Abstract
Adeno-associated virus (AAV) vectors have emerged as a promising tool in the development of gene therapies for various neurological diseases, including Alzheimer’s disease and Parkinson’s disease. However, the blood-brain barrier (BBB) poses a significant challenge to successfully delivering AAV vectors to the brain. Strategies that can overcome the BBB to improve the AAV delivery efficiency to the brain are essential to successful brain-targeted gene therapy. This review provides an overview of existing strategies employed for AAV delivery to the brain, including direct intraparenchymal injection, intra-cerebral spinal fluid injection, intranasal delivery, and intravenous injection of BBB-permeable AAVs. Focused ultrasound has emerged as a promising technology for the noninvasive and spatially targeted delivery of AAV administered by intravenous injection. This review also summarizes each strategy’s current preclinical and clinical applications in treating neurological diseases. Moreover, this review includes a detailed discussion of the recent advances in the emerging focused ultrasound-mediated AAV delivery. Understanding the state-of-the-art of these gene delivery approaches is critical for future technology development to fulfill the great promise of AAV in neurological disease treatment.
Keywords: Intraparenchymal injection, intra-cerebral spinal fluid injection, intranasal delivery, blood-brain barrier permeable AAV, intravenous injection, focused ultrasound, neurological diseases, gene therapy
Graphical Abstract
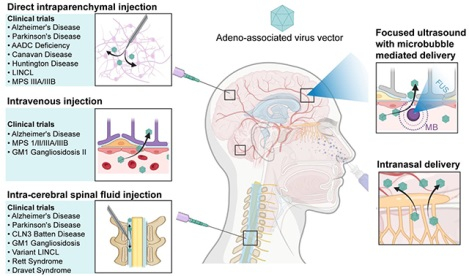
1. Introduction
Neurological diseases are the leading cause of physical and cognitive disability across the globe, currently affecting approximately 15% of the worldwide population [1]. These conditions, ranging from neurodegenerative diseases like Alzheimer’s and Parkinson’s disease to neurodevelopmental disorders like Rett syndrome, Fragile X syndrome, and Lysosome storage diseases, lead to a wide spectrum of physical and cognitive disabilities. Conventional pharmacological methods have been mainly unsuccessful in curing or managing these diseases, as effective pharmaceutical solutions either do not exist or fall short of halting disease progression. Gene therapy is a promising approach to treating diseases that have proven untreatable through conventional pharmacological strategies [2]. Gene therapy uses genetic constructs to introduce healthy and functional genes into targeted cells for gene replacement, modification, augmentation, and inactivation. This either corrects or compensates for the faulty, mutated, or damaged genes, or it nullifies the effect of a dysfunctional gene, potentially providing an innovative approach to treat challenging central nervous system (CNS) diseases.
Genetic constructs can be classified into two main categories: non-viral and viral vectors. Non-viral vectors, such as plasmid DNA and plasmid-containing nanoparticles, offer the advantage of reduced immunogenicity; however, the resulting expression is typically transient and unstable, thus requiring repeated administration [3,4]. In contrast, viral vectors, including herpes simplex virus, lentivirus, adenovirus, and adeno-associated virus (AAV), provide high transfection efficiency with relative long-term gene expression but may present safety concerns. Both adenovirus and herpes simplex viruses are pathogenic in humans and can infect human cells, replicate their genetic material, and disrupt normal cellular functions, leading to various symptoms and diseases. Lentivirus, an RNA retrovirus, integrates its genetic payload into the host genome, carrying the risk of causing insertional mutagenesis [5]. Among these, AAV has emerged as the most successful gene construct due to its high efficiency and stability in gene transduction and low immunogenicity and toxicity [6–8].
AAV vectors have two main components: the capsid, whose structure varies among AAV serotypes and determines what tissue and cell to target (tropism), and a genome containing a promoter and a transgene. AAVs used in gene therapy are all recombinant AAVs, which are AAVs with the genome encoding the viral protein replaced by the promoter and therapeutic gene. The size of the recombinant AAVs is around 20–26 nm with a packaging capacity of 4.7–6 kb linear single-stranded DNA genomes (Figure 1) [9,10]. Recombinant AAVs share the same properties as wild-type AAVs, enabling them to traverse the cell membrane and deliver genes into the nucleus of a cell. However, the transgenes carried by recombinant AAVs cannot integrate into the host genome due to the lack of the Rep gene, which is essential for site-specific integration into the host cell chromosome [11]. Instead, they form double-stranded circular concatemers that persist as extrachromosomal elements in the nucleus of transduced cells [12].
Figure. 1.
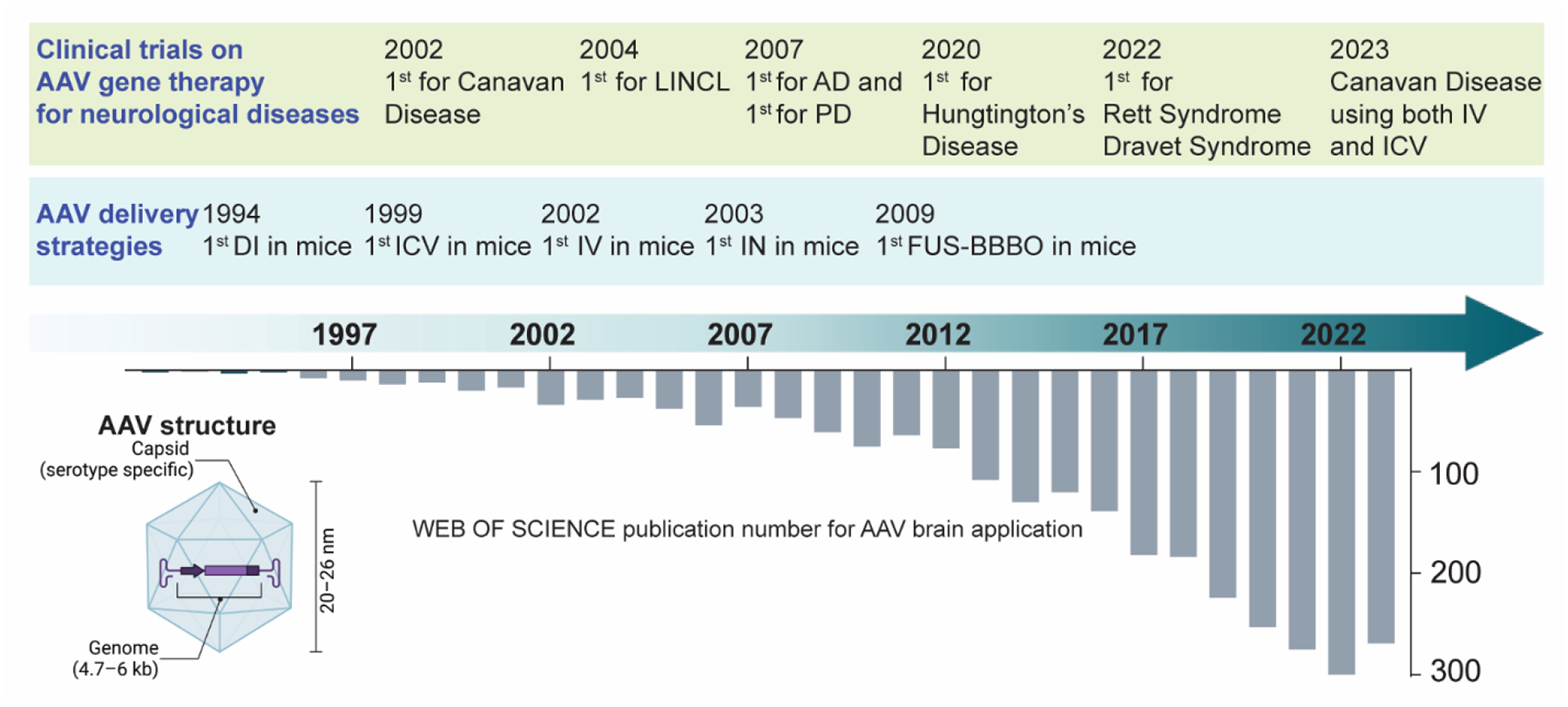
Schematic diagram to show the history and development of AAV delivery to the brain. The bottom graph displays the annual number of publications from 1992, with key milestones in preclinical and clinical studies highlighted at the top. LINCL, late infantile neuronal ceroid lipofuscinosis, is one type of lysosomal storage disease. AD: Alzheimer’s disease. PD: Parkinson’s disease. IV: intravenous injection. ICV: intraventricular delivery. DI: direct intraparenchymal injection. IN: intranasal delivery. FUS-BBBO: focused ultrasound with microbubble-mediated blood-brain barrier opening.
AAVs have been used in over 300 human trials, covering thousands of patients [13]. The field of AAV-mediated gene therapy gained significant momentum following the European Medicines Agency’s 2012 approval of Glybera®, the first AAV gene therapy product, for treating hereditary lipoprotein lipase deficiency [14]. In the subsequent decade, four additional AAV therapies received approval. These include Luxturna™, approved by the United States Food and Drug Administration for a rare form of inherited retinal dystrophy [15]; Zolgensma® (AVXS-101), approved for spinal muscular atrophy [16]; Hemgenix®, approved for adult hemophilia B [17]; and Strimvelis, greenlighted by the European Medicines Agency for severe combined immunodeficiency due to adenosine deaminase deficiency [18]. All these AAV products have been clinically approved for the treatment of diseases outside the brain, while no AAV products have been approved for treating brain diseases.
Sufficient and safe delivery of AAV to the brain is widely recognized as the key to successful AAV gene therapy in treating brain diseases. However, a unique brain barrier called the blood-brain barrier (BBB) hinders the adequate delivery of AAV vectors to the brain through systemic delivery. The BBB is a distinct vascular structure in the brain, composed of endothelial cells, astrocyte end feet, and pericytes embedded in the basement membrane [19]. It is a physiological, cellular, and metabolic barrier that restricts the free movement of substances between the blood and the extracellular fluid of the brain. As a result, the BBB prevents most pharmaceuticals, including 98% of small-molecule drugs and 100% of large-molecule drugs administered through systemic circulation, from reaching the brain [20–22]. Likewise, it poses significant challenges for AAV delivery to the brain.
Several strategies have been developed to overcome the BBB for AAV delivery to the brain, including bypassing the BBB [e.g., direct intraparenchyma injection (DI), intra-cerebrospinal fluid (intra-CSF) injection and intranasal (IN) delivery], disrupting the BBB [e.g., focused ultrasound with microbubble-induced BBB opening (FUS-BBBO)], or designing BBB-permeable AAV through intravenous (IV) injection. Our review examines these strategies employed for AAV delivery to the brain, including DI, intra-CSF, IV, IN delivery, and FUS-BBBO. For intra-CSF delivery, we will specifically discuss intracerebroventricular (ICV), intrathecal (IT), and intra-cisterna magna (ICM) injections. We describe each delivery procedure and compare the advantages and limitations of each approach. Furthermore, we explore each delivery strategy’s preclinical and clinical applications for treating various brain diseases. Figure 1 summarizes the annual number of publications on AAV delivery to the brain, with critical milestones highlighted.
2. Existing AAV delivery strategies to the brain
This session will introduce the following AAV brain delivery strategies: DI, intra-CSF, IV, and IN. Table 1 summarizes the advantages and disadvantages of these existing strategies.
Table 1.
Comparison of advantages and disadvantages of different AAV brain delivery strategies.
| Strategy | Advantages | Disadvantages |
|---|---|---|
| DI |
|
|
| Intra-CSF |
|
|
| IV |
|
|
| IN |
|
|
| FUS-BBBO |
|
|
2.1. Direct intraparenchymal injection (DI)
DI is the most used AAV delivery method in preclinical and clinical studies for treating brain diseases. This approach involves removing the scalp and drilling a burr hole in the designated location on the skull. Accurately identifying injection sites is paramount in DI injection, usually accomplished via a stereotactic head frame or magnetic resonance imaging guidance [23,24]. Convection-enhanced delivery can be employed to expand the diffusion volume of the DI-injected AAV [25,26]. Convection-enhanced delivery generates a pressure gradient using a pump at the infusion catheter’s tip [26]. Recent advancements in the development of real-time magnetic resonance imaging-guided convection-enhanced delivery achieved enhanced coverage of the targeted brain region, enabling personalized administration attuned to individual patient anatomy and facilitating adjustments to the administration rate and dosage based on real-time assessments of intraparenchymal distribution. This image-guided delivery method offers a notable advantage over traditional stereotactic techniques, setting a path for future gene delivery using DI injection [27,28]. In addition to convection-enhanced delivery, co-injecting heparin or mannitol with AAV can also enlarge the spread pattern of AAV distribution by chemically enhancing BBB permeability [29–31].
DI allows for precise targeting and delivery of AAV to diseased brain regions. It offers the dual advantages of high delivery efficiency to targeted areas and limited systemic distribution to peripheral organs, potentially minimizing the risk of systemic immune responses or adverse effects on peripheral organs and healthy brain regions. Furthermore, the delivery dosage needed is relatively low since the AAV is directly administered to the targeted area.
However, DI does present significant drawbacks. Firstly, it involves an invasive surgical procedure, carrying risks of viral or bacterial infection, hemorrhage, and edema. A clinical study on Parkinson’s disease patients (NCT00400634) reported severe side effects associated with this surgical procedure, raising safety concerns regarding hemorrhaging and pathogen contamination [32]. Infusion failure or inaccurate catheter tip placement could also compromise study results [33]. Apart from these surgical risks, there is also a significant chance for DI delivery of AAV to result in neurotoxicity and cause substantial neuronal loss at the infusion site if the dose of administration is too high [34]. Secondly, DI injections can only influence a limited range of brain regions. While it can be a viable option for diseases with localized pathology, such as Parkinson’s disease, it shows clear limitations for treating brain diseases that affect multiple brain regions, like Alzheimer’s disease (AD) and Lysosomal storage diseases (LSDs). Although DI of AAV can be achieved via multiple site injections, this requires drilling of multiple burr holes for AAV to be deposited at separate locations; however, more than ten separate injections raised serious safety concerns in clinical studies [35,36]. Therefore, while DI of AAV is actively utilized in clinical trials, its clinical translation remains challenging due to both the inherent risks of the surgical intervention and, crucially, the restricted spread of transgene expression.
2.2. Intra-cerebral spinal fluid injection (intra-CSF)
Injecting the AAV into the CSF compartment is another effective brain delivery strategy. The CSF delivery is performed mainly through three approaches: (1) intracerebroventricular injection (ICV), (2) intrathecal injection (IT), and (3) intra-cisterna magna injection (ICM).
ICV refers to direct administration into ventricles in the brain, predominantly used in preclinical studies. In preclinical studies, it is worth pointing out that the effectiveness of this method varies depending on the subject’s age. In neonatal animals (newborns), the ICV method is particularly effective in distributing the AAV throughout the brain because the ependymal barrier between the CSF and the brain tissue is not yet fully developed. This allows the AAV to permeate the barrier and spread widely across different brain regions [37–40]. When applied to adult animals, the AAV primarily targets ependymal cells. In adult animals, the ependymal barrier is mature, which limits AAV penetration through it. The AAV primarily affects the ependymal cells lining the ventricles in these cases. Despite this limitation, the affected ependymal cells can act as “biological pumps,” producing and releasing high amounts of the proteins (e.g., specifically enzymes) into the surrounding brain tissue and CSF [41]. These proteins could potentially correct specific pathological or functional abnormalities [42]. However, the quick turnover rates of the ependymal cells limited the long-term therapeutic effectiveness of this strategy [43,44].
IT administration delivers AAV via injection into the spinal canal through the lumbar puncture, specifically at the base of the spinal cord. This process begins with cleaning the skin over the lumbar region, followed by a mid-sagittal incision through the skin to expose the muscle and spine. A needle or catheter is delicately inserted between the lumbar vertebrae, usually between L4 and L5. Once the needle or catheter is accurately positioned, the AAV solution is gradually injected into the CSF. For repeated administrations, an intrathecal catheter can be surgically implanted in the spinal cord. The volume of the injected solution typically ranges from a few microliters to a few milliliters. For larger injection amounts, an osmotic pump can be employed. This pump can be implanted beneath the skin and removed upon completion of the injection. Studies have shown that using an osmotic pump for IT delivery can reduce systemic leakage into peripheral organs, notably the liver and heart tissue, compared with bolus injection [45,46].
ICM delivery is a technique like IT administration, with the primary distinction being the injection site: the cisterna magna (CM). The CM is a subarachnoid space below the cerebellum and behind the medulla oblongata, between the base of the brain and the top of the spinal cord. It is primarily filled with CSF. The procedure for ICM delivery requires a stereotactic frame for accurate targeting and mechanical manipulator to immobilize the needle. The head of the patient (or animal subject) is secured in a prone position, and the back of the neck is sanitized. A needle attached to a syringe is manually guided into the cisterna magna, with correct placement verified by aspiration of a small volume of CSF into the syringe. This syringe is then secured to a micromanipulator. A small volume of CSF is again aspirated following vector infusion to ensure the needle’s continued placement in the CM [47]. Recently, the adoption of magnetic resonance (MR) contrast imaging during CSF injection has been reported. This technology mixes MR contrast agents with the injection solution and performs contrast-enhanced MR to monitor agent distribution [48,49]. Adjustments to the infusion rate and volume can be made in reference to the subject’s body weight to mitigate potential adverse effects.
All three intra-CSF injection approaches use the CSF pathway for delivery; however, they produce varying gene transduction effects within the brain. IT injection, while considered safer and less invasive than ICV and ICM methods, often leads to lower AAV distribution and gene transduction in the brain. In contrast, ICM injections tend to result in broader vector distribution and improved penetration to both the brain and spinal cord when used in non-human primates (NHPs) [49–51]. It was hypothesized that the degree of exposure to the CSF determines the level of transduction, with greater exposure leading to more efficient transduction [46]. A study conducted in sheep further supports this observation, revealing that IT injection of methylene blue primarily colored the spinal cord and cerebellum, with minimal distribution in the cerebrum. On the other hand, ICM injection led to a more substantial distribution to the cerebellar and midbrain regions [48]. These findings corroborate the understanding that IT typically leads to stronger transduction in the spinal cord, while ICM provides broader brain distribution.
Intra-CSF delivery has several advantages. Intra-CSF can achieve more widespread gene transfection throughout the CNS than DI. Intra-CSF delivery has the advantage of bypassing the BBB compared to IV injection [46]. As intra-CSF delivery does not rely on blood circulation, it avoids the liver’s first-pass clearance and significantly reduces systemic leakage into peripheral organs, particularly the liver and heart. Furthermore, intra-CSF delivery mitigates the problem of circulating antibodies in the bloodstream, which can neutralize the therapeutic AAV. This feature makes intra-CSF delivery a potentially favorable method for patients with high neutralizing antibody levels.
Intra-CSF delivery of AAV faces several challenges. One key obstacle is insufficient coverage, leading to difficulties in reaching deeper brain regions such as the caudate, putamen, and thalamus [48,52]. Ependymal barriers, which act as a protective barrier between the CSF and the brain, are not as strong as the BBB in preventing substance entry. Nevertheless, they can still hinder the penetration of AAV into the brain tissue [53–55]. Moreover, the ICV and ICM delivery need surgery invention, especially the CM is in proximity to critical brain structures, including the brainstem, thus may induce complications after the surgical procedure. The mechanical damage by the needle or incorrect placement of the needle into these critical structures not only leads to inefficient CSF delivery but also could cause medullary injury, life-threatening complications, or sudden death [56]. Another limitation is the rapid circulation and clearance of CSF. The choroid plexus constantly produces the CSF, circulating throughout the brain via the glymphatic system and cleared into the lymphatic system. The CSF clearance rate was estimated to be three to five times daily in humans, leading to AAV clearance [57,58].
