyton J. Quisqualate and L-glutamate inhibit retinal horizontal-cell responses to kainate. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1837–1841. doi: 10.1073/pnas.82.6.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahr C. E., Stevens C. F. Glutamate activates multiple single channel conductances in hippocampal neurons. Nature. 1987 Feb 5;325(6104):522–525. doi: 10.1038/325522a0. [DOI] [PubMed] [Google Scholar]
- Johnson J. W., Ascher P. Glycine potentiates the NMDA response in cultured mouse brain neurons. Nature. 1987 Feb 5;325(6104):529–531. doi: 10.1038/325529a0. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. Tetrodotoxin and neuromuscular transmission. Proc R Soc Lond B Biol Sci. 1967 Jan 31;167(1006):8–22. doi: 10.1098/rspb.1967.0010. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. The statistical nature of the acetycholine potential and its molecular components. J Physiol. 1972 Aug;224(3):665–699. doi: 10.1113/jphysiol.1972.sp009918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura H., Okamoto K., Sakai Y. Pharmacological evidence for L-aspartate as the neurotransmitter of cerebellar climbing fibres in the guinea-pig. J Physiol. 1985 Aug;365:103–119. doi: 10.1113/jphysiol.1985.sp015761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Sugimori M. Electrophysiological properties of in vitro Purkinje cell somata in mammalian cerebellar slices. J Physiol. 1980 Aug;305:171–195. doi: 10.1113/jphysiol.1980.sp013357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathers D. A., Barker J. L. Chemically induced ion channels in nerve cell membranes. Int Rev Neurobiol. 1982;23:1–34. doi: 10.1016/s0074-7742(08)60620-0. [DOI] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L. The physiology of excitatory amino acids in the vertebrate central nervous system. Prog Neurobiol. 1987;28(3):197–276. doi: 10.1016/0301-0082(87)90011-6. [DOI] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L., Vyklický L., Jr Sites of antagonist action on N-methyl-D-aspartic acid receptors studied using fluctuation analysis and a rapid perfusion technique. J Neurophysiol. 1988 Aug;60(2):645–663. doi: 10.1152/jn.1988.60.2.645. [DOI] [PubMed] [Google Scholar]
- Moonen G., Neale E. A., Macdonald R. L., Gibbs W., Nelson P. G. Cerebellar macroneurons in microexplant cell culture. Methodology, basic electrophysiology, and morphology after horseradish peroxidase injection. Brain Res. 1982 Sep;281(1):59–73. doi: 10.1016/0165-3806(82)90113-4. [DOI] [PubMed] [Google Scholar]
- Nowak L., Bregestovski P., Ascher P., Herbet A., Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984 Feb 2;307(5950):462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- Sandoval M. E., Cotman C. W. Evaluation of glutamate as a neurotransmitter of cerebellar parallel fibers. Neuroscience. 1978;3(2):199–206. doi: 10.1016/0306-4522(78)90101-x. [DOI] [PubMed] [Google Scholar]
- Segal M., Barker J. L. Rat hippocampal neurons in culture: voltage-clamp analysis of inhibitory synaptic connections. J Neurophysiol. 1984 Sep;52(3):469–487. doi: 10.1152/jn.1984.52.3.469. [DOI] [PubMed] [Google Scholar]
- Somogyi P., Halasy K., Somogyi J., Storm-Mathisen J., Ottersen O. P. Quantification of immunogold labelling reveals enrichment of glutamate in mossy and parallel fibre terminals in cat cerebellum. Neuroscience. 1986 Dec;19(4):1045–1050. doi: 10.1016/0306-4522(86)90121-1. [DOI] [PubMed] [Google Scholar]
- Wiklund L., Toggenburger G., Cuénod M. Aspartate: possible neurotransmitter in cerebellar climbing fibers. Science. 1982 Apr 2;216(4541):78–80. doi: 10.1126/science.6121375. [DOI] [PubMed] [Google Scholar]