2.3. Intravenous (IV) injection of blood-brain barrier (BBB)-permeable AAV
IV delivery involves directly introducing AAV into the bloodstream via IV injection. This method’s efficacy largely hinges on the ability of AAV to cross the BBB. The capability of AAV to penetrate the BBB is predominantly determined by the properties of the AAV capsid. The AAV capsid has 60 subunits [59], and variations in the amino acid sequence of each subunit determine receptor binding, transportation, and antigenicity of the AAV. To date, 11 naturally occurring AAV serotypes and over 1000 AAV variants isolated from various animal species have been identified [60]. AAV serotypes 1–9 originate from humans, while others like AAVrh.8, AAVrh.10, and AAVrh.43 are derived from NHPs. These naturally existing serotypes and variants have distinct tropism profiles and efficacies in gene delivery. Notably, only a few of these naturally existing AAVs have demonstrated the capability to cross the BBB effectively and target the CNS. Recognizing these limitations, recent research efforts have been directed toward engineering novel AAV capsids capable of crossing the BBB to enhance brain transduction.
Among the naturally existing AAV, AAV9 is currently the most commonly used AAV serotype for CNS transduction in preclinical and clinical studies. AAV9 is a naturally exist AAV and was proven to achieve spreaded transduction in the brain. Although the mechanism remains unclear, it is believed that AAV9 crosses the BBB by active-transport mechanisms, such as receptor-mediated vesicular transport [61,62] This is supported by evidence that co-injection with mannitol, a BBB-disrupting agent that alters endothelial cells and tight junction integrity, significantly increased transgene expression of AAV2 but not AAV9 in adult mice [55,63–66]. However, although proven the most effective in transducing the brain, the transduction efficiency of IV administered AAV9 is age-dependent. AAV9 transgene is widely distributed in neonatal animals, predominantly in neurons across numerous brain regions (olfactory bulb, striatum, cerebral cortex, hippocampus, and brainstem) [67,68]. In contrast, most transduced cells for adult mice are glial (astrocytes or endothelial cells) with sparse neuronal transduction [68–73]. For instance, one study found that when administered at birth, AAV9 infected approximately 60% of motor neurons and 30% of astrocytes in amyotrophic lateral sclerosis mice; however, when injected into the adult mice, AAV9 more efficiently transduced astrocytes (around 50%), compared to motor neurons (8%) [71]. Similar trends have also been observed in NHPs [47,64,67,69,74]. The mechanisms underlying these age-related differences in transduction are not fully understood but could be related to developmental changes in the brain. Factors such as the neuron-to-glia ratio and the development stage of astrocytic end feet could be potential causes. Once astrocytic end feet fully develop in neonatal brains as mice mature, they may trap AAV9, preventing its widespread distribution [75]. The preferential transduction of astrocytes in adults also suggests that AAV9 may be trapped by and directly infect the perivascular astrocys after crossing the BBB endothelial cells [68].
Several methods have been employed to develop novel BBB-permeable AAV capsids. While the engineering methods for creating novel AAV capsids are beyond the scope of this review and thus won’t be detailed here, a comprehensive exploration can be found in recent reviews by Wang et al. [59] and Lin et al [76]. Among these methods, directed evolution was proven to be the most successful in enhancing the biological characteristics of AAV capsids [59,76]. Directed evolution involves introducing mutations into the genes of wild-type AAV capsids to generate extensive genetic libraries. Subsequent selection rounds allow for the accumulation of beneficial mutations or genetic modifications that enhance specific biological properties, even without understanding the underlying mechanistic basis [77]. Recently, this technique has been successfully utilized to engineer serotypes capable of crossing the BBB, such as AAV.PHP.B, AAV-PHP.eB, and AAV-PHP.S. All these engineered serotypes effectively transduce neurons and glia throughout the CNS [78]. However, recent reports suggest that the enhanced CNS tropism of AAV.PHP.B and AAV-PHP.eB is dependent on strain-specific Ly6a receptor expression and appears to be limited to a specific mouse model (C57BL/6J), with less effectiveness in NHPs and other mouse models [79,80]. Different novel AAV variants have been developed, including AAV-CAP-B10, AAV-CAP-B22, AAV-MaCPNS1, AAV-MaCPNS2, and AAV-CAP-Mac, which exhibit improved transduction in both the CNS and peripheral nervous system in NHPs. Nevertheless, the effectiveness of these variants in humans still requires further exploration, considering potential species-specific differences in tropism [81].
IV delivery of AAV has several advantages. Firstly, the procedure is straightforward, non-invasive, and can be repeated. Secondly, IV delivery is particularly suited to diseases where AAV vectors need to target both the CNS and peripheral tissues, such as the liver, spleen, kidney, and skeletal muscles. This is pertinent in neurological disorders such as amyotrophic lateral sclerosis (ALS), which not only present a primary CNS phenotype but may also exhibit secondary effects that impact the entire organism. Thirdly, AAV delivered via IV injection may cover an extensive range of brain regions, making this method beneficial for managing neurodegenerative diseases like Huntington’s disease (HD), AD, and several LSD. These disorders often affect multiple brain structures and even peripheral organs.
IV delivery of AAV also faces some challenges. First, when AAV vectors travel through the bloodstream, organs like the spleen and liver serve as first-pass filters, removing a substantial quantity of the vectors before they can reach the brain [82,83]. This necessitates the injection of large vector doses to attain a therapeutic level of transduction, simultaneously raising concerns of systemic toxicity, such as off-target effects and even severe hepatotoxicity [84–88]. Second, immune responses can be triggered upon IV injection of AAV, which can compromise the efficacy of gene transduction [89–91]. Many adults (around 90%) have already been exposed to AAV, meaning they might have antibodies that can neutralize the AAV vectors, making the therapy less effective [92]. Moreover, a high-dose injection of AAV during the initial delivery could negatively impact the therapeutic effects of later administrations due to the higher risk of activating immune responses [93,94].
2.4. Intranasal (IN) delivery
IN delivery directly delivers the AAV through the nose to the brain, bypassing the BBB and minimizing systemic exposure [95]. IN delivery has been used in multiple clinical studies to deliver small molecule agents to the brain, including oxytocin [96], insulin [97], erythropoietin [98], perillyl alcohol [99], and neurotrophic factor [100,101], for treating neurodegenerative diseases and brain tumors. Recently, IN delivery has gained attention since the coronavirus 2 has been found to be transported to the brain via the olfactory bulb [102]. Despite this progress, IN delivery of AAV remains primarily in preclinical studies. For mice, a micropipette is often employed to dispense eight 3 μl drops, alternating between each nostril, resulting in a total of 24 μl injection volume per mouse [103]. While the exact pathway from nose to brain for AAV delivery has not been extensively studied, existing literature suggests that IN-delivered agents reach the brain via olfactory and trigeminal nerves through intracellular and extracellular routes [104]. In the intracellular route, the agent could be transmitted through the olfactory or trigeminal nerves to the olfactory bulb or the brainstem, respectively. In the extracellular route, the agent could move through supporting cells, passing through tight junctions, paracellular clefts, the lamina propria, perineural space, and finally reaching the subarachnoid space [105,106].
IN delivery of AAV has been successfully employed to transduce epithelial tissues in olfactory and respiratory regions in the nose, providing effective treatments for conditions like olfactory dysfunction [107,108]. Recent explorations have aimed to leverage IN delivery of AAV beyond the olfactory bulb for brain gene therapy. One study demonstrated that IN administration of AAV8 and AAV9 encoding α-L-iduronidase in adult α-L-iduronidase-deficient mice elevated the α-L-iduronidase enzyme throughout the brain. The most significant elevations in enzyme levels were detected within the olfactory region and the trigeminal ganglion—key entry points for IN delivery [103,109,110]. Interestingly, when green fluorescence protein was encoded into AAV9 and delivered intranasally, green fluorescence protein expression was restricted to the olfactory region. This suggests that rather than enabling direct access of AAV to the brain, IN delivery may convert the olfactory bulb and nasal epithelium into a “storage depot.” Vector transduction and enzyme expression can occur within these regions, followed by enzyme diffusion and uptake by other brain regions [103,109]. Further studies involving IN delivery of AAV-encoded proteins or peptides, such as brain-derived neurotrophic factor (BDNF), observed significantly elevated protein levels in the hippocampus [111–114]. Nonetheless, the mechanisms and distribution patterns of AAV in the brain post-IN delivery still require further exploration to fully leverage this promising delivery route.
IN delivery has the advantages of being non-invasive and easy to perform. The olfactory bulb and brainstem, as the entry points of the brain from the olfactory and trigeminal pathways, respectively, always exhibit more accumulation of the IN-delivered agents. IN delivery can be performed repeatedly, even by patients themselves, to increase the delivery amounts. However, IN delivery also holds limitations. The IN delivery of AAV is generally associated with low efficiency, and only a limited volume can be administered through the nose each time. Based on current research findings, it is highly likely that the AAV, not the gene product, cannot directly enter the brain after IN delivery. Moreover, the cells along the delivery pathways, such as olfactory sensory neurons and trigeminal nerves, may also be transduced and cause off-target effects. This issue can be addressed by designing the AAV with a cell-type specific promoter to avoid the transduction of cells along the delivery pathway. In addition, disease conditions in the nasal cavity, such as upper respiratory tract infections, may impact IN delivery to the brain. These factors should be carefully considered when applying this method for preclinical or clinical research studies [115].
3. Emerging focused ultrasound with microbubble-mediated BBB opening (FUS-BBBO) AAV delivery to the brain
Focused ultrasound is the only existing technique capable of noninvasively delivering energy through the skull to target specific brain regions with high precision without ionizing radiation. The unique capability has opened various applications, including gene delivery to the brain through FUS-BBBO. FUS-BBBO provides a safe, non-invasive, spatially targeted method for temporally opening the BBB, facilitating AAV delivery from the bloodstream to the brain. In the following, we introduce FUS-BBBO, its application in AAV delivery, and its advantages and limitations.
3.1. Introduction to FUS-BBBO
Ultrasound refers to sound waves with frequencies higher than the upper limit of human hearing, typically around 20 kHz [116]. It can penetrate the human skull noninvasively and reach virtually any area in the entire brain. Focused ultrasound concentrates ultrasound energy into a small ellipsoid focal volume, typically measuring about 1 mm × 10 mm, enabling spatially precise targeting of specific brain regions [117]. Microbubbles are used in the clinic as ultrasound contrast agents. They are micron-size gas spheres encapsulated by lipid or protein shells. Upon IV injection, they remain confined within the vasculature due to their relatively large sizes. When subjected to ultrasound sonication, microbubbles undergo cavitation, a process involving expansion, contraction, and collapse of the microbubbles. Microbubble cavitation concentrates and amplifies ultrasound effects on the vasculature, leading to increased permeability of the BBB. Several cellular and molecular mechanisms have been proposed to explain FUS-BBBO, including (1) disruption of the tight junction, facilitating enhanced paracellular transport; (2) upregulation of caveolin, promoting increased transcellular transport; and (3) suppression of P-glycoprotein expression to inhibit efflux transport [118]. Nevertheless, the exact mechanisms remain incompletely understood.
The FUS-BBBO procedure comprises four key components: treatment planning, treatment delivery, treatment monitoring, and treatment assessment. Treatment planning ensures accurate targeting of the ultrasound focus on the desired brain regions. Magnetic resonance imaging or neuronavigation systems often guide treatment planning in clinical studies. In preclinical rodent studies, guidance can be achieved using ultrasound imaging with fiducial markers or stereotactic frames. Treatment delivery involves using focused ultrasound devices, including single-element concave-shaped transducers, multi-element phased array transducers, and flat transducers coupled with acoustic lenses. Treatment monitoring is crucial in ensuring the procedure’s safety and consistency. The most used treatment monitoring techniques are passive cavitation detection using a single-element sensor and passive cavitation imaging using multi-element imaging arrays. These methods aim to detect acoustic emissions from the cavitating microbubbles to characterize their behavior, offering insights into the procedure’s safety and efficacy. Treatment assessment can be performed in vivo using contrast-enhanced MRI to observe the leakage of MRI contrast agents from the bloodstream into the brain tissue through the opened BBB. Treatment assessment can also be performed ex vivo for preclinical research by analyzing harvested brain tissue post-treatment to quantify the BBB permeability and agent delivery outcomes.
Hynynen and his colleagues introduced the FUS-BBBO technique in 2001 [119]. Since then, it has evolved into a versatile technique for delivering a broad spectrum of therapeutic agents across the BBB, including small molecule drugs [120], proteins [121–123], and even cells [124,125] in rodents and NHPs. Early-phase clinical trials have provided evidence of the feasibility and safety of FUS-BBBO in patients with various diseases, including glioblastoma [126], AD [127], ALS [128], and PD [129].
3.2. FUS-BBBO for AAV delivery
Since its advent in 2001, FUS-BBBO has been predominantly utilized for drug delivery. The technique’s adaptation for AAV delivery was first reported in 2012 [130], and numerous subsequent studies demonstrated its potential across mice, rats, and NHPs, as summarized in Table 2. These studies indicate that the targeted brain location, AAV parameters, and FUS treatment parameters influence AAV delivery outcomes.
Table 2.
Summary of AAV delivery by FUS-BBBO in mice, rats, and NHPs.
| Animal | AAV parameters | FUS parameters | Ref | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Animal | Target | Serotype | Gene | Dose (vg/g) | Microbubble (MB) Dose | Frequency (MHZ) |
Pressure (MPa) |
PRF (Hz) |
Pulse length (ms) |
Duration (s) | |
| Mouse | CT, STR, HP, TH | AAV9 | GFP | 5×108 2.5×109 1.25×1010 |
40–80 μL/kg | 0.558 1.18 |
0.3 0.53–0.6 |
1 | 10 | 120 | [130] |
| HP, CT | AAV9 | mcherry-ChR2 | 4×1011 vg/animal | 5 μL | 1.5 | 0.74 | 5 | 10 | 120 | [145] | |
| HP, SN, OB, DMV | AAV9 | 1.25×1010 | 0.02 mL/kg | 1.68 | 1.0 ± 0.2 1.1 ± 0.2 |
1 | 10 | 120 | [146] | ||
| STR, HP, midbrain | AAV9 |
6.7×109
1.3×109 |
1.5×106 MB | 1.5 | 0.33 | 1 | 10 | 120 | [147] | ||
| Whole brain | AAV9 | GFP | 1.1×1011 vg/animal |
8 μL/mL at 100 μL volume | 1.5 | 0.57 | 10 | 99 | 150 | [148] | |
| HP | AAV9 | GFP | 6.5×1010
1.0×1010 1.3×1011 vg/animal |
3.6×108 MBs/mL | 1.5 | 1.0 | 0.33 | 1.0 3.33 6.67 |
120 | [149] | |
| STR, TH | AAV9 | GFP | 1×1011
1.3×1010 |
0.02 mL/kg | 1.78 | 0.06 0.12 0.25 0.38 |
0.5 | 10 | 120 | [150] | |
| Large brain regions | AAV9 | GFP | 1×1010 | 1.5×106 MBs | 1.53 | 0.27 | 1 | 10 | 30 | [151] | |
| HP | AAV9-hM4Di | mcherry | 1×1010 | 1.5×106 MBs | 1.5 | 0.42 | 1 | 10 | 120 | [152] | |
| CT | AAV9, PHP.B |
1×1011 vg/animal |
1.5×107 MBs in 50 μL volume | 1.5 | 0.51 | 5 | 1.0 | 660 | [153] | ||
| CT, STR, TH | AAV9, PHP.B |
GFP | 1×1010
1×1011 |
0.02 mL/kg | 1.68 to 1.78 | ~0.5 – 2.0 | 1 | 10 | 120 | [139] | |
| HP | AAV9 AAV1 |
mcherry-ChR2 | 2.48×1012 vg/animal |
~2.5×107 MBs | 1.5 | 0.45 | 5 | 6.7 | 60 | [154] | |
| STR, TH | AAV9 AAV2-HBKO |
GFP | 1.7×109 3.3×109 1.7×1010 |
0.02 mL/kg | 0.58 | NA | 1 | 10 | 120 | [155] | |
| STR, HP, TH, CT | AAV6 AAV1/2 AAV9 |
GFP | 3×109 | 0.02 mL/kg | 1.68 | NA | 1 | 10 | 120 | [156] | |
| CT, STR, TH, HP | AAV1, 2, 5, 8, 9, rg | TdTomatoGFP | 3.33×109
1.67×1010 |
0.02 mL/kg | 0.58 | <0.9 | 1 | 10 | 180 | [131] | |
| STR | AAV2 | hrGFP | 1×109 | 0.3 mL/kg | 1.5 | 0.44 0.53 0.7 |
1 | 10 | 120 | [136] | |
| CPu | AAV1 | GFP | 1.1×1011 vg/animal |
~2.5×107 MBs | 1.5 | 0.45 | 5 | 20 | 300 | [138] | |
| CT, HP | AAV1/2 | GFP | 3×109 | 0.02 mL/kg | 1.68 | NA | 1 | 10 | 120 | [157] | |
| HP | AAV9-Cu64 | PKM2 | 2×1011 5×1011 vg/animal |
2.5×108 MBs/kg | 1.5 | 0.42 0.6 0.74 |
NA | NA | 120 | [158] | |
| Rat | Left brain | AAV2/1 | LacZ | 1.06×1011 vg/animal | 0.2×109 MBs/mL | 0.5 | 1.1 | 1 | 10 | 40+ | [159] |
| STR | AAV1/2 | GFP | 1×109 | 0.4×108 − 0.64×108 MBs/kg | 0.97 | 1 | 10 | 200 | [137] | ||
| STR, SN | AAV2 | NA | 2.96×1012 − 3.94×1012 vg/animal |
NA | 0.58 | 0.4 | 1 | 10 | 120 | [160] | |
| NHP | CT, TH, ST midbrain | AAV9-PHP.eB AAV9.2-PHP.eB |
GFP | 5×1013 vg/animal |
4 μL/kg | 0.220 | 1 Watts | NA | NA | 60 to 150 | [143] |
| CT | AAV2 AAV9 |
EGFP mCherry |
NA | 200 μL/kg | 1.46 | 1.17 | 1 | 10 | 60 | [144] | |
CT: Cortex; STR: Striatum; HP: Hippocampus; TH: Thalamus; CPu: Caudate putamen; Cd: Caudate nucleus; SN: Substantia nigra; OB: Olfactory bulb; DMV: Dorsal motor nucleus of the vagus; STN: Subthalamic nucleus; NHP: Non-human primates.
The AAV delivery outcomes by FUS-BBBO appear to vary across brain regions, potentially due to anatomical, cellular, and vascular differences [131–135]. A study reported differential GFP expression in the hippocampal region (58% for neurons, 36% for astrocytes, and 6% for other cell types) compared to the striatal region (18% for neurons, 63% for astrocytes, and 19% for other cell types) [130]. This finding highlights the complexity of AAV delivery by FUS-BBBO.
The effectiveness of FUS-BBBO in enhancing AAV delivery is contingent upon AAV serotype and dosage. FUS-BBBO has shown promise in improving the brain delivery of several AAV serotypes, such as AAV1, AAV2, and AAV1/2. Still, it has been most effective in enhancing the delivery of AAV9, which is known to naturally traverse the BBB to some extent [136–138]. One comparative study analyzed the transduction efficacy of AAV9, AAV1, AAV2, AAV5, AAV8, and AAVrg after IV injection at the same dose and FUS-BBBO treatment parameters. AAV9, AAV1, and AAV8 exhibited relatively better transduction efficacy, while AAV2 and AAV5 showed relatively poorer efficacy [131]. Interestingly, a study involving AAV9 and AAV-PHP.B (AAV9 variant known for crossing the BBB in Ly6A receptor-expressing animals) showed that FUS-BBBO only enhances AAV-PHP.B delivery to the brain in animals with reduced Ly6A receptor expression [139].
FUS parameters also significantly affect BBB opening, directly impacting AAV delivery efficiency [140–142]. Table 2 lists the FUS parameters used in each reported study. It shows that a wide range of FUS parameters have been used for AAV delivery, highlighting the importance of optimizing FUS parameters for efficient and safe AAV delivery. For instance, Hsu et al. demonstrated that increased FUS pressure correlates with enhanced AAV delivery and gene expression [136]. The study revealed a 32.3% increase in gene expression at the lowest pressure (0.44 MPa) and a 124.3% increase at the highest pressure (0.7 MPa) compared to the control region. This can be explained by the increased BBB permeability due to higher acoustic pressure, allowing for more AAV to pass through the BBB at the target region.
Although FUS-mediated AAV delivery has been extensively tested in rodents, its application in NHPs is just emerging. Blesa et al. successfully delivered AAV9 and AAV-PHP.eB via FUS-BBBO in healthy and Parkinsonian NHPs [143]. Crucially, pre-screening for neutralizing antibodies is imperative to ensure effective AAV brain delivery in NHPs. Additionally, Parks et al. have recently demonstrated that AAV2 and AAV9 can be precisely delivered to the frontal cortex of marmosets via FUS-BBBO, indicating a significant advancement in precision delivery techniques for primate brain research [144].
3.3. Advantages and limitations of FUS-BBBO for AAV delivery
FUS-BBBO offers significant benefits for AAV delivery to the brain. It allows for precise targeting of specific brain regions akin to DI but without the associated invasive procedures. Its noninvasiveness permits the delivery of AAV to large brain areas by enlarging the ultrasound sonication volumes [146]. A recent study demonstrated the ability of FUS-BBBO to safely deliver AAV across large brain regions, including simultaneous targeting of up to 105 points to cover almost the whole mouse brain [151].
Despite these advantages, FUS-BBBO has its challenges. One primary concern is the potential for off-target side effects and toxicity due to IV injection. High peripheral absorption of the AAV reduces its bioavailability in the brain. Brain-specific promoters and optimized AAV serotypes have been proposed to mitigate peripheral transgene expression and toxicity. AAV2-HBKO, engineered for diminished liver absorption and improved brain parenchyma diffusion, showed superior brain transduction levels post-FUS delivery compared to AAV9 [155]. In a novel approach, FUS has been combined with IN AAV administration, providing an enhanced method for brain delivery. Ye et al. demonstrated that this combination increased brain transduction and reduced peripheral distribution compared to traditional FUS-BBBO AAV delivery [161]. These findings suggest that when used with FUS sonication, the IN route is a promising avenue for targeted AAV delivery to the brain.
4. Preclinical and clinical studies of AAV delivery for the treatment of brain diseases
Efficient delivery of therapeutic genes to the desired target cells is crucial for successful gene therapy, especially for brain diseases, where the BBB represents a significant obstacle. To date, although no AAV agent has received regulatory approval for clinical use, hundreds of preclinical studies and over 100 clinical trials have been conducted or are ongoing, exploring various routes to deliver AAV for the treatment of neurologic diseases through gene supplementation, replacement, and inactivation [13] (Fig. 2). As discussed in the previous chapter, each delivery route has its advantages and disadvantages, varying in efficiency, target region, and biodistribution. Therefore, optimum AAV delivery requires carefully selecting administration routes and weighing disease specifics, such as manifested symptoms, affected brain regions, the intended function of the gene therapy, and potential risks associated with the delivery method. This chapter provides an overview of CNS diseases targeted by AAV-based gene therapy, including neurodegenerative diseases, lysosomal storage disorders, and neurodevelopmental disorders. Each of these diseases has shown considerable potential for treatment using AAV-based gene therapy. We detail the specific pathogens and therapeutic genes involved in each disease, the various AAV delivery routes utilized, and discuss the outcomes achieved by each route. Table 3 summarizes preclinical studies, and Table 4 offers a comprehensive summary of clinical studies. It is worth mentioning that while this summary provides a broad perspective, AAV-based gene therapy has been extended to address diseases beyond the scope discussed here, for example Down syndrome [162] and Prader-Willi syndrome [163].
Figure 2.

Summary of preclinical and clinical applications of AAV delivery for treating CNS diseases through different mechanisms, including gene inactivation, gene supplementation, gene replacement. These diseases include neurodegenerative diseases (Parkinson’s disease, Alzheimer’s diseases, Canavan diseases, and Huntington’s disease), lysosomal storage disease (LSDs), and neurodevelopmental disorders (Rett syndrome, Fragile X syndrome, Dravet Syndrome, and Angelman syndrome).
Table 3.
Preclinical studies of AAV-mediated gene therapy of brain diseases
| Animal | Delivery | Targeted sites | Serotype | Transgene | Dosage (vg) | Ref |
|---|---|---|---|---|---|---|
| Parkinson’s disease | ||||||
| Rat | DI | Striatum | AAV2 | AADC | 2.5×1010 | [25] |
| Rat | DI | Striatum | AAV2 | AADC | 4×1010 | [164] |
| NHP | DI | Striatum | AAV2 | AADC | 3.6×1011 | [165] |
| Rat | DI | STN | AAV2 | GAD | 1.0×108 | [166] |
| NHP | DI | STN | AAV2 | GAD | 0.6–1.2×1010 | [167] |
| Mouse | DI | Striatum | AAV9 | miniSINEUP-GDNF | 7.0×109 | [168] |
| Rat | DI | Striatum | AAV5, AAV6 | GDNF | 3.0×108 | [169] |
| Rat | DI | Striatum | AAV2 | CDNF/GDNF | 4.0×107, 2.0×108, 1.0×109 | [170] |
| NHP | DI | Striatum | AAV2 | GDNF | 9.9×1011 | [171] |
| NHP | DI | SN Striatum |
AAV2 | GDNF | 5.5×1010, 5.5×1011 | [172] |
| NHP | DI | Striatum | AAV2 | NTN | 3.0×1011 | [173] |
| NHP | DI | Striatum | AAV2 | NTN | 1.7×1011 | [174] |
| Mouse | DI | SN | AAV9 | GBA1 | 2.0×1010 | [175] |
| NHP | DI | SN | AAV9 | GBA1 | 5.0×1010 | [175] |
| Mouse | FUS-BBBO | HP/SN/OB/DMN | AAV9 | shRNA | 3.75×1011 | [146] |
| Mouse | FUS-BBBO | STN Striatum |
AAV1 | GDNF | 1.2×1011 | [176] |
| Alzheimer’s disease | ||||||
| Mouse | DI | HP | AAV2/1 | IL-10 | 3.0×109 | [177] |
| Mouse | DI | HP | AAV2/1 | IL-4 | 2.0×09 | [178] |
| Mouse | DI | HP | AAV2/1 | 7ND | 2.0×109 | [179] |
| Mouse | ICV | Ventricle | AAV4 | APOE2 | 1.0×1010 | [180] |
| NHP | DI ICV ICM |
HP Ventricle CM |
AAVrh.10 | APOE2 | DI: 5.0×1012 ICV: 5.0×1013 ICM: 5.0×1013 |
[50] |
| Mouse | DI | HP | AAV8 | IGF1/IGF2 | NA | [181] |
| Mouse | IV | Left Heart Ventricle | AAV9 | NEP | 0.5–15×1011 | [182] |
| Mouse | ICV | Ventricle | AAV1 | Anti-Aβ scFvs | 2.0×1010 | [183] |
| Mouse | IN | NA | AAV2 | CBD3 | 5.0×1011 | [111] |
| Huntington’s disease | ||||||
| Rat | DI | Striatum | AAV2 | GDNF | 4.5 ×109 | [184] |
| Rat | DI | Striatum | AAV2 | BDNF and GDNF | 1.4×1010 | [185] |
| Mouse | DI | Striatum | AAV2/5 | NeuroD1 or Dlx2 | 6.9 ×1010 | [186] |
| Rat | DI | Striatum | AAV1/2 | BDNF | 2×109 | [187] |
| Minipig | DI | Putamen thalamus | AAV5 | miHTT | 1–3×1013 | [188] |
| Mouse | IV | NA | AAV-AS AAV9 |
miRHtt | 5.0×1011 | [73] |
| Mouse | DI | Striatum | AAV5 AAVrh10 |
CYP46A1 | 3×109 | [189] |
| Canavan disease | ||||||
| Mouse | DI | Striatum thalamus |
AAV2 | ASPA | 1.68×109 | [190] |
| Rat | DI | Caudate thalamus |
AAV2 | ASPA | 3.2×1010 | [191] |
| Mouse | IV | NA | AAV9, rh.8, rh.10 | ASPA | 4.0×1011, 8.0×1011, 1.2×1012, 4.0×1012 | [192] |
| Mouse | IV ICV |
NA | AAV9, rh.8, rh.10 | ASPA | IV:4.0×1011 ICV:2.0×1010 |
[193] |
| Mouse | IV | NA | AAV9 | ASPA | 4.0×1010, 1.33×1011, 4.0×1011 | [194] |
| Lysosomal storage disease | ||||||
| Rat NHP |
DI | Rat: Striatum NHP: Striatum |
AAVrh.10 | hCLN2 | Rat:1.0×1011 NHP:1.8×1012 |
[195] |
| Mouse | DI | Multiple sites | AAV1 | hCLN2 | 6.0×1010 | [196] |
| Rat | DI | Striatum | AAV2, 5, 8, rh.10 | hCLN2 | 2.5×109 | [197] |
| Mouse | DI | Multiple sites | AAV2, AAV5 | hCLN2 | 3.6×109 | [198] |
| Mouse | DI | Striatum | AAV5 | TPP-I | 5.0×109 | [199] |
| Mouse | ICV | Ventricle | AAV8 | IDUA | 2.0×1010 | [200] |
| Mouse | IT or IV | NA | AAV2 | IDUA | 4.0×109 to 4.0×1010 | [201] |
| Cat | ICM | Cisterna magna | AAV9 | IDUA | 1.0×1012/kg | [202] |
| Mouse | IN | NA | AAV9 | IDUA | 2.0×1011 | [103] |
| Mouse | IN | NA | AAV8 | IDUA | 9.3×1010 | [109] |
| Mouse | ICV | Ventricle | AAV9 | IDS | 3×108, 3×09, 3×1010 | [203] |
| Mouse | DI | Striatum | NA | βgluc | 1.0×107 | [204] |
| Mouse | ICV | Ventricle | AAV5 | Adbgluc/Adbgal | 2.0×107 | [54] |
| Dog | IT IV |
NA | AAV9 AAVrh.10 |
GUSB | IV: 2.0×1013/kg IT: 5.0 or 2.8×1012/kg |
[205] |
| Mouse | IV | NA | AAV9 AAVPFG | βgluc | 1.0×1012 | [206] |
| Dog | ICM | CM | AAV9 | rβGpA | 2.0 × 1013 | [207] |
| Mouse Dog |
ICV ICM |
Ventricle CM |
AAV9 | Sgsh | Mouse: 5.0×109 or 5.0×1010 dog: 2.0×1013 |
[208] |
| Mouse | ICM IV |
CM | AAV2 | hNaGlu | IV:4.0×1011 ICM:5.0×1010 |
[53] |
| Mouse | ICM | CM | AAV9 | Gns | 5.0×1010 | [209] |
| Mouse | DI | Thalamus or Thalamus & DCN | AAV2/1 | GLB1 | 4.8×1010, 7.2×1010 | [210] |
| Mouse | IV | NA | AAV9 | GLB1 | 1.0×1011, 3.0×1011 | [211] |
| Mouse | DI ICV |
DI: Thalamus ICV: Ventricle | AAVrh.8 | cmHexa cmHexb | 1.1×1011, 3.2×1011, 3.2×1012 |
[212] |
| Mouse | DI | DCN | AAV1, 2, 5, 7, 8 | ASM | 1.86×1010 | [213] |
| Mouse | IV | NA | AAV9 | NPC1 | 1.2–1.3×1012 | [214] |
| Sheep | ICM | CM | AAVrh.8 | HEXA HEXB | 1×1014, 5×1013 | [48] |
| Rett syndrome | ||||||
| Mouse | IV | NA | AAV9 | MECP2 | 1×1012 | [215] |
| Mouse | P0–3: DI 4–5 wks.: IV |
NA | AAV9 | MECP2 | DI: 4.8 × 1010 IV: 1.0 × 1011 ,5.0 × 1011 |
[216] |
| Mouse | P0–3: DI 4–5 wks.: IV |
NA | AAV9 | MECP2 | DI: 4.8 × 1010 IV: 1.0 × 1011 ,5.0 × 1011 |
[217] |
| Mouse | ICM | CM | AAV9 | MECP2 | 1.0×1012 | [218] |
| Mouse | IV | NA | AAV9 | MCO | 2.0 × 1011 | [219] |
| Mouse | IV | NA | AAV-PHP.eB | iMECP2 | 1.0×109–1.0×1012 | [220] |
| Mouse | IT and ICM | CM | AAV9 PHP.B |
miniMECP2 | 1×1012 | [221] |
| Mouse | IV | NA | AAV9 | MCO | 1× 1011 ,5 × 1011,1 × 1012 | [222] |
| Fragile X syndrome | ||||||
| Mouse | DI | HP | AAV5 | FMR1 | 1×109 | [223] |
| Mouse | ICV | Ventricle | AAV9 | FMR1 | 1×109 | [224] |
| Mouse | ICV | Ventricle | AAV9 | FMR1 | 0.375–1×109 | [225] |
| Mouse | ICV | Ventricle | AAV2/9 | FMR1 | 1×109 | [226] |
| Mouse/Rat | ICV, ICM | ICV: Ventricle | AAV9 | FMR1 | NA | [227] |
| Mouse | DI, IV | DI: Striatum, HP IV: Retro-bulbar space |
DI: AAVrh.10 IV:AAV-PHP.eB |
DGKk | DI: 2.5×109 ,IV: 8×108 | [228] |
| Mouse | IV | NA | AAV-PHP.eB | FMR1 | 5×1013 | [229] |
| Mouse | IT | NA | NA | FMR1 | 1.3×1011 ,2.3×1011, 5.0×1011 | [230] |
| Dravet Syndrome | ||||||
| Mouse | ICV, ICM | Ventricle, CM | AAV9 | NaVβ1 | 8.0×1010 | [231] |
| Mouse | ICV | Ventricle | AAV9 | REGABA-eTFSCN1A | 1.7×1010–5.1×1010 | [232] |
| NHP | ICV | Ventricle | AAV9 | REGABA-eTFSCN1A | 4.8×1013–8×1013 | [232] |
| Mouse | ICV | Ventricle | AAV9 | SCN1A-dCas9A | 5.0×1013 | [233] |
| Mouse | IV | NA | AAV-PHP.eB | SCN1A-dCas9A | 1.8×1011 | [234] |
| Angelman syndrome | ||||||
| Mouse | DI | HP | AAV9 | UBE3A | 1.5×109 | [235] |
| Mouse | ICV | Ventricle | PHP.B | UBE3A | 1.6×1011 | [236] |
| Mouse | DI | DI: HP ICV: Ventricle |
AAV9 | STUB | 2.0×1011 | [237] |
| Rat | DI, ICV | DI: HP ICV: Ventricle |
AAV9 | UBE3A/STUB | UBE3A DI: 4.0×1010
UBE3A ICV: 2.0×1011 STUB DI: 6.8×1010 STUB ICV: 3.0×1011 |
[237] |
| Mouse | ICV | Ventricle | AAV9 | UBE3A-ATS SpCas9 | 1.5×1010 | [238] |
| Mouse | ICV,IV | ICV:Ventricle | ICV:hu68 IV: PHP.B |
UBE3A-ATS-dCas9 | ICV: 1.0×1011
IV: 1.0×1012 |
[239] |
| Mouse | IV | NA | PHP.eB | ATF-S1K | 1.0×1012 | [240] |
DI: Direct intraparenchyma injection; IN: Intranasal delivery; ICV: Intracerebroventricular injection; IT: Intrathecal injection; ICM: Intra-cisterna magna injection; IV: Intravenous injection; FUS-BBBO: Focused ultrasound with microbubble-induced BBB opening; STN: Subthalamic nucleus; SN: Substantia nigra; HP: Hippocampus; OB: Olfactory bulb; DMN: Dorsal motor nucleus of the vagus; CM: Cisterna magna; DCN: Deep cerebellar nuclei.
Table 4.
Clinical trials on AAV-mediated gene therapy for neurological diseases.
| ID | Delivery | Year | Phase | Patient age (y) | Target Sites | Serotype | Transgene | AAV dosage (vg) |
|---|---|---|---|---|---|---|---|---|
| Parkinson’s disease | ||||||||
| NCT00229736 | DI | 2004–2013 | 1 | 40–75 | Striatum | AAV2 | AADC | 9×1010 or 3×1011 |
| NCT01973543 | DI | 2013–2020 | 1 | 40–70 | Striatum | AAV2 | AADC | ≤7.5×1011, ≤1.5×1012, or ≤4.7×1012 |
| NCT02418598 | DI | 2015–2019 | 2 | 35–70 | Striatum | AAV2 | AADC | 3×1011 or 9×1011 |
| NCT03562494 | DI | 2018–present | 2 | 40–75 | Striatum | AAV2 | AADC | ≤3.6×1012 |
| NCT03733496 | DI | 2018–present | NA | 40–75 | Striatum | AAV2 | AADC | NA |
| NCT03065192 | DI | 2017–present | 1 | 40–75 | Striatum | AAV2 | AADC | 9.4×1012 |
| NCT00195143 | DI | 2003–2005 | 1 | 25–75 | SN | AAV2 | GAD | 1×1011, 3×1011, or 1×1012 /mL |
| NCT00643890 | DI | 2008–2012 | 2 | 30–75 | SN | AAV2 | GAD | 1×1012 |
| NCT01301573 | DI | 2011–2012 | NA | 30–90 | SN | AAV2 | GAD | NA |
| NCT00252850 | DI | 2005–2007 | 1 | 35–75 | Striatum | AAV2 | NRTN | 1.3×1011 or 5.4×1011 |
| NCT00400634 | DI | 2006–2008 | 2 | 35–75 | Striatum | AAV2 | NRTN | 5.4×1011 |
| NCT00985517 | DI | 2009–2017 | 2 | 35–70 | Striatum & SN | AAV2 | NRTN | 9.4×1011 or 2.4×1012 |
| NCT04167540 | DI | 2020–present | 1 | 35–75 | Striatum | AAV2 | GDNF | NA |
| NCT01621581 | CED | 2012–present | 1 | ≥18 | Striatum | AAV2 | GDNF | 9×1010, 3×1011, 9×1011, or 3×1012 |
| NCT04127578 | ICM | 2019–present | 2 | 40–75 | NA | AAV9 | GBA1 | NA |
| Alzheimer’s disease | ||||||||
| NCT00087789 | DI | 2004–2010 | 1 | 50–80 | NBM | AAV2 | NGF | 2.0×1010, 1.0×1011, or 2.0×1011 |
| NCT00876863 | DI | 2008–2015 | 2 | 55–80 | NBM | AAV2 | NGF | 2.0×1011 |
| NCT03634007 | ICM | 2019–present | 2 | ≥50 | CM | AAVrh.10 | APOE2 | 8.0×1010, 2.5×1011, or 8.0×1011 |
| NCT04133454 | IV/IT | 2019–present | 1 | ≥45 | NA | NA | hTERT | NA |
| Huntington’s disease | ||||||||
| NCT04120493 | DI | 2019–2021 | 2 | 25–65 | Striatum | AAV5 | miHTT | 6.0×1012 or 6.0×1013 |
| NCT05243017 | DI | 2022–2027 | 2 | 25–65 | Striatum | AAV5 | miHTT | 6.0×1012 or 6.0×1013 |
| NCT05541627 | DI | 2022–2029 | 2 | 18–65 | Striatum | AAVrh10 | CYP46A1 | NA |
| Canavan disease | ||||||||
| NA ref.[241] | DI | 2001–2005 | 3mo-96mo | Frontal, periventricular, and occipital lobes | AAV2 | ASPA | 8.0×1011 or 1.0v1012 | |
| NA ref.[242] | DI | 2001–2005 | 3mo-96mo | Frontal, parietal, and occipital regions | AAV2 | ASPA | 9.0×1011 | |
| NCT04998396 | IV/ICV | 2021–2028 | ≤30mo | NA | AAV9 | ASPA | NA | |
| Lysosomal storage diseases | ||||||||
| NCT00151216 | DI | 2004–2019 | 1 | 3–18 | Multiple sites | AAV2 | CLN2 | 3.2×1012 |
| NCT01414985 | DI | 2010–2017 | 2 | 3–18 | NA | AAVrh.10 | CLN2 | 9.0×1011or 2.85×1011 |
| NCT01161576 | DI | 2010–2020 | 1 | 2–18 | Multiple sites | AAVrh.10 | CLN2 | 9.0×1011or 2.85×1011 |
| NCT02725580 | IT | 2016–2021 | 2 | ≥1 | NA | NA | CLN6 | NA |
| NCT02702115 | IV | 2017–2022 | 2 | >18 | NA | AAV2/6 | IDUA | 1.0×1011 or 5.0×1013 /kg |
| NCT03041324 | IV | 2017–2021 | 2 | >18 | NA | AAV2/6 | IDS | 5.0×1012, 1.0×1013 or 5.0×1013 /kg |
| NCT03612869 | DI | 2018–2022 | 3 | ≥0.5 | NA | AAVrh.10 | SGSH | 7.2×1012 |
| NCT02716246 | IV | 2016–present | 3 | ≥0.5 | NA | scAAV9 | SGSH | 0.5×1013 , 1.0×1013 or 3.0×1013 /kg |
| NCT01474343 | DI | 2011–2014 | 2 | 1.5–6 | Multiple sites | AAVrh.10 | SGSH SUMF1 | 7.2×1011 |
| NCT04088734 | IV | 2019–2022 | 2 | 2–18 | NA | scAAV10 | SGSH | 3.0×1013 /kg |
| ISRCTN19853672 | DI | 2013–2015 | 2 | 1.5 –5 | Multiple sites | AAV2/5 | NAGLU | 4.0×1012 |
| NCT03315182 | IV | 2017–2022 | 2 | All | NA | AAV9 | NAGLU | 2.0×1013, 5.0×1013, 1.0×1014 /kg |
| NCT04273269 | ICM | 2020–2023 | 2 | up to 3 | NA | AAVrh.10 | GLB1 | 8.0×1012 /kg |
| NCT03952637 | IV | 2019–2028 | 2 | 0.5–12 | NA | AAV9 | GLB1 | 1.5×1013 /kg |
| NCT03770572 | IT | 2018–2024 | 2 | 3–10 | NA | scAAV9 | CLN3 | ≤6.0×1013 or ≤1.2×1014 |
| NCT01801709 | DI | 2014–2029 | 2 | 0.5–5 | Multiple sites | AAVrh.10 | ARSA | 1.0×1012 or 4.0×1012 |
| Rett Syndrome | ||||||||
| NCT05606614 | IT | 2023–2032 | 1/2 | ≥12 | NA | AAV9 | miniMECP2 | Initial dose: 5.0×1014
Second dose: 1.0×1015 |
| NCT05898620 | ICV | 2023–2029 | 1/2 | 4–10 | Ventricle | AAV9 | MECP2 | 1.0×1015 |
| Dravet Syndrome | ||||||||
| NCT05419492 | ICV | 2022–present | 1/2 | 6mo-35mo | Ventricle | AAV9 | NA | NA |
DI: Direct intraparenchyma injection; CED: Convection enhanced delivery; ICV: Intracerebroventricular injection; IT: Intrathecal injection; ICM: Intra-cisterna magna injection; IV: Intravenous injection; STN: Subthalamic nucleus; SN: Substantia nigra, CM: Cisterna magna, NBM: Basal forebrain region containing the nucleus basalis of meynert.
4.1. Parkinson’s disease (PD)
PD is a neurodegenerative disease characterized by the progressive loss of dopaminergic neurons in the substantia nigra, a brain region integral to movement control. These neurons are crucial for producing dopamine, a neurotransmitter essential for neuronal communication, particularly in the striatum, which encompasses the caudate nucleus and the putamen. Once dopaminergic neurons are lost to a certain level (usually 60%–80%), the dopamine receptors in the striatum become under-stimulated, triggering PD symptoms such as tremors, movement slowness, stiffness, and balance issues [243]. The pathology involves altered activity in two neural pathways: decreased activity in the direct pathway from the striatum to the internal segment of the globus pallidus and the thalamus and increased activity in the indirect pathway involving the external segment of the globus pallidus and the subthalamic nucleus (STN). Advanced gene therapies using AAV have been developed to address this imbalance, focusing on three primary strategies: (1) Introducing the enzyme aromatic l-amino acid decarboxylase (AADC) to enhance dopamine levels in the substantia nigra; (2) Delivering glutamic acid decarboxylase (GAD) to the STN to mitigate the overactive glutamatergic output by converting it into the inhibitory neurotransmitter GABA, thereby helping to restore motor function; and (3) Infusing neurotrophic factors like GDNF and neurturin (NRTN) to improve neuronal functions in the substantia nigra and striatum.
The delivery of AADC using AAV vectors, particularly AAV2-hAADC, has shown promising results in preclinical and clinical studies. AADC is an enzyme that is crucial for the synthesis of dopamine from its precursor, L-DOPA. In rodent models of Parkinson’s disease, DI of AAV2-hAADC to striatum significantly improved the conversion of L-DOPA to dopamine, leading to restored motor function and a measurable behavioral outcome [164]. In NHPs (n=2), DI delivery of the AAV-mediated AADC gene to the substantia nigra resulted in up to eight years of AADC expression with no signs of neuroinflammation and other side effects. In vivo PET imaging analysis revealed a significant reduction of AADC to 34.4% of the baseline on the non-injected side. Conversely, the injected side restored the signal up to 90.3% of the baseline at 96 months post-injection. Intriguingly, post-mortem analysis revealed that only about 5.6 ± 1% and 6.6 ± 1% of neurons within the transduced areas of the striatum were directly affected [165]. It was suspected that a low percentage of neurons were needed to be transduced to secrete the AADC to influence a much larger surrounding region. Transitioning from preclinical models to clinical trials, the AAV-mediated AADC delivery has continued to show encouraging outcomes. Patients receiving intracranial injections of AAV2-hAADC exhibit improvements in motor function, as evidenced by objective motor rating scales [27,244]. The therapy has been well-tolerated, with a safety profile highlighting its feasibility for clinical use. However, it is essential to note that while the overall results are positive, there are associated risks, including intracranial hemorrhage and transient headaches in some cases [244]. The current state of the art suggests that while AAV-mediated AADC delivery holds potential as a transformative treatment for PD, further refinement in delivery methods and long-term efficacy studies are needed to optimize its therapeutic impact.
The second enzyme-based strategy is to deliver GAD to the subthalamic nucleus. This approach is grounded in its potential to modulate the excessive glutamatergic activity in the substantia nigra by converting it to GABA, thus restoring inhibitory balance in a region where dopaminergic loss has led to circuit disruption. Promising preclinical research in rodent and NHP models has demonstrated the potential of AAV-GAD gene therapy in treating PD [166,167]. In these models, AAV-GAD delivered to the STN led to behavioral improvements. Clinical trials have been initiated following encouraging preclinical outcomes, focusing on evaluating the safety and efficacy of AAV2-GAD infusion in the human STN. AAV2-GAD delivery to the substantia nigra has led to notable motor improvements. These improvements have been quantified using the Unified Parkinson’s Disease Rating Scale (UPDRS) and sustained for up to a year post-treatment. PET imaging provides evidence of improved neural connectivity [33,245–247]. While these initial results are promising, it’s important to note that comprehensive long-term data and larger patient cohorts are needed to conclusively determine the therapeutic value of AAV2-GAD. However, the current clinical evidence supports further investigation into AAV2-GAD as a potential treatment modality for the motor symptoms of PD.
The third approach explores the delivery of neurotrophic factors, substances that support neurons’ growth, survival, and differentiation. Preclinical studies have shown the promise of DI of AAV vectors encoding neurotrophic factors such as the GDNF and NRTN in the substantia nigra and striatum of rodent and NHP models [168,171–173,248]. When AAV2-GDNF was injected into the NHP striatum, it effectively rescued dopaminergic neurons in the SN, likely due to the interconnectedness of neurons between the striatum and substantia nigra [248]. Administering AAV encoding human NRTN (AAV2-hNRTN) to the substantia nigra and striatum in NHPs significantly mitigated MPTP-induced motor deficits, showing 80 to 90% mprovements. Despite these promising preclinical results, the expected clinic outcome has not been achieved. For example, DI of AAV2-hNRTN into the putamen failed to produce notable benefits in PD patients [32]. The follow-up studies injected AAV to both the putamen and SN similarly did notyield significant improvements [24,249]. Notably, patients diagnosed with PD within five years appeared to benefit more from AAV2-NRTN therapy than those diagnosed >10 years. This discrepancy raises concerns that axonal transport deficiencies in later-stage patients might prevent the transport of AAV and the expressed neurotrophic factors from reaching and covering the entirety of the PD-affected regions. Insufficient delivery could cause the failure of the clinical trials. As such, the field continues refining the AAV vector delivery techniques, aiming to maximize the therapeutic reach within the targeted regions and improve outcomes for PD patients.
Alongside mainstream treatments, alternative treatments involve the delivery of the β-glucocerebrosidase (GBA) gene to the brain. This strategy addresses mutations in the GBA gene, which disrupts the enzyme glucocerebrosidase (GCase) production. The rationale is that the lack of GCase leads to an accumulation of lipid substrates such as glucosylceramide and glucosyl sphingosine in brain cells, contributing to neuronal damage and potentially exacerbating PD progression [250]. Preclinical studies have shown promising results by delivering AAV9 vectors carrying the GBA1 gene directly into the substantia nigra of both mice and non-human primates. These studies demonstrated that enhanced GCase activity reduced alpha-synuclein levels, a protein closely associated with PD and supported the survival of dopaminergic neurons [175]. Building on these findings, a clinical study (NCT04127578) is investigating the effects of ICM delivery of AAV9-GBA1 in patients to restore GCase function and improve neuron health.
Beyond invasive approaches, FUS-BBBO is an emerging noninvasive approach for AAV delivery in treating PD. A recent study has showcased the potential of this approach to noninvasively enable the delivery of AAV1-encoded GDNF to the substantia nigra and striatum. Utilizing MR guidance for precise targeting, this method has confirmed the accurate disruption of the BBB at the targeted location. Mice treated with the AAV-GDNF injection via the tail vein, coupled with unilateral FUS sonication, exhibited a notable increase in tyrosine hydroxylase activity, an enzyme pivotal in the dopamine synthesis. Substantial behavioral enhancements observed twelve weeks post-delivery suggest the therapeutic promise of this approach [176]. Additionally, FUS-BBBO has been applied to deliver an α-synuclein (α-syn) gene silencing vector (AAV9- hSNCA-shRNA) to four brain regions: the hippocampus, substantia nigra, olfactory bulb, and dorsal motor nucleus, in a mouse model of PD. It aims to mitigate the progression of the disease by this gene silencing approach to reduce α-synuclein accumulation, which is known to contribute to neuronal damage [146]. While the definitive therapeutic benefits of this approach remain to be confirmed, it holds the potential for neuroprotective effects in PD. Although FUS-BBBO is currently confined to the preclinical stage for PD, the feasibility and safety of BBB opening in Parkinson’s patients have been demonstrated in clinical trials, paving the way for its potential application in AAV delivery.
In summary, AAV-mediated gene therapy holds promise for revolutionizing PD treatment with strategies to replenish dopamine, restore neuronal balance, and protect neuronal health. While clinical applications are still under investigation, these approaches may lead to significant advancements in the management of PD. Further refinement of these techniques and detailed long-term studies will be crucial to optimize their therapeutic potential.
4.2. Alzheimer’s disease (AD)
AD is a progressive neurodegenerative disorder characterized by the accumulation of amyloid β and Tau proteins in the brain, which leads to the deterioration of cognitive functions and impaired learning abilities. This condition originates in the entorhinal cortex and hippocampus and progressively spreads to the cerebral cortex, leading to widespread brain atrophy. Preclinical and clinical studies have explored various strategies for AAV-mediated gene therapy for the treatment of AD. Clinical trials have focused on using DI, ICM, IV, and IT injection of AAV for delivering neurotrophic factors, nerve growth factor (NGF) to support neuronal survival and function, targeting genetic risk factors, such as the ε2 allele of apolipoprotein E (APOE2), and expressing active telomerase (hTERT) as an anti-aging strategy. Preclinical research also explored additional strategies, including inflammatory modulation and amyloid-beta clearance.
AAV vectors have delivered neurotrophic factors to support neuronal survival and function. Preclinical studies have demonstrated the efficacy of AAV-mediated neurotrophic factor delivery in enhancing neuronal survival and function [251]. A phase I/II clinical trial (NCT00087789) utilized stereotactic-guided DI of AAV2-NGF into the basal forebrain, targeting cholinergic neurons severely affected in AD. The cholinergic neurons of the basal forebrain experience significant degeneration in AD. An elevated NGF expression was observed in the cholinergic neurons of the injected area, with the expression enduring for up to two years. This expression was substantially higher compared to the neighboring neurons. Although the trial demonstrated the safety and feasibility of the approach, the cognitive benefits were inconclusive. This lack of benefit may be attributed to injection inaccuracies: over two-thirds of the injections missed their targets, likely due to the limited precision of the human stereotaxic method used. Additionally, the NGF spread minimally from the injection site, covering a mere 1 mm radius. This highlights the need for more precise delivery methods, such as real-time MRI-guided convection-enhanced delivery, to improve gene transfer accuracy and the need to enhance spread [252,253].
Genetic risk factors for AD, such as the apolipoprotein E (APOE) ε4 allele, have also been targeted by delivering protective alleles like APOE2 using AAV vectors. APOE4 is a critical genetic risk factor associated with the sporadic form of AD, which leads to the massive accumulation of Aβ in the AD brain, accelerates the likelihood of AD occurrence, and reduces the age of onset. Conversely, the APOE2 offers a protective effect against AD, countering the effects of APOE4 [180]. AAV-mediated delivery of APOE2 has shown promise in preclinical studies. DI delivery of AAV9-APOE2 to the thalamus has achieved a broad brain distribution and reductions in Aβ burden across several brain regions, including the thalamus, hippocampus, and cortex. The widespread distribution might be attributed to the AAV9’s retrograde and anterograde transportation capability. Additionally, the thalamus is extensively connected to various brain regions, which might facilitate this distribution [254]. AAVrh.10-APOE2 administered bilaterally into the hippocampus of NHPs resulted in persistent APOE2 expression and a dose-dependent reduction in Aβ burden. A recent study on NHPs evaluated AAVrh.10-mediated HA-tagged human APOE2 (AAVrh.10h-APOE2) by DI, ICM, and ICV delivery. The DI dose was set at a magnitude significantly lower than the ICM and ICV doses to prevent potential inflammatory reactions due to high vector concentrations in the hippocampal region. All routes resulted in efficient APOE2-HA protein expression within the hippocampus and entorhinal cortex. While DI led to localized expression around the injection areas, ICM and ICV produced broader brain-wide expressions. Specifically, ICM mainly impacted the brainstem, whereas ICV predominantly influenced the cortex. Though ICM typically precedes ICV in viral vector dispersion, both pathways showed similar mRNA and protein expression levels across the brain. ICM and ICV deliveries induced the formation of anti-AAVrh10 antibodies, with ICM generating marginally fewer antibodies than ICV [50]. The ICM delivery of AAVrh.10-hAPOE2 is being tested in AD clinical trials (NCT03634007). The results of this trial could provide insight into the therapeutic promise of this approach.
Another innovative strategy is the anti-aging approach, which uses AAV to express hTERT to potentially counteract cellular aging linked to AD. Although it has not been extensively studied yet, AAV9-mediated delivery of the TERT gene therapy extended lifespan and improved physiological markers in mice, such as insulin sensitivity, osteoporosis, and neuromuscular coordination, along with several molecular biomarkers of aging, including CyclinD1 and p16 [255]. Currently, both IV and IT delivery of AAV encoding hTERT are being evaluated in a phase I clinical trial (NCT04133454).
Beyond these, preclinical studies investigate the modulation of inflammation, a common feature in AD. The modulation has been achieved by overexpressing anti-inflammatory cytokines such as IL-4 or downregulating pro-inflammatory cytokines like CCL2 using AAV vectors. Bilateral DI of AAV1/2-IL-4 and AAV1/2–7ND in mouse hippocampus resulted in long-term, dose-dependent neuronal expression of IL-4 in the hippocampus and dentate gyrus [178] and CCL2/7ND in hippocampus and cortex, respectively [179]. Notably, the expression extended beyond the hippocampus, hinting at intraneuronal connections. Post DI delivery, the encoded cytokine expression in the hippocampus increased over time, peaking at 12 weeks and remaining stable up to 24 weeks. Suppressed glial accumulation, gliosis, and β-amyloidosis at the hippocampus with behavior efficacy were observed.
Amyloid-beta clearance remains a crucial target in AD gene therapy. AAV9 vectors encoding neprilysin, an enzyme that degrades Aβ peptides, have been used to reduce Aβ levels and improve spatial learning and memory in AD mouse models. A one-time intracardiac injection of AAV9-neprilysin into the left ventricle of heart of adult mice led to extensive neprilysin distribution across the brain limbic region, including the neocortex and hippocampus. This notably diminished Aβ levels and improved AD mice’s spatial learning and memory function. However, the study did not detail neprilysin’s distribution in other brain areas [182]. In very early work, people also developed an AAV vaccine to stimulate anti-amyloid antibody production. They showed reduced amyloid accumulation and astrocytosis in transgenic mice, improving their behavior [256]. However, there has been no follow-up of this study since 2003.
In conclusion, AAV gene therapy for AD is multifaceted, encompassing neurotrophic support, genetic targeting, anti-aging strategies, inflammatory modulation, and Aβ clearance. Various delivery methods, including DI, intra-CSF, and IV, are critical to these therapeutic approaches. The ongoing clinical trials and preclinical studies are paving the way for potential breakthroughs in AD treatment, offering hope for a disease that currently has no cure.
4.3. Huntington’s disease (HD)
HD is a genetic disorder caused by a mutation in the HTT gene, producing a mutant huntingtin protein. This aberrant protein accumulates in the basal ganglia, instigating neurodegeneration that manifests as motor and cognitive impairments. AAV-mediated gene therapies in clinical studies have included DI of AAV-miRNA to silence the mutant HTT gene, and a pioneering approach aims to express an enzyme to correct cholesterol metabolism disturbances linked to HD. Further, preclinical research has also explored AAV delivery of neurotrophic factors like BDNF to support affected neurons.
The primary strategy for HD gene therapy involves using DI of AAV to deliver microRNAs that silence the mutant HTT gene, thereby reducing the production of the toxic huntingtin protein. AAV5-miHTT, an AAV5 variant carrying a transgene for a designed miRNA against HTT mRNA, has demonstrated a significant dose-responsive decline in mutant huntingtin protein levels up to 39% in preclinical studies [257–260]. AAV5-miHTT affected substantial transduction in the striatum and distal brain structures when administered to minipigs through DI and IT injection. The DI was performed by multisite injection in the striatum, providing insights for clinical HD study [188]. Phase I/II clinical trials (NCT04120493, NCT05243017) evaluate the safety and efficacy of AAV5-miHTT (AMT-130) administered into the striatum of early-stage HD patients. Preliminary findings indicate a 68% reduction in mutated HTT following a single dose in patient-derived neuronal cells [261]. Additionally, IV delivery of AAV9 and AAV-AS vectors, both encoding artificial microRNAs targeting HTT (miRHtt), has been explored. This approach led to the suppression of HTT in various brain regions, including the striatum, thalamus, and motor cortex. AAV-AS is a novel serotype created by the insertion of a poly-alanine peptide into the AAV9 capsid, which enhances neuronal gene transfer in adult mice. AAV-AS was found to be 6-fold and 15-fold more efficient than AAV9 in gene transfer within the spinal cord and cerebrum, respectively [63,73].
Moreover, a newly launched approach is investigating the potential of CYP46A1 in treating HD. Cholesterol, essential for normal brain function and critical in neuron structure and signaling, becomes dysregulated in HD. This dysregulation is characterized by increased cholesterol accumulation in striatal neurons and reduced levels of cholesterol metabolic precursors [189]. CYP46A1 is an enzyme pivotal for converting cholesterol into 24-hydroxycholesterol, which is the primary pathway through which cholesterol is expelled from the brain. This process is crucial because disruptions in cholesterol metabolism, noted in HD due to the. Increasing the levels of CYP46A1 could potentially help restore normal cholesterol metabolism in the neurons of HD patients, potentially mitigating some of the pathological processes associated with the disease. With promising results in preclinical studies [189], the current clinical study assesses the effects of AAVrh10.CAG.hCYP46A1 administered via DI for early-stage HD patients (NCT05541627).
Although not tested in clinical trials, AAV delivery of neurotrophic factors to support neural survival and function has been investigated in preclinical studies. Specifically, bilateral injections of AAV encoding BDNF and GDNF into the striatum of adult rat models of HD demonstrated neuroprotective effects and behavioral benefits [184,185]. In one study, bilateral injections of AAV vectors carrying BDNF into the striatum of a transgenic rat model for HD resulted in localized increases in BDNF protein levels in the treated areas, leading to a reduction in both motor and cognitive impairments. Interestingly, one study revealed a gender-specific effect: female transgenic HD rats exhibited less functional impairment than their male counterparts [187].
In summary, AAV-mediated gene therapy presents a promising approach to combat HD. The promising results from preclinical studies and the encouraging data from ongoing clinical trials provide a hopeful outlook for the application of gene therapy in HD. Future research should aim to further elucidate the efficacy and safety of these therapies, optimize delivery methods, and explore the sex-specific responses observed in treatment outcomes.
4.4. Canavan disease (CD)
CD is a fatal genetic disorder marked by the deterioration of white matter in the brain, leading to severe neurological impairment. It stems from mutations in the aspartoacylase (ASPA) gene, which disrupts the breakdown of N-acetyl-L-aspartic acid (NAA), causing progressive damage to the brain’s myelin sheath. The disease predominantly affects infants, presenting with a decline in psychomotor development and a range of neurological symptoms. Currently, no cure exists for CD, with treatments primarily focusing on symptom management. However, recent advancements in gene therapy offer a glimpse of hope for CD patients. Existing preclinical and clinical studies all focus on one strategy: delivering AAV encoding normal ASPA by DI, IV, and ICV delivery.
DI of AAV2-ASPA has obtained promising data in preclinical and clinical studies. Using the rodent model, the delivery of AAV2-ASPA into the striatum and thalamus of CD mice resulted in reduced vacuolization and a decline in whole-brain NAA levels, alongside notable behavioral improvements [190,191]. Interestingly, ASPA-positive cells extended beyond the injection site, reaching areas like the cortex and white matter tracts. This widespread presence could be attributed to the axonal transport of the protein. Transitioning to human applications, a phase I/II clinical trial performed DI in six brain areas in the frontal, periventricular, and occipital regions, aiming for extensive distribution of the therapeutic gene. Given the disease’s pervasive nature and the considerable size of the human brain, this extensive approach was taken to ensure broad treatment coverage. The results were promising. Patients treated with AAV2-ASPA showed decreased NAA levels, especially in the periventricular and frontal regions, where the DI was performed. This clinical study demonstrated notable efficacy, such as modest but significant improvement in motor functions, such as rolling. A 5-year follow-up showed that AAV2-ASPA gene therapy decreased elevated NAA levels and slowed the progression of brain atrophy, with some improvement in seizure frequency and stabilizing the overall clinical status of the patients [242].
Further explorations into the route of administration have led to the evaluation of IV and ICV methods. Mouse studies used various AAV serotypes for ASPA delivery, including rh8, 9, and rh10. These studies highlighted the critical importance of early intervention, with the most significant therapeutic outcomes observed when treatments were administered in the neonatal period [192,193]. While both IV and ICV routes demonstrated efficacy in mitigating neuropathology and extending lifespan, the ICV method proved more potent, requiring a considerably lower dose. Subsequent optimization of this gene therapy has successfully prevented the onset of CD in mouse models and has also reversed the symptoms after their occurrence [194]. Building on these preclinical successes, an ongoing Phase 1/2 clinical trial assesses the safety and efficacy of a combined IV and ICV injection approach using AAV9-hASPA. Early results from this trial are encouraging, showing an 80% decline in NAA levels in the CSF over four years. Additionally, patients improved motor functions and visual capacity for up to two years post-treatment [262].
In summary, AAV-mediated gene therapy represents a promising frontier in treating CD. The advancements from preclinical models to clinical trials have consistently demonstrated the potential of AAV gene therapy to address the underlying genetic defect of CD. The ability of AAV vectors to deliver the ASPA gene effectively into the CNS, resulting in metabolic correction and clinical improvement, underscores the transformative potential of this therapeutic approach. Continued research is vital to refine these therapies, improve delivery methods, and understand the long-term outcomes. These interventions’ success in preclinical and clinical settings provides a foundation for the future of CD treatment. It offers hope to patients and families affected by this challenging disorder.
4.5. Lysosomal storage diseases (LSDs)
LSDs represent a complex group of inherited metabolic conditions stemming from a genetic mutation that leads to a specific enzyme deficiency within the lysosomes—essential cellular organelles responsible for breaking down waste materials. These deficiencies result in the accumulation of undigested or partially digested macromolecules, such as lipids, glycoproteins, and mucopolysaccharides, progressively leading to cellular dysfunction and clinical symptoms. The clinical manifestations of LSDs are diverse, often involving multiple organ systems, and can range from mild to severe. Due to the intrinsic genetic nature of these diseases, conventional treatments have been limited, prompting a shift toward gene therapy approaches. AAV-mediated gene therapy has emerged as a promising strategy to correct the underlying genetic defects by introducing functional copies of the gene responsible for the missing or dysfunctional enzyme, thereby tackling the root cause of these disorders.
Preclinical studies have underscored the potential of gene therapy for LSDs by employing AAV to compensate for deficient enzymes. This approach promises to correct enzyme levels within a range of cells and extends the therapeutic benefits to cell distal to the point of delivery. For instance, DI delivery of various AAV serotypes (1, 2, 5, 8, and rh10) encoding the hCLN2 gene has effectively produced the TPP-1 enzyme, with enzyme levels reaching up to 100 times that of healthy mice [195,199,204]. Other delivery routes have also been explored with success. CSF and IN delivery have been shown to enhance enzyme levels, reduce lysosomal storage, improve cognitive and motor functions, and prolonged survival [103,109,201–203,205,207,208]. Additionally, IV delivery of AAV9, although utilized to a lesser extent, has shown promise for achieving more uniform distribution and clearance of lysosomal storage across various brain regions and has been associated with improved behavioral outcomes [205,206,211,214]. IV administration is particularly relevant for systemic diseases such as Niemann-Pick disease (NPC), which affects both the brain and peripheral organs. Neonatal mice treated with IV-delivered AAV9 containing the NPC1 gene exhibited reduced cholesterol storage and mitigated loss of Purkinje neurons, indicative of therapeutic potential [214]. Similarly, in GM1 gangliosidosis, IV delivery of AAV9 improved neurological functions and resulted in high β-galactosidase activity and clearance of lysosomal storage in the liver, cortex, and spinal cord [211]. It’s crucial to clarify that the distribution of therapeutic enzymes does not necessarily coincide with the distribution of the virus vector. For instance, ICV injections of AAV4 carrying the green fluorescent protein (GFP) gene resulted in limited GFP expression, mainly in a small number of neurons, suggesting that AAV4 may not efficiently traverse the ependymal layer lining the ventricles to access the broader brain parenchyma. However, when AAV4 is used to deliver therapeutic enzymes, the transduced ependymal cells can effectively function as “biological pumps,” secreting high levels of enzymes that then permeate the entire brain [41].
Clinical studies have highlighted the potential of AAV-mediated gene therapy in treating LSDs. A notable phase I/II trial employed AAVrh.10-CUh-CLN2 to combat late infantile neuronal ceroid lipofuscinosis (LINCL), revealing a significant decline in disease progression as gauged by neurological rating scales. In this trial, ten children with LINCL underwent DI of the therapeutic AAV into twelve cortical locations via six burr holes (2 locations at 2 depths through each hole). Despite the overall positive trend, the trial faced challenges, including the death of one child 49 days after the procedure following an episode of status epilepticus on day 14. However, there were no indications of CNS inflammation. Due to safety considerations, there are constraints on the volume injection per unit time, vector concentration, and injection site numbers. Thus, the quest to distribute the vector across a larger brain volume is an ongoing challenge [263]. Opting for serotypes that provide extensive coverage, such as AAVrh.10, which exhibited vast NHP brain distribution and LINCL treatment efficacy [195], might offer a solution, mainly when used in ongoing clinical trials (NCT01414985, NCT01161576) [264]. For safety, AAVrh.10 dosages were curtailed to avert hyperintensities previously detected by T2 MRI images. These hyperintensities are indicative of changes such as edema or inflammation at the sites of vector administration [264].
While DI remains a commonly used method, the increasing trend toward less invasive methods like IT, ICM, and IV delivery is notable. These methods have been employed across extensive animal studies and clinical trials for a variety of LSDs, reflecting a broader approach to treatment delivery. For instance, IV administration of AAV9 in MPS IIIB showed promising results, with rapid and sustained decreases in biomarkers and a considerable reduction in liver volume, indicating therapeutic benefits for both the CNS and peripheral organs [265]. These outcomes demonstrate the potential for gene therapy to confer benefits to both the CNS and peripheral organs affected by LSDs.
In summary, AAV-mediated gene therapy represents a transformative approach to treating LSDs, with the potential to not only alleviate symptoms but also address the underlying enzyme deficiencies. The progression from preclinical to clinical studies has been marked by encouraging outcomes, suggesting that gene therapy could play a crucial role in the future management of LSDs. Ongoing research is focused on optimizing delivery methods and dosages to maximize therapeutic efficacy and safety to provide a lasting solution for patients suffering from these challenging disorders.
4.6. Rett syndrome (RTT)
RTT is a rare genetic neurological disorder that primarily affects young girls. It is caused by mutations in the MECP2 gene, which is vital for brain development and function. MECP2 is predominantly active in the nucleus and is crucial for neuronal differentiation, maturation, circuit refinement, and synaptic maintenance. Mutations in MECP2 lead to widespread neuronal dysfunction, manifesting as severe impairments in speech, motor skills, cognitive development, and other neurological issues. Although there is no cure for RTT, recent advancements in gene therapy offer hope. The primary AAV-based gene therapy for RTT focuses on gene replacement by introducing a functional MECP2 gene, which resides on the X chromosome. In recent years, innovative strategies such as gene editing and reactivation of the silenced X-chromosome have been explored, primarily in in vitro settings, for their potential applications in gene therapy for RTT. For those interested in a detailed review of the state-of-the-art in gene therapy for RTT, including these emerging approaches, the excellent review papers by Nicolas et al. [266] and Michela et al. [267] offer extensive insights. Our discussion will primarily focus on AAV brain delivery strategies in the treatment of RTT.
Early preclinical studies involved administering AAV2/9-MECP2 by DI into the brains of newborn MECP2-null mice. This method achieved significant transduction in crucial brain areas, such as the thalamus, hypothalamus, motor cortex, and hippocampus, leading to improved motor functions and extended lifespan [216]. The same study explored IV injection of AAV9-MECP2 in juvenile mice, which showed less efficiency in brain transduction (less than 5%) and raised concerns about liver toxicity due to MECP2 overexpression [216]. However, this strategy faces challenges, as overexpression of MECP2 can cause severe neurological defects and liver damage. Therefore, researchers are exploring methods to precisely control gene expression. Subsequent IV delivery of AAV9-codon-optimized MECP2e1 (MCO) in older heterozygous female mice increased the transduction efficiency in the CA3, brain stem, and cortex to 20–25%, without liver damage [219]. However, higher doses of this AAV9-MCO also led to severe off-target effects [222]. These findings underscore the need for balancing transduction efficiency with potential side effects in IV delivery.
Intra-CSF delivery was then explored for treating RTT for the purpose to minimize peripheral transgene expression and increased delivery efficiency to brain. ICV injection of AAV9-MECP2 into neonatal MECP2-null mice resulted in higher brain transduction efficiency compared to IV delivery, enhancing survival, and reducing RTT-like symptoms [217,268]. Similarly, ICM delivery of the same vector to juvenile MECP2 mutant mice showed improvements in lifespan and body weight, although behavioral traits were not consistently rescued [218]. It is important to note that high-dose of intra-CSF delivery can potentially lead to CNS side effects, such as neurological distress [221]. Recent preclinical advancements have focused on integrating synthetic vectors like AAV-PHP.B with regulatory elements to optimize delivery efficiency and mitigate the challenge of gene overexpression. AAV-PHP.B vectors, recognized for their increased BBB permeability in adult mice, have been paired with miR-responsive auto-regulatory elements (miRARE). miRARE serves as an inhibitory element that is activated in response to excessive MECP2 expression, thereby enhancing the safety of the miniMECP2 gene transfer. MiniMECP2 is a truncated version of the MECP2 gene, retaining essential functional domains while being shorter than the full-length gene [221]. Incorporating miRARE ensures the effectiveness of gene transfer while mitigating potential safety concerns. Both ICM delivery of AAV-PHP.B-miniMECP2-miRARE and IT delivery of AAV9-miniMECP2-miRARE have been successful in reducing the occurrence of adverse effects typically associated with unregulated miniMECP2 gene transfer, while maintaining therapeutic benefits [221]. Moreover, IV delivery of AAV-PHP.eB with an instability-prone MECP2 (iMecp2) transgene cassette has shown promising results. This approach provides widespread and efficient gene transfer while maintaining physiological Mecp2 protein levels in the brain, presenting a potentially effective and safe method for gene therapy in RTT [220].
The clinical trials for RTT have been launched in 2023 based on intra-CSF delivery of MECP2 gene, representing a significant advancement in applying these preclinical insights. The first trial investigated TSHA-102 (NCT05606614), an AAV9-delivered gene therapy that uses the miniMECP2-miRARE to regulate and control the overexpression of the MECP2 gene. TSHA-102 is administered by IT to evaluate its safety and preliminary efficacy in adult females diagnosed with RTT. Concurrently, another trial (NCT05898620) focused on NGN-401, a gene therapy containing a full-length human MECP2 gene, administered via ICV injection. NGN-401 is designed to express therapeutic levels of the MECP2 protein without causing overexpression, thus minimizing toxicity associated with it. These clinical trials are pivotal in translating preclinical findings into clinical settings, potentially offering effective treatment options for RTT.
In summary, AAV-mediated gene therapy has emerged as a promising approach in treating RTT, with significant advancements transitioning from preclinical studies to clinical trials. The utilization of AAV vectors for the effective delivery of the MECP2 gene into the CNS has the potential to correct the underlying genetic defect in RTT, showcasing the transformative potential of this therapeutic method. The incorporation of regulatory elements such as miRARE, which aids in controlling gene expression, further enhances this potential. Although the focus here is primarily on gene replacement therapy, the exploration of novel gene editing techniques and the reactivation of dormant genes also open new avenues for upcoming preclinical and clinical studies. Moreover, the results from ongoing clinical trials are keenly anticipated to further assess the efficacy and safety of these innovative gene therapy methods in treating RTT.
4.7. Fragile X syndrome (FXS)
FXS is a neurodevelopmental disorder linked to the X chromosome, primarily caused by a mutation in the Fragile X Messenger Ribonucleoprotein 1 (FMR1) gene. This mutation leads to the absence of the fragile X messenger ribonucleoprotein (FMRP), a vital mRNA-binding protein necessary for normal brain development. The lack of FMRP results in various symptoms, including developmental delays (like delayed sitting, walking, or speaking), learning disabilities, and social and behavioral challenges. Intellectual disability of varying degrees is often observed in males with FXS, while females may have either normal intelligence or some level of intellectual disability. While there is currently no cure for FXS, various treatments and therapies are employed to assist in skill development and manage symptoms. A promising area in the treatment of FXS is AAV-mediated gene therapy, which focuses on gene replacement to address the core molecular deficiencies of the disorder [269]. This therapy typically involves delivering a functional copy of the FMR1 gene to compensate for the lack of FMRP [269]. A recent and promising approach targets diacylglycerol kinase kappa (DGKk), a key factor implicated in the pathogenesis of FXS, to potentially mitigate FXS-related behavioral abnormalities [228]. It’s important to note that while multiple isoforms of the FMR1 gene have been used in different studies, they will all be referred to as the FMR1 gene in this context, without delving into the specifics of these isoforms.
Multiple AAV delivery methods have been employed to deliver the FMR1 gene for the treatment of FXS, each showing varying degrees of benefit or improvement in behavior. The initial in vivo AAV-based gene therapy involved the delivery of an AAV5 vector encoding the FMR1 gene directly into the hippocampus of 5-week-old FMR1 knockout mice. This approach successfully corrected the abnormally enhanced long-term depression observed in these mice [223]. However, considering that FXS affects the entire brain, a broad distribution of AAV delivery is preferred. Subsequent explorations shifted to using ICV injection of AAV9-FMR1 in neonatal mice on postnatal day 5, achieving long-term FMRP expression in the forebrain regions near the ventricles [224]. Later advancements refined this approach, finding that performing the injection on postnatal day 0–2 led to superior diffusion of the vector within the forebrain. It is hypothesized that the ependymal lining surrounding the brain’s ventricles is still immature in the first two days after birth. Therefore, AAV vectors can diffuse more effectively to distal regions within the brain parenchyma when injected at this early time point, compared to injections made a few days later [225]. Additionally, this study also found that while moderate overexpression of FMRP was beneficial and safe, excessive overexpression (more than double of normal levels) worsened motor hyperactivity [225]. Although ICV injections offer broader distribution than DI, it remains challenging to achieve widespread AAV distribution in brain regions away from the ventricles. Recent investigations explored other delivery methods, including a combination of ICV and ICM, IT, and IV delivery of BBB permeable AAVs. The ICV+ICM method was thought to achieve extensive transduction across large brain areas. However, the increase in FMRP expression in the caudal regions of the brain was relatively modest compared to ICV injection alone [227]. For IT delivery, a dose-dependent AAV9-FMR1 transcription of the transgene was found, with the high dose group showing broader distribution throughout the brain, including the hippocampus, cerebral cortex, midbrain, and brainstem [230]. It worths to note that that all intra-CSF injections in these studies were performed at an early age in rodents, aiming for more widespread diffusion of AAV-FMR1 [224,227,270]. IV delivery of AAV-PHP.eB encoding FMR1 to adult mouse showed high efficiency in delivering FMR1 gene in all tested brain regions, including the cortex, hippocampus, and cerebellum. It exceeded control levels of FMRP across all tested brain regions and led to partial improvements in FXS-related behaviors. These findings highlight the feasibility and efficacy of IV delivery in adult mice.
In addition to directly introducing the FMR1 gene, an alternative innovative method was to introduce DGKk through AAV delivery. DGKk is a primary mRNA target of FMRP and is involved in brain lipid signaling control. It was found that DGKk was downregulated in the brains of FXS patients and FMR1 knockout mouse models. DI of AAVRh10-DGKk into the cerebral region was shown to restore DGKk expression and function, effectively reversing abnormal behavioral alterations in adolescent FMR1-null mice. Contrastingly, IV delivery of AAV-PHP.eB-DGKk did not successfully rescue the FMR1-KO phenotype at the administered dose. This lack of efficacy could be attributed to insufficient brain expression levels of DGKk and the limited dose of the AAV vector delivered through retro-orbital injection, a method commonly used in small animals like mice that involves administering a substance into the vein-rich area behind the eye for quick systemic delivery [228]. This highlights the importance of both the gene being delivered and the delivery method in gene therapy approaches for FXS.
In summary, AAV-mediated gene therapy for FXS represents a significant advancement in preclinical studies. The introduction of the functional FMR1 gene through gene replacement therapy aims to correct the fundamental genetic defect in FXS. DGKk targets disrupted signaling pathways associated with the absence of FMRP, offering a novel therapeutic angle. Both methods have demonstrated efficacy in preclinical models, showing improvements in rodents’ FXS-related behaviors and neurological functions. The use of various AAV vector serotypes and delivery methods further underscores the versatility of this therapeutic strategy. Future clinical studies are anticipated to explore further and refine these therapeutic strategies.
4.8. Dravet syndrome (DS)
DS is a severe neurodevelopmental disorder characterized by mutations in the SCN1A gene, which encodes the alpha subunit of the voltage-gated sodium channel Nav1.1. These genetic alterations disrupt sodium channel function, leading to recurrent seizures that typically begin in infancy and are accompanied by a spectrum of neurological and developmental impairments, such as cognitive deficits, motor dysfunction, and behavioral abnormalities. Delivering a functional copy of the SCN1A gene to correct DS is challenging due to the large and unstable nature of SCN1A cDNA [271]. AAV-based gene therapy has shifted towards strategies that supplement the existing cellular mechanisms rather than replace the SCN1A gene. These gene supplementation approaches focus on delivering auxiliary components that bolster the function of the existing proteins. Additionally, gene editing techniques, particularly those utilizing CRISPR technology, have been adapted to perform targeted modifications that enhance the function of the native sodium channels compromised by SCN1A mutations. This method of gene editing can be viewed as a sophisticated form of gene supplementation, as it seeks to optimize and augment the existing gene’s function without introducing a new gene, thereby improving clinical outcomes in individuals with DS.
One strategy involves delivering an auxiliary sodium channel subunit, Navb1, encoded by the pGad1-Navb1-myc AAV9 vector. In preclinical studies using a DS mouse model, bilateral ICV and ICM injections of this vector resulted in increased survival rates, particularly in females, and reduced seizure frequency in males. However, behavioral improvements were observed only in male mice, highlighting potential sex-specific differences in treatment response [231]. Another strategy involves AAV-mediated delivery of an engineered transcription factor, eTFSCN1A, aimed at increasing expression of the SCN1A gene specifically in GABAergic interneurons. In both preclinical mouse models and non-human primate studies, ICV administration of AAV9-REGABA-eTFSCN1A led to enhanced SCN1A expression, reduced seizure susceptibility, and improved survival rates [232]. These promising results have paved the way for clinical trials (NCT05419492) to evaluate the safety and efficacy of this approach in DS patients.
Gene editing techniques utilizing CRISPR-based approaches, particularly employing dead Cas9 (dCas9) fused with transcriptional activators like VP64 or VPR, have been devised to boost SCN1A gene expression [233,234]. One method entails employing a dual AAV9 vector system (TRE-dCas9-VP64 and U6-sgRNA-mDlx5/6-tTA-tdTomato) that targets the proximal promoter of SCN1A using a single guide RNA (gRNA). These AAVs were administered via ICV injection to neonatal DS mice (P0). This intervention effectively augmented SCN1A gene expression in forebrain GABAergic interneurons, elevated Nav1.1 levels, and improved electrophysiological responses. Despite the treatment leading to heightened seizure thresholds and reduced seizure severity and duration, complete suppression wasn’t achieved, likely due to the limited co-infection efficiency of the two separate AAVs [233]. In another study, IV administration of AAV-PHP.eB carrying four tandem gRNAs (AAV-4xU6-sgRNA: mSC1U1, mSC1U2, hmSC1U3, and mSC1U4) was performed in adult SCN1A mice with neuron-specific expression of dCas9-VPR. This intervention significantly upregulated SCN1A mRNA and Nav1.1 protein levels, resulting in elevated seizure thresholds, prolonged latency to clonic seizures, and improved behavioral outcomes and survival. Although the use of transgenic CRISPR-ON mice presents challenges in direct clinical translation, these findings underscore that enhancing SCN1A expression in inhibitory neurons can effectively ameliorate disease phenotypes. Moreover, the IV injection of AAV-PHP.eB carrying the transgene even makes the treatment feasible when initiated post-juvenile stages [233,234].
In summary, AAV-mediated gene therapy has emerged as a promising approach for treating DS, with significant progress moving from preclinical studies to clinical trials initiated in 2022. The use of AAV vectors has demonstrated effectiveness in delivering auxiliary subunits that regulate ion channel activities and engineered transcription factors to enhance SCN1A expression. Additionally, CRISPR-based techniques have also been explored to achieve precise targeting and increase expression of the SCN1A gene. While not all of these techniques are directly translating to clinical practice, they offer promising prospects for developing effective treatment strategies and potentially finding a cure for DS.
4.9. Angelman syndrome (AS)
AS is a neurodevelopmental disorder primarily stemming from the lack of expression of the UBE3A gene in the brain, a gene responsible for encoding the ubiquitin ligase E3A enzyme. In healthy individuals, the UBE3A gene from the father is naturally silent while the maternal UBE3A gene is active, playing a crucial role in brain development. However, in individuals with AS, this critical maternal gene expression is lost. Although a functional paternal UBE3A gene is present, it remains inactive, silenced by a long non-coding antisense transcript known as UBE3A-ATS. This leads to reduced levels of the UBE3A protein, which is essential for proper neurological development. Individuals with AS typically experience intellectual deficits, seizures, ataxic movements, and significant challenges with speech and language. In addressing AS, current AAV-based gene therapy strategies primarily focus on two approaches: introducing UBE3A isoforms directly into the brain or activating the silenced paternal UBE3A allele by modifying the genomic DNA to enhance its expression.
Gene replacement therapy for AS focuses on introducing functional UBE3A isoforms directly into the brain to compensate for the inactive maternal gene. Initial studies utilized AAV9 vectors to deliver the UBE3A gene to the hippocampus of AS mice, co-administered with 20% mannitol to enhance uptake [235]. These studies achieved partial restoration of learning capabilities and synaptic function. However, this method did not significantly increase UBE3A expression in the cerebellum, nor did it impact motor symptoms [235]. In addition, the potential UBE3A overexpression by DI also raised the concern to cause autism-like symptoms [272]. Subsequent research explored AAV9-derived PHP.B vectors to deliver a codon-optimized human UBE3A transgene via ICV injection to neonatal AS mice. This approach resulted in widespread expression of human UBE3A in dorsal forebrain neurons early in development, offering promise for mitigating AS-like phenotypes from an early stage and continuing into adulthood [236]. A novel approach for gene therapy has recently been developed using a secreted form of UBE3A, termed STUB. This method allows transduced cells to not only produce UBE3A but also to secrete it, enabling neighboring non-transduced cells to uptake the protein. ICV injections of the STUB vector have shown to be more effective than traditional non-secreted UBE3A vectors in rescuing behavioral and electrophysiological deficits in a rat model of AS [237].
In addition to replacing the gene, another set of strategies focuses on unsilencing the paternal allele of UBE3A that is naturally inactive in the brain by the UBE3A-ATS. CRISPR-Cas9 technology has been particularly instrumental in this approach, allowing precise targeting of the regulatory regions near UBE3A-ATS to reduce its expression and thereby increase the expression of the paternal UBE3A allele. Studies have demonstrated the effectiveness of ICV injection of AAV9-SpCas9-gRNAs in neonatal mice, which effectively reduced Ube3a-ATS expression and unsilenced paternal UBE3A, leading to improvements in AS phenotypes [238]. Comparisons between ICV and IV delivery of CRISPR systems highlighted that ICV injections in neonates achieved higher editing frequencies and were more suitable for efficient gene editing [239]. In addition to CRISPR-based strategies, recent advancements have utilized Zinc Finger Proteins (ZFPs) equipped with a Kruppel-associated box (KRAB) domain to engineer artificial transcription factors (ATFs), specifically ATF-S1K, to selectively inhibit the UBE3A-ATS. IV injection of AAV-PHP.eB-ATF-S1K in 6-week-old AS mice resulted in widespread brain transduction and restored UBE3A protein levels to about 25% of wild-type levels, which significantly improved exploratory locomotion behaviors [240].
In summary, AAV-mediated gene therapy has demonstrated significant promise in preclinical studies for treating AS, employing both gene replacement and gene silencing techniques. Selecting appropriate delivery methods is crucial to optimize gene expression levels without causing overexpression. The timing of gene delivery is also critical and should be carefully considered in relation to the developmental stage of the patient. Future clinical studies are poised to further refine these therapeutic approaches, aiming to develop effective treatments for individuals with AS. This will include advancing the exploration of delivery techniques, optimizing dosages, and determining the most beneficial age for administering gene therapy.
5. Conclusion
Gene therapy using AAV vector systems is emerging as a formidable tool in the battle against neurological diseases. This comprehensive review provides an in-depth look into the diverse strategies developed for delivering AAV into the brain, each with distinct advantages and limitations. Invasive approaches, including DI and intra-CSF delivery, are efficient in AAV delivery but carry surgical risks. They are associated with limitations in their capability to broadly distribute AAV vectors, especially problematic for diseases that affect multiple brain areas. Recognizing these challenges, there has been a surge in research aiming to engineer specialized capsids that can be introduced through IV, allowing them to cross the BBB. Yet, these innovative techniques are still in their early stages of development and face hurdles towards clinical adoption. Some challenges include the varying ability of engineered capsids to penetrate the BBB across different species and potential adverse effects, such as liver toxicity. Additionally, this review spotlights the emerging technique FUS-BBBO for AAV delivery, underscoring its potential for noninvasive AAV delivery based on early research findings.
A key takeaway from this review is the complexity of factors that influence the choice of AAV delivery method. This includes the nature and size of the brain lesion, the therapeutic action of the AAV gene, the efficiency of the protein expressed in neurological functions, and patient-specific considerations like disease type. The research landscape suggests that a one-size-fits-all solution is impractical; a tailored approach is necessary for each unique therapeutic context.
In conclusion, the journey of developing AAV gene therapy for neurological diseases is marked by significant potential yet accompanied by complex challenges. This review serves as a thorough guide, outlining the current state of AAV delivery methods and their applications in treating various neurological diseases. It is a crucial resource for researchers and clinicians exploring the promising yet intricate world of AAV-mediated gene therapies for brain disorders.
Acknowledgments
Supported by National Institutes of Health R01CA276174, UG3MH126861, R01EB027223, R01EB030102, and R01NS128461.
Footnotes
Declaration of generative AI in scientific writing
During the preparation of this review, the authors used OpenAi (Chat GPT) to enhance the clarity and conciseness of extensive or verbose sentences. After using this tool/service, the authors reviewed and edited the content as needed and take full responsibility for the content of the publication.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- [1].Feigin VL, Vos T, Nichols E, Owolabi MO, Carroll WM, Dichgans M, Deuschl G, Parmar P, Brainin M, Murray C, The global burden of neurological disorders: translating evidence into policy, Lancet Neurol. 19 (2020) 255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Piguet F, Alves S, Cartier N, Clinical Gene Therapy for Neurodegenerative Diseases: Past, Present, and Future, Hum. Gene Ther 28 (2017) 988–1003. [DOI] [PubMed] [Google Scholar]
- [3].Kantor B, Bailey RM, Wimberly K, Kalburgi SN, Gray SJ, Methods for Gene Transfer to the Central Nervous System, in: Adv. Genet, 2014: pp. 125–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Ingusci S, Verlengia G, Soukupova M, Zucchini S, Simonato M, Gene Therapy Tools for Brain Diseases, Front. Pharmacol 10 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Schambach A, Zychlinski D, Ehrnstroem B, Baum C, Biosafety features of lentiviral vectors, Hum. Gene Ther 24 (2013) 132–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Choudhury SR, Hudry E, Maguire CA, Sena-Esteves M, Breakefield XO, Grandi P, Viral vectors for therapy of neurologic diseases, Neuropharmacology. 120 (2017) 63–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Lentz TB, Gray SJ, Samulski RJ, Viral vectors for gene delivery to the central nervous system, Neurobiol. Dis 48 (2012) 179–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Bedbrook CN, Deverman BE, Gradinaru V, Viral Strategies for Targeting the Central and Peripheral Nervous Systems, Annu. Rev. Neurosci 41 (2018) 323–348. [DOI] [PubMed] [Google Scholar]
- [9].Kyostio-Moore S, Berthelette P, Piraino S, Sookdeo C, Nambiar B, Jackson R, Burnham B, O’Riordan CR, Cheng SH, Armentano D, The impact of minimally oversized adeno-associated viral vectors encoding human factor VIII on vector potency in vivo, Mol. Ther. - Methods Clin. Dev 3 (2016) 16006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Penaud-Budloo M, François A, Clément N, Ayuso E, Pharmacology of Recombinant Adeno-associated Virus Production, Mol. Ther. - Methods Clin. Dev 8 (2018) 166–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Rapti K, Grimm D, Adeno-Associated Viruses (AAV) and Host Immunity – A Race Between the Hare and the Hedgehog, Front. Immunol 12 (2021) 1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Flotte TR, Gene therapy progress and prospects: Recombinant adeno-associated virus (rAAV) vectors, Gene Ther. 11 (2004) 805–810. [DOI] [PubMed] [Google Scholar]
- [13].Ling Q, Herstine JA, Bradbury A, Gray SJ, AAV-based in vivo gene therapy for neurological disorders, Nat. Rev. Drug Discov 22 (2023). [DOI] [PubMed] [Google Scholar]
- [14].Watanabe N, Yano K, Tsuyuki K, Okano T, Yamato M, Re-examination of regulatory opinions in Europe: possible contribution for the approval of the first gene therapy product Glybera, Mol. Ther. Methods Clin. Dev 2 (2015) 14066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Darrow JJ, Luxturna: FDA documents reveal the value of a costly gene therapy, Drug Discov. Today 24 (2019) 949–954. [DOI] [PubMed] [Google Scholar]
- [16].Hoy SM, Onasemnogene Abeparvovec: First Global Approval, Drugs. 79 (2019) 1255–1262. [DOI] [PubMed] [Google Scholar]
- [17].Heo YA, Etranacogene Dezaparvovec: First Approval, Drugs. 83 (2023) 347–352. [DOI] [PubMed] [Google Scholar]
- [18].Schimmer J, Breazzano S, Investor Outlook: Rising from the Ashes; GSK’s European Approval of Strimvelis for ADA-SCID, Hum. Gene Ther. Clin. Dev 27 (2016) 57–61. [DOI] [PubMed] [Google Scholar]
- [19].Wu D, Chen Q, Chen X, Han F, Chen Z, Wang Y, The blood–brain barrier: structure, regulation, and drug delivery, Signal Transduct. Target. Ther 8 (2023) 217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Hawkins BT, Davis TP, The blood-brain barrier/neurovascular unit in health and disease, Pharmacol. Rev 57 (2005) 173–185. [DOI] [PubMed] [Google Scholar]
- [21].Daneman R, Prat A, The blood–brain barrier, Cold Spring Harb. Perspect. Biol 7 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ballabh P, Braun A, Nedergaard M, The blood – brain barrier : an overview Structure , regulation , and clinical implications, 16 (2004) 1–13. [DOI] [PubMed] [Google Scholar]
- [23].Fiandaca MS, Varenika V, Eberling J, McKnight T, Bringas J, Pivirotto P, Beyer J, Hadaczek P, Bowers W, Park J, Federoff H, Forsayeth J, Bankiewicz KS, Real-time MR imaging of adeno-associated viral vector delivery to the primate brain, Neuroimage. 47 (2009) T27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Bartus RT, Baumann TL, Siffert J, Herzog CD, Alterman R, Boulis N, Turner DA, Stacy M, Lang AE, Lozano AM, Warren Olanow C, Safety/feasibility of targeting the substantia nigra with AAV2-neurturin in Parkinson patients, Neurology. 80 (2013) 1698–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Sanftner LM, Suzuki BM, Doroudchi MM, Feng L, McClelland A, Forsayeth JR, Cunningham J, Striatal delivery of rAAV-hAADC to rats with preexisting immunity to AAV, Mol. Ther 9 (2004) 403–409. [DOI] [PubMed] [Google Scholar]
- [26].Bankiewicz KS, Eberling JL, Kohutnicka M, Jagust W, Pivirotto P, Bringas J, Cunningham J, Budinger TF, Harvey-White J, Convection-enhanced delivery of AAV vector in Parkinsonian monkeys; in vivo detection of gene expression and restoration of dopaminergic function using pro-drug approach, Exp. Neurol 164 (2000) 2–14. [DOI] [PubMed] [Google Scholar]
- [27].Christine CW, Bankiewicz KS, Van Laar AD, Richardson RM, Ravina B, Kells AP, Boot B, Martin AJ, Nutt J, Thompson ME, Larson PS, Magnetic resonance imaging–guided phase 1 trial of putaminal AADC gene therapy for Parkinson’s disease, Ann. Neurol 85 (2019) 704–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Salegio LEA, Samaranch, Kells AP, Forsayeth J, and Bankiewicz* KS, Guided delivery of adeno-associated viral vectors into the primate brain, Adv Drug Deliv Rev. 23 (2011) 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Mastakov MY, Baer K, Kotin RM, During MJ, Recombinant adeno-associated virus serotypes 2-and 5-mediated gene transfer in the mammalian brain: Quantitative analysis of heparin co-infusion, Mol. Ther 5 (2002) 371–380. [DOI] [PubMed] [Google Scholar]
- [30].Brown RC, Egleton RD, Davis TP, Mannitol opening of the blood-brain barrier: Regional variation in the permeability of sucrose, but not 86Rb+ or albumin, Brain Res. 1014 (2004) 221–227. [DOI] [PubMed] [Google Scholar]
- [31].Carty N, Lee D, Dickey C, Ceballos-Diaz C, Jansen-West K, Golde TE, Gordon MN, Morgan D, Nash K, Convection-enhanced delivery and systemic mannitol increase gene product distribution of AAV vectors 5, 8, and 9 and increase gene product in the adult mouse brain, J. Neurosci. Methods 194 (2010) 144–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Marks WJ, Bartus RT, Siffert J, Davis CS, Lozano A, Boulis N, Vitek J, Stacy M, Turner D, Verhagen L, Bakay R, Watts R, Guthrie B, Jankovic J, Simpson R, Tagliati M, Alterman R, Stern M, Baltuch G, Starr PA, Larson PS, Ostrem JL, Nutt J, Kieburtz K, Kordower JH, Olanow CW, Gene delivery of AAV2-neurturin for Parkinson’s disease: A double-blind, randomised, controlled trial, Lancet Neurol. 9 (2010) 1164–1172. [DOI] [PubMed] [Google Scholar]
- [33].LeWitt PA, Rezai AR, Leehey MA, Ojemann SG, Flaherty AW, Eskandar EN, Kostyk SK, Thomas K, Sarkar A, Siddiqui MS, Tatter SB, Schwalb JM, Poston KL, Henderson JM, Kurlan RM, Richard IH, Van Meter L, Sapan CV, During MJ, Kaplitt MG, Feigin A, AAV2-GAD gene therapy for advanced Parkinson’s disease: A double-blind, sham-surgery controlled, randomised trial, Lancet Neurol. 10 (2011) 309–319. [DOI] [PubMed] [Google Scholar]
- [34].Albert K, Voutilainen MH, Domanskyi A, Airavaara M, AAV vector-mediated gene delivery to substantia nigra dopamine neurons: Implications for gene therapy and disease models, Genes (Basel). 8 (2017) 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Janson C, McPhee S, Bilaniuk L, Haselgrove J, Testaiuti M, Freese A, Wang DJ, Shera D, Hurh P, Rupin J, Saslow E, Goldfarb O, Goldberg M, Larijani G, Sharrar W, Liouterman L, Camp A, Kolodny E, Samulski J, Leone P, Gene therapy of Canavan disease: AAV-2 vector for neurosurgical delivery of aspartoacylase gene (ASPA) to the human brain, Hum. Gene Ther 13 (2002) 1391–1412. [DOI] [PubMed] [Google Scholar]
- [36].Tardieu M, Zérah M, Gougeon ML, Ausseil J, de Bournonville S, Husson B, Zafeiriou D, Parenti G, Bourget P, Poirier B, Furlan V, Artaud C, Baugnon T, Roujeau T, Crystal RG, Meyer C, Deiva K, Heard JM, Intracerebral gene therapy in children with mucopolysaccharidosis type IIIB syndrome: an uncontrolled phase 1/2 clinical trial, Lancet Neurol. 16 (2017) 712–720. [DOI] [PubMed] [Google Scholar]
- [37].Passini MA, Wolfe JH, Widespread gene delivery and structure-specific patterns of expression in the brain after intraventricular injections of neonatal mice with an adeno-associated virus vector., J. Virol 75 (2001) 12382–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Passini MA, Watson DJ, Vite CH, Landsburg DJ, Feigenbaum AL, Wolfe JH, Intraventricular Brain Injection of Adeno-Associated Virus Type 1 (AAV1) in Neonatal Mice Results in Complementary Patterns of Neuronal Transduction to AAV2 and Total Long-Term Correction of Storage Lesions in the Brains of -Glucuronidase-Deficient Mice, J. Virol 77 (2003) 7034–7040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Chakrabarty P, Rosario A, Cruz P, Siemienski Z, Ceballos-Diaz C, Crosby K, Jansen K, Borchelt DR, Kim JY, Jankowsky JL, Golde TE, Levites Y, Capsid Serotype and Timing of Injection Determines AAV Transduction in the Neonatal Mice Brain, PLoS One. 8 (2013) 0–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Kim JY, Grunke SD, Levites Y, Golde TE, Jankowsky JL, Intracerebroventricular viral injection of the neonatal mouse brain for persistent and widespread neuronal transduction, J. Vis. Exp (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Dodge JC, Treleaven CM, Fidler JA, Hester M, Haidet A, Handy C, Rao M, Eagle A, Matthews JC, Taksir TV, Cheng SH, Shihabuddin LS, Kaspar BK, AAV4-mediated expression of IGF-1 and VEGF within cellular components of the ventricular system improves survival outcome in familial ALS mice, Mol. Ther 18 (2010) 2075–2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Liu G, Martins I, Wemmie JA, Chiorini JA, Davidson BL, Functional correction of CNS phenotypes in a lysosomal storage disease model using adeno-associated virus type 4 vectors, J. Neurosci 25 (2005) 9321–9327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Davidson BL, From the Cover: Recombinant adeno-associated virus type 2, 4, and 5 vectors: Transduction of variant cell types and regions in the mammalian central nervous system, Proc. Natl. Acad. Sci 97 (2000) 3428–3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Hudry E, Vandenberghe LH, Therapeutic AAV Gene Transfer to the Nervous System: A Clinical Reality, Neuron. 101 (2019) 839–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Gong Y, Berenson A, Laheji F, Gao G, Wang D, Ng C, Volak A, Kok R, Kreouzis V, Dijkstra IM, Kemp S, Maguire CA, Eichler F, Intrathecal Adeno-Associated Viral Vector-Mediated Gene Delivery for Adrenomyeloneuropathy, Hum. Gene Ther 30 (2019) 544–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Guo Y, Wang D, Qiao T, Yang C, Su Q, Gao G, Xu Z, A Single Injection of Recombinant Adeno-Associated Virus into the Lumbar Cistern Delivers Transgene Expression Throughout the Whole Spinal Cord, Mol. Neurobiol 53 (2016) 3235–3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Samaranch L, Salegio EA, San Sebastian W, Kells AP, Foust KD, Bringas JR, Lamarre C, Forsayeth J, Kaspar BK, Bankiewicz KS, Adeno-associated virus serotype 9 transduction in the central nervous system of nonhuman primates, Hum. Gene Ther 23 (2011) 382–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Taghian T, Marosfoi MG, Puri AS, Cataltepe OI, King RM, Diffie EB, Maguire AS, Martin DR, Fernau D, Batista AR, Kuchel T, Christou C, Perumal R, Chandra S, Gamlin PD, Bertrand SG, Flotte TR, McKenna-Yasek D, Tai PWL, Aronin N, Gounis MJ, Sena-Esteves M, Gray-Edwards HL, A Safe and Reliable Technique for CNS Delivery of AAV Vectors in the Cisterna Magna, Mol. Ther 28 (2020) 411–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Bey K, Deniaud J, Dubreil L, Joussemet B, Cristini J, Ciron C, Hordeaux J, Le Boulc’h M, Marche K, Maquigneau M, Guilbaud M, Moreau R, Larcher T, Deschamps JY, Fusellier M, Blouin V, Sevin C, Cartier N, Adjali O, Aubourg P, Moullier P, Colle MA, Intra-CSF AAV9 and AAVrh10 Administration in Nonhuman Primates: Promising Routes and Vectors for Which Neurological Diseases?, Mol. Ther. Methods Clin. Dev 17 (2020) 771–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Rosenberg JB, Kaplitt MG, De BP, Chen A, Flagiello T, Salami C, Pey E, Zhao L, Ricart Arbona RJ, Monette S, Dyke JP, Ballon DJ, Kaminsky SM, Sondhi D, Petsko GA, Paul SM, Crystal RG, AAVrh.10-Mediated APOE2 Central Nervous System Gene Therapy for APOE4-Associated Alzheimer’s Disease, Hum. Gene Ther. Clin. Dev 29 (2018) 24–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Hinderer C, Bell P, Vite CH, Louboutin JP, Grant R, Bote E, Yu H, Pukenas B, Hurst R, Wilson JM, Widespread gene transfer in the central nervous system of cynomolgus macaques following delivery of AAV9 into the cisterna magna, Mol. Ther. - Methods Clin. Dev 1 (2014) 14051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Kordasiewicz HB, Stanek LM, Wancewicz EV, Mazur C, McAlonis MM, Pytel KA, Artates JW, Weiss A, Cheng SH, Shihabuddin LS, Hung G, Bennett CF, Cleveland DW, Sustained Therapeutic Reversal of Huntington’s Disease by Transient Repression of Huntingtin Synthesis, Neuron. 74 (2012) 1031–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Fu H, Kang L, Jennings JS, Moy SS, Perez A, DiRosario J, McCarty DM, Muenzer J, Significantly increased lifespan and improved behavioral performances by rAAV gene delivery in adult mucopolysaccharidosis IIIB mice, Gene Ther. 14 (2007) 1065–1077. [DOI] [PubMed] [Google Scholar]
- [54].Ghodsi A, Stein C, Derksen T, Martins I, Anderson RD, Davidson BL, Systemic hyperosmolality improves β-glucuronidase distribution and pathology in murine MPS VII brain following intraventricular gene transfer, Exp. Neurol 160 (1999) 109–116. [DOI] [PubMed] [Google Scholar]
- [55].Fu H, Muenzer J, Samulski RJ, Breese G, Sifford J, Zeng X, McCarty DM, Self-complementary adeno-associated virus serotype 2 vector: Global distribution and broad dispersion of AAV-mediated transgene expression in mouse brain, Mol. Ther 8 (2003) 911–917. [DOI] [PubMed] [Google Scholar]
- [56].Katz N, Goode T, Hinderer C, Hordeaux J, Wilson JM, Standardized Method for Intra-Cisterna Magna Delivery Under Fluoroscopic Guidance in Nonhuman Primates, Hum. Gene Ther. Methods 29 (2018) 212–219. [DOI] [PubMed] [Google Scholar]
- [57].Benatti HR, Gray-Edwards HL, Adeno-Associated Virus Delivery Limitations for Neurological Indications, Hum. Gene Ther 33 (2022) 1–7. [DOI] [PubMed] [Google Scholar]
- [58].Murlidharan G, Crowther A, Reardon RA, Song J, Asokan A, Glymphatic fluid transport controls paravascular clearance of AAV vectors from the brain, JCI Insight. 1 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Wang D, Tai PWL, Gao G, Adeno-associated virus vector as a platform for gene therapy delivery, Nat. Rev. Drug Discov 18 (2019) 358–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Wu Z, Asokan A, Samulski RJ, Adeno-associated Virus Serotypes: Vector Toolkit for Human Gene Therapy, Mol. Ther 14 (2006) 316–327. [DOI] [PubMed] [Google Scholar]
- [61].Merkel SF, Andrews AM, Lutton EM, Mu D, Hudry E, Hyman BT, Maguire CA, Ramirez SH, Trafficking of adeno-associated virus vectors across a model of the blood-brain barrier; a comparative study of transcytosis and transduction using primary human brain endothelial cells, J. Neurochem 140 (2017) 216–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Weber‐Adrian D, Heinen S, Silburt J, Noroozian Z, Aubert I, The human brain endothelial barrier: transcytosis of AAV9, transduction by AAV2, J. Neurochem 140 (2017) 192–194. [DOI] [PubMed] [Google Scholar]
- [63].Dufour BD, Smith CA, Clark RL, Walker TR, Mcbride JL, Intrajugular VEIN DELIVERY OF AAV9-RNAi prevents neuropathological changes and weight loss in huntington’s disease mice, Mol. Ther 22 (2014) 797–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Gray SJ, Matagne V, Bachaboina L, Yadav S, Ojeda SR, Samulski RJ, Preclinical differences of intravascular aav9 delivery to neurons and glia: A comparative study of adult mice and nonhuman primates, Mol. Ther 19 (2011) 1058–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].McCarty DM, DiRosario J, Gulaid K, Muenzer J, Fu H, Mannitol-facilitated CNS entry of rAAV2 vector significantly delayed the neurological disease progression in MPS IIIB mice, Gene Ther. 16 (2009) 1340–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Burks SR, Kersch CN, Witko JA, Pagel MA, Sundby M, Muldoon LL, Neuwelt EA, Frank JA, Blood-brain barrier opening by intracarotid artery hyperosmolar mannitol induces sterile inflammatory and innate immune responses, Proc. Natl. Acad. Sci. U. S. A 118 (2021) 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Bevan AK, Duque S, Foust KD, Morales PR, Braun L, Schmelzer L, Chan CM, McCrate M, Chicoine LG, Coley BD, Porensky PN, Kolb SJ, Mendell JR, Burghes AHM, Kaspar BK, Systemic gene delivery in large species for targeting spinal cord, brain, and peripheral tissues for pediatric disorders, Mol. Ther 19 (2011) 1971–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Foust KD, Nurre E, Montgomery CL, Hernandez A, Chan CM, Kaspar BK, Intravascular AAV9 preferentially targets neonatal neurons and adult astrocytes, Nat. Biotechnol 27 (2009) 59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Foust KD, Wang X, McGovern VL, Braun L, Bevan AK, Haidet AM, Le TT, Morales PR, Rich MM, Burghes AHM, Kaspar BK, Rescue of the spinal muscular atrophy phenotype in a mouse model by early postnatal delivery of SMN, Nat. Biotechnol 28 (2010) 271–274. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [70].Miyake K, Miyake Noriko, Shimada T, Gene Delivery into the Central Nervous System (CNS) Using AAV Vectors, in: Intech, 2012: p. 13. [Google Scholar]
- [71].Foust KD, Salazar DL, Likhite S, Ferraiuolo L, Ditsworth D, Ilieva H, Meyer K, Schmelzer L, Braun L, Cleveland DW, Kaspar BK, Therapeutic AAV9-mediated suppression of mutant SOD1 slows disease progression and extends survival in models of inherited ALS, Mol. Ther 21 (2013) 2148–2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Saraiva J, Nobre RJ, Pereira de Almeida L, Gene therapy for the CNS using AAVs: The impact of systemic delivery by AAV9, J. Control. Release 241 (2016) 94–109. [DOI] [PubMed] [Google Scholar]
- [73].Choudhury SR, Harris AF, Cabral DJ, Keeler AM, Sapp E, Ferreira JS, Gray-Edwards HL, Johnson JA, Johnson AK, Su Q, Stoica L, DiFiglia M, Aronin N, Martin DR, Gao G, Sena-Esteves M, Widespread central nervous system gene transfer and silencing after systemic delivery of novel AAV-AS vector, Mol. Ther 24 (2016) 726–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Dehay B, Dalkara D, Dovero S, Li Q, Bezard E, Systemic scAAV9 variant mediates brain transduction in newborn rhesus macaques, Sci. Rep 2 (2012) 253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Saunders NR, Joakim Ek C, Dziegielewska KM, The neonatal blood-brain barrier is functionally effective, and immaturity does not explain differential targeting of AAV9, Nat. Biotechnol 27 (2009) 804. [DOI] [PubMed] [Google Scholar]
- [76].Kang L, Jin S, Wang J, Lv Z, Xin C, Tan C, Zhao M, Wang L, Liu J, AAV vectors applied to the treatment of CNS disorders: Clinical status and challenges, J. Control. Release 355 (2023) 458–473. [DOI] [PubMed] [Google Scholar]
- [77].Bartel MA, Weinstein JR, Schaffer DV, Directed evolution of novel adeno-associated viruses for therapeutic gene delivery, Gene Ther 19 (2012) 694–700. [DOI] [PubMed] [Google Scholar]
- [78].Challis RC, Ravindra Kumar S, Chan KY, Challis C, Beadle K, Jang MJ, Kim HM, Rajendran PS, Tompkins JD, Shivkumar K, Deverman BE, Gradinaru V, Systemic AAV vectors for widespread and targeted gene delivery in rodents, Nat. Protoc 14 (2019) 379–414. [DOI] [PubMed] [Google Scholar]
- [79].Hordeaux J, Wang Q, Katz N, Buza EL, Bell P, Wilson JM, The Neurotropic Properties of AAV-PHP.B Are Limited to C57BL/6J Mice, Mol. Ther 26 (2018) 664–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Chen W, Yao S, Wan J, Tian Y, Huang L, Wang S, Akter F, Wu Y, Yao Y, Zhang X, BBB-crossing adeno-associated virus vector: An excellent gene delivery tool for CNS disease treatment., J. Control. Release 333 (2021) 129–138. [DOI] [PubMed] [Google Scholar]
- [81].Campos LJ, Arokiaraj CM, Chuapoco MR, Chen X, Goeden N, Gradinaru V, Fox AS, Advances in AAV technology for delivering genetically encoded cargo to the nonhuman primate nervous system, Curr. Res. Neurobiol (2023) 100086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Peng KW, Pham L, Ye H, Zufferey R, Trono D, Cosset FL, Russell SJ, Organ distribution of gene expression after intravenous infusion of targeted and untargeted lentiviral vectors, Gene Ther. 8 (2001) 1456–1463. [DOI] [PubMed] [Google Scholar]
- [83].Ganesan LP, Mohanty S, Kim J, Clark KR, Robinson JM, Anderson CL, Rapid and Efficient Clearance of Blood-borne Virus by Liver Sinusoidal Endothelium, PLoS Pathog. 7 (2011) e1002281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Kügler S, Meyn L, Holzmüller H, Gerhardt E, Isenmann S, Schulz JB, Bähr M, Neuron-specific expression of therapeutic proteins: Evaluation of different cellular promoters in recombinant adenoviral vectors, Mol. Cell. Neurosci 17 (2001) 78–96. [DOI] [PubMed] [Google Scholar]
- [85].Shevtsova Z, Malik JMI, Michel U, Bähr M, Kügler S, Promoters and serotypes: Targeting of adeno-associated virus vectors for gene transfer in the rat central nervous system in vitro and in vivo, in: Exp. Physiol, Blackwell Publishing Ltd, 2005: pp. 53–59. [DOI] [PubMed] [Google Scholar]
- [86].Hedegaard C, Kjaer-Sorensen K, Madsen LB, Henriksen C, Momeni J, Bendixen C, Oxvig C, Larsen K, Porcine synapsin 1: SYN1gene analysis and functional characterization of the promoter, FEBS Open Bio. 3 (2013) 411–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].McLean JR, Smith GA, Rocha EM, Hayes MA, Beagan JA, Hallett PJ, Isacson O, Widespread neuron-specific transgene expression in brain and spinal cord following synapsin promoter-driven AAV9 neonatal intracerebroventricular injection, Neurosci. Lett 576 (2014) 73–78. [DOI] [PubMed] [Google Scholar]
- [88].Cho W, Hagemann TL, Johnson DA, Johnson JA, Messing A, Dual transgenic reporter mice as a tool for monitoring expression of glial fibrillary acidic protein, J. Neurochem 110 (2009) 343–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Mingozzi F, High K, Immune Responses to AAV in Clinical Trials, Curr. Gene Ther 7 (2007) 316–324. [DOI] [PubMed] [Google Scholar]
- [90].Ertl HCJ, Immunogenicity and toxicity of AAV gene therapy, Front. Immunol 13 (2022) 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Ronzitti G, Gross DA, Mingozzi F, Human Immune Responses to Adeno-Associated Virus (AAV) Vectors, Front. Immunol 11 (2020) 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Mingozzi F, High KA, Immune Responses to AAV in Clinical Trials, Curr. Gene Ther 11 (2011) 321–330. [DOI] [PubMed] [Google Scholar]
- [93].Calcedo R, Morizono H, Wang L, McCarter R, He J, Jones D, Batshaw ML, Wilson JM, Adeno-associated virus antibody profiles in newborns, children, and adolescents, Clin. Vaccine Immunol 18 (2011) 1586–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Calcedo R, Vandenberghe LH, Gao G, Lin J, Wilson JM, Worldwide Epidemiology of Neutralizing Antibodies to Adeno‐Associated Viruses, J. Infect. Dis 199 (2009) 381–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Gadenstaetter AJ, SCHMUTZLER L, GRIMM D, LANDEGGER LD, Intranasal application of adeno-associated viruses: a systematic review, Transl. Res 248 (2022) 87–110. [DOI] [PubMed] [Google Scholar]
- [96].Goodin BR, Anderson AJB, Freeman EL, Bulls HW, R MT, Ness TJ, Administration of intranasal oxytocin may augment endogenous pain nhibitory capacity and reduce anxiety, Clin. J. Pain 31 (2015) 757–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].a Reger M, Watson GS, Green PS, Baker LD, Cholerton B, a Fishel M, Plymate SR, Cherrier MM, Schellenberg GD, Frey WH, Craft S, Intranasal insulin administration dose-dependently modulates verbal memory and plasma amyloid-beta in memory-impaired older adults., J. Alzheimers. Dis 13 (2008) 323–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Santos-Morales O, Díaz-Machado A, Jiménez-Rodríguez D, Pomares-Iturralde Y, Festary-Casanovas T, González-Delgado CA, Pérez-Rodríguez S, Alfonso-Muñoz E, Viada-González C, Piedra-Sierra P, García-García I, Amaro-González D, García-Rodríguez JC, Sosa-Testé I, Lagarto-Parra A, Barrero-Viera L, David-Baldo M, Tamayo-Rodríguez M, Rivero-Vázquez I, González-Gamiz G, Martín-Trujillo A, Rodríguez-Fernández Y, Ledo-de la Luz AA, Álvarez-Delgado M, Howland-Álvarez I, Cruz-Gómez Y, Nasal administration of the neuroprotective candidate NeuroEPO to healthy volunteers: a randomized, parallel, open-label safety study, BMC Neurol. 17 (2017) 129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Da Fonseca CO, Masini M, Futuro D, Caetano R, Rocha Gattass C, Quirico-Santos T, Anaplastic oligodendroglioma responding favorably to intranasal delivery of perillyl alcohol: a case report and literature review, Surg. Neurol 66 (2006) 611–615. [DOI] [PubMed] [Google Scholar]
- [100].de Bellis A, de Bellis M, Aloe L, Long-Term Non-Invasive Treatment via Intranasal Administration of Nerve Growth Factor Protects the Human Brain in Frontotemporal Dementia associated with Corticobasal Syndrome: A Pilot Study, J. Alzheimer’s Dis. Reports 2 (2018) 67–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Chiaretti A, Conti G, Falsini B, Buonsenso D, Crasti M, Manni L, Soligo M, Fantacci C, Genovese O, Calcagni ML, Di Giuda D, Mattoli MV, Cocciolillo F, Ferrara P, Ruggiero A, Staccioli S, Colafati GS, Riccardi R, Intranasal Nerve Growth Factor administration improves cerebral functions in a child with severe traumatic brain injury: A case report, Brain Inj 31 (2017) 1538–1547. [DOI] [PubMed] [Google Scholar]
- [102].Meinhardt J, Radke J, Dittmayer C, Franz J, Thomas C, Mothes R, Laue M, Schneider J, Brünink S, Greuel S, Lehmann M, Hassan O, Aschman T, Schumann E, Chua RL, Conrad C, Eils R, Stenzel W, Windgassen M, Rößler L, Goebel HH, Gelderblom HR, Martin H, Nitsche A, Schulz-Schaeffer WJ, Hakroush S, Winkler MS, Tampe B, Scheibe F, Körtvélyessy P, Reinhold D, Siegmund B, Kühl AA, Elezkurtaj S, Horst D, Oesterhelweg L, Tsokos M, Ingold-Heppner B, Stadelmann C, Drosten C, Corman VM, Radbruch H, Heppner FL, Olfactory transmucosal SARS-CoV-2 invasion as a port of central nervous system entry in individuals with COVID-19, Nat. Neurosci 24 (2021) 168–175. [DOI] [PubMed] [Google Scholar]
- [103].Belur LR, Temme A, Podetz-Pedersen KM, Riedl M, Vulchanova L, Robinson N, Hanson LR, Kozarsky KF, Orchard PJ, Frey WH, Low WC, Scott McIvor R, Intranasal Adeno-Associated Virus Mediated Gene Delivery and Expression of Human Iduronidase in the Central Nervous System: A Noninvasive and Effective Approach for Prevention of Neurologic Disease in Mucopolysaccharidosis Type i, Hum. Gene Ther 28 (2017) 576–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Lochhead JJ, Thorne RG, Intranasal delivery of biologics to the central nervous system., Adv. Drug Deliv. Rev 64 (2012) 614–628. [DOI] [PubMed] [Google Scholar]
- [105].Erdő F, Bors LA, Farkas D, Bajza Á, Gizurarson S, Evaluation of intranasal delivery route of drug administration for brain targeting, Brain Res. Bull 143 (2018) 155–170. [DOI] [PubMed] [Google Scholar]
- [106].Bors LA, Erdö F, Overcoming the blood-brain barrier. Challenges and tricks for CNS drug delivery, Sci. Pharm 87 (2019). [Google Scholar]
- [107].Quinn K, Quirion MR, Lo CY, Misplon JA, Epstein SL, Chiorini JA, Intranasal administration of adeno-associated virus type 12 (AAV12) leads to transduction of the nasal epithelia and can initiate transgene-specific immune response, Mol. Ther 19 (2011) 1990–1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Williams CL, Uytingco CR, Green WW, McIntyre JC, Ukhanov K, Zimmerman AD, Shively DT, Zhang L, Nishimura DY, Sheffield VC, Martens JR, Gene Therapeutic Reversal of Peripheral Olfactory Impairment in Bardet-Biedl Syndrome, Mol. Ther 25 (2017) 904–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Wolf DA, Hanson LR, Aronovich EL, Nan Z, Low WC, Frey WH, McIvor RS, Lysosomal enzyme can bypass the blood-brain barrier and reach the CNS following intranasal administration, Mol. Genet. Metab 106 (2012) 131–134. [DOI] [PubMed] [Google Scholar]
- [110].Belur LR, Romero M, Lee J, Podetz-Pedersen KM, Nan Z, Riedl MS, Vulchanova L, Kitto KF, Fairbanks CA, Kozarsky KF, Orchard PJ, Frey WH, Low WC, McIvor RS, Comparative Effectiveness of Intracerebroventricular, Intrathecal, and Intranasal Routes of AAV9 Vector Administration for Genetic Therapy of Neurologic Disease in Murine Mucopolysaccharidosis Type I, Front. Mol. Neurosci 14 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Qi B, Yang Y, Cheng Y, Sun D, Wang X, Khanna R, Ju W, Nasal delivery of a CRMP2-derived CBD3 adenovirus improves cognitive function and pathology in APP/PS1 transgenic mice, Mol. Brain 13 (2020) 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Chen C, Dong Y, Liu F, Gao C, Ji C, Dang Y, Ma X, Liu Y, A Study of Antidepressant Effect and Mechanism on Intranasal Delivery of BDNF-HA2TAT/AAV to Rats with Post-Stroke Depression, Neuropsychiatr. Dis. Treat Volume 16 (2020) 637–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Liu F, Liu Y, Lei G, Liu P, Chu Z, Gao C, Dang Y, Antidepressant effect of recombinant NT4-NAP/AAV on social isolated mice through intranasal route, Oncotarget 8 (2017) 10103–10113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Ma XC, Liu P, Zhang XL, Jiang WH, Jia M, Wang CX, Dong YY, Dang YH, Gao CG, Intranasal Delivery of Recombinant AAV Containing BDNF Fused with HA2TAT: A Potential Promising Therapy Strategy for Major Depressive Disorder, Sci. Rep 6 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Kirange RH, Chaudhari RB, Utilizing Mucoadhesive Polymers for Nasal Drug Delivery System, Int. J. Pharm. Sci. Res. IJPSR 8 (2017) 1012–1022. [Google Scholar]
- [116].Corso JF, Bone-Conduction Thresholds for Sonic and Ultrasonic Frequencies, J. Acoust. Soc. Am 35 (1963) 1738–1743. [Google Scholar]
- [117].Ebbini ES, Ter Haar G, Ultrasound-guided therapeutic focused ultrasound: Current status and future directions, Int. J. Hyperth 31 (2015) 77–89. [DOI] [PubMed] [Google Scholar]
- [118].Chen S, Nazeri A, Baek H, Ye D, Yang Y, Yuan J, Rubin JB, Chen H, A review of bioeffects induced by focused ultrasound combined with microbubbles on the neurovascular unit, J. Cereb. Blood Flow Metab 42 (2022) 3–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Hynynen K, McDannold N, Vykhodtseva N, Jolesz FA, Noninvasive MR imaging-guided focal opening of the blood-brain barrier in rabbits., Radiology. 220 (2001) 640–646. [DOI] [PubMed] [Google Scholar]
- [120].Park J, Aryal M, Vykhodtseva N, Zhang Y-Z, McDannold N, Evaluation of permeability, doxorubicin delivery, and drug retention in a rat brain tumor model after ultrasound-induced blood-tumor barrier disruption, J. Control. Release 250 (2016) 77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Jordão JF, Ayala-Grosso CA, Markham K, Huang Y, Chopra R, McLaurin J, Hynynen K, Aubert I, Antibodies targeted to the brain with image-guided focused ultrasound reduces amyloid-beta plaque load in the TgCRND8 mouse model of Alzheimer’s disease., PLoS One. 5 (2010) e10549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Wang X, Cui G, Yang X, Zhang Z, Shi H, Zu J, Hua F, Shen X, Intracerebral administration of ultrasound-induced dissolution of lipid-coated GDNF microbubbles provides neuroprotection in a rat model of Parkinson’s disease, Brain Res. Bull 103 (2014) 60–65. [DOI] [PubMed] [Google Scholar]
- [123].Kinoshita Manabu, McDannold N, Jolesz FA, Noninvasive localized delivery of Herceptin to the mouse brain by MRI-guided focused ultrasound-induced blood-brain barrier disruption., Proc. Natl. Acad. Sci. U. S. A 103 (2006) 11719–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Burgess A, Ayala-Grosso CA, Ganguly M, Jordão JF, Aubert I, Hynynen K, Targeted delivery of neural stem cells to the brain using MRI-guided focused ultrasound to disrupt the blood-brain barrier, PLoS One. 6 (2011) e27877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Shen W-B, Anastasiadis P, Nguyen B, Yarnell D, Yarowsky PJ, Frenkel V, Fishman PS, Magnetic Enhancement of Stem Cell–Targeted Delivery into the Brain Following MR-Guided Focused Ultrasound for Opening the Blood–Brain Barrier, Cell Transplant. 26 (2017) 1235–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Carpentier A, Canney M, Vignot A, Reina V, Beccaria K, Horodyckid C, Karachi C, Leclercq D, Lafon C, Chapelon JY, Capelle L, Cornu P, Sanson M, Hoang-Xuan K, Delattre JY, Idbaih A, Clinical trial of blood-brain barrier disruption by pulsed ultrasound, Sci. Transl. Med 8 (2016). [DOI] [PubMed] [Google Scholar]
- [127].Lipsman N, Meng Y, Bethune AJ, Huang Y, Lam B, Masellis M, Herrmann N, Heyn C, Aubert I, Boutet A, Smith GS, Hynynen K, Black SE, Blood–brain barrier opening in Alzheimer’s disease using MR-guided focused ultrasound, Nat. Commun 9 (2018) 2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Abrahao A, Meng Y, Llinas M, Huang Y, Hamani C, Mainprize T, Aubert I, Heyn C, Black SE, Hynynen K, Lipsman N, Zinman L, First-in-human trial of blood-brain barrier opening in amyotrophic lateral sclerosis using MR-guided focused ultrasound., Nat. Commun 10 (2019) 4373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Pineda-Pardo JA, Gasca-Salas C, Fernández-Rodríguez B, Rodríguez-Rojas R, del Álamo M, Obeso I, Hernández-Fernández F, Trompeta C, Martínez-Fernández R, Matarazzo M, Mata-Marín D, Guida P, Duque A, Albillo D, Plaza de las Heras I, Montero JI, Foffani G, Toltsis G, Rachmilevitch I, Blesa J, Obeso JA, Striatal Blood–Brain Barrier Opening in Parkinson’s Disease Dementia: A Pilot Exploratory Study, Mov. Disord 37 (2022) 2057–2065. [DOI] [PubMed] [Google Scholar]
- [130].Thévenot E, Jordão JF, O’Reilly MA, Markham K, Weng Y, Foust KD, Kaspar BK, Hynynen K, Aubert I, Targeted Delivery of Self-Complementary Adeno-Associated Virus Serotype 9 to the Brain, Using Magnetic Resonance Imaging-Guided Focused Ultrasound, Hum. Gene Ther 23 (2012) 1144–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Kofoed RH, Dibia CL, Noseworthy K, Xhima K, Vacaresse N, Hynynen K, Aubert I, Efficacy of gene delivery to the brain using AAV and ultrasound depends on serotypes and brain areas, J. Control. Release 351 (2022) 667–680. [DOI] [PubMed] [Google Scholar]
- [132].Ross JM, Kim C, Allen D, Crouch EE, Narsinh K, Cooke DL, Abla AA, Nowakowski TJ, Winkler EA, The Expanding Cell Diversity of the Brain Vasculature, Front. Physiol 11 (2020) 600767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Agarwal N, Carare RO, Cerebral Vessels: An Overview of Anatomy, Physiology, and Role in the Drainage of Fluids and Solutes, Front. Neurol 11 (2021) 1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Keller D, Erö C, Markram H, Cell densities in the mouse brain: A systematic review, Front. Neuroanat 12 (2018) 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].O’Muircheartaigh J, Jbabdi S, Concurrent white matter bundles and grey matter networks using independent component analysis, Neuroimage. 170 (2018) 296–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Hsu P-H, Wei K-C, Huang C-Y, Wen C-J, Yen T-C, Liu C-L, Lin Y-T, Chen J-C, Shen C-R, Liu H-L, Noninvasive and targeted gene delivery into the brain using microbubble-facilitated focused ultrasound., PLoS One. 8 (2013) e57682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Stavarache MA, Petersen N, Jurgens EM, Milstein ER, Rosenfeld ZB, Ballon DJ, Kaplitt MG, Safe and stable noninvasive focal gene delivery to the mammalian brain following focused ultrasound, J. Neurosurg 130 (2019) 989–998. [DOI] [PubMed] [Google Scholar]
- [138].Wang S, Olumolade OO, Sun T, Samiotaki G, Konofagou EE, Noninvasive, neuron-specific gene therapy can be facilitated by focused ultrasound and recombinant adeno-associated virus, Gene Ther. 22 (2015) 104–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Kofoed RH, Heinen S, Silburt J, Dubey S, Dibia CL, Maes M, Simpson EM, Hynynen K, Aubert I, Transgene distribution and immune response after ultrasound delivery of rAAV9 and PHP.B to the brain in a mouse model of amyloidosis, Mol. Ther. - Methods Clin. Dev 23 (2021) 390–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Kofoed RH, Aubert I, Focused ultrasound gene delivery for the treatment of neurological disorders, Trends Mol. Med 30 (2024) 263–277. [DOI] [PubMed] [Google Scholar]
- [141].Chen H, Konofagou EE, The size of blood-brain barrier opening induced by focused ultrasound is dictated by the acoustic pressure, J. Cereb. Blood Flow Metab 34 (2014) 1197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].McDannold N, Vykhodtseva N, Hynynen K, Effects of Acoustic Parameters and Ultrasound Contrast Agent Dose on Focused-Ultrasound Induced Blood-Brain Barrier Disruption, Ultrasound Med. Biol 34 (2008) 930–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Blesa J, Pineda-Pardo JA, Inoue KI, Gasca-Salas C, Balzano T, Del Rey NLG, Reinares-Sebastián A, Esteban-García N, Rodríguez-Rojas R, Márquez R, Ciorraga M, Del Álamo M, García-Cañamaque L, Ruiz de Aguiar S, Rachmilevitch I, Trigo-Damas I, Takada M, Obeso JA, BBB opening with focused ultrasound in nonhuman primates and Parkinson’s disease patients: Targeted AAV vector delivery and PET imaging, Sci. Adv 9 (2023) eadf4888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Parks TV, Szczupak D, Choi S-H, Schaeffer DJ, Noninvasive focal transgene delivery with viral neuronal tracers in the marmoset monkey, Cell Reports Methods. 4 (2024) 100709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Wang S, Kugelman T, Buch A, Herman M, Han Y, Karakatsani ME, Hussaini SA, Duff K, Konofagou EE, Non-invasive, Focused Ultrasound-Facilitated Gene Delivery for Optogenetics, Sci. Rep 7 (2017) 39955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Xhima K, Nabbouh F, Hynynen K, Aubert I, Tandon A, Noninvasive delivery of an α-synuclein gene silencing vector with magnetic resonance–guided focused ultrasound, Mov. Disord 33 (2018) 1567–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Li H, Heath JE, Trippett JS, Shapiro MG, Szablowski JO, Engineering Viral Vectors for Acoustically Targeted Gene Delivery, BioRxiv. (2021) 2021.07.26.453904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Felix MS, Borloz E, Metwally K, Dauba A, Larrat B, Matagne V, Ehinger Y, Villard L, Novell A, Mensah S, Roux JC, Ultrasound-Mediated Blood-Brain Barrier Opening Improves Whole Brain Gene Delivery in Mice, Pharmaceutics. 13 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Batts AJ, Ji R, Noel RL, Kline-Schoder AR, Bae S, Kwon N, Konofagou EE, Using a novel rapid alternating steering angles pulse sequence to evaluate the impact of theranostic ultrasound-mediated ultra-short pulse length on blood-brain barrier opening volume and closure, cavitation mapping, drug delivery feasibility, and safety, Theranostics. 13 (2023) 1180–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Kofoed RH, Simpson EM, Hynynen K, Aubert I, Sonoselective delivery using ultrasound and microbubbles combined with intravenous rAAV9 CLDN5-GFP does not increase endothelial gene expression, Gene Ther. (2023) 2–6. [DOI] [PubMed] [Google Scholar]
- [151].Nouraein S, Lee S, Saenz VA, Del Mundo HC, Yiu J, Szablowski JO, Acoustically targeted noninvasive gene therapy in large brain volumes, Gene Ther. (2023). [DOI] [PubMed] [Google Scholar]
- [152].Szablowski JO, Harb M, Focused Ultrasound Induced Blood-Brain Barrier Opening for Targeting Brain Structures and Evaluating Chemogenetic Neuromodulation, JoVE (Journal Vis. Exp 2020 (2020) e61352. [DOI] [PubMed] [Google Scholar]
- [153].Foiret J, Zhang H, Fite BZ, Ilovitsh T, Mahakian LM, Tam S, Beitnere U, Pyles B, Segal DJ, Ferrara KW, Blood-brain barrier disruption for the delivery of non-infectious viral vectors and proteins, preliminary study, IEEE Int. Ultrason. Symp. IUS (2017). [Google Scholar]
- [154].Wang S, Buch A, Hussaini SA, Acosta C, Konofagou E, Focused ultrasound facilitated adenoviral delivery for optogenetic stimulation, 2015 IEEE Int. Ultrason. Symp. IUS 2015 (2015) 1–4. [Google Scholar]
- [155].Kofoed RH, Noseworthy K, Wu K, Sivadas S, Stanek L, Elmer B, Hynynen K, Shihabuddin LS, Aubert I, The engineered AAV2-HBKO promotes non-invasive gene delivery to large brain regions beyond ultrasound targeted sites, Mol. Ther. Methods Clin. Dev 27 (2022) 167–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Weber-Adrian D, Kofoed RH, Silburt J, Noroozian Z, Shah K, Burgess A, Rideout S, Kügler S, Hynynen K, Aubert I, Systemic AAV6-synapsin-GFP administration results in lower liver biodistribution, compared to AAV1&2 and AAV9, with neuronal expression following ultrasound-mediated brain delivery, Sci. Reports 2021 111 11 (2021) 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Weber-Adrian D, Kofoed RH, Chan JWY, Silburt J, Noroozian Z, Kügler S, Hynynen K, Aubert I, Strategy to enhance transgene expression in proximity of amyloid plaques in a mouse model of Alzheimer’s disease, Theranostics. 9 (2019) 8127–8137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Ajenjo J, Seo JW, Foiret J, Wu B, Raie MN, Wang J, Fite BZ, Zhang N, Malek R, Beinat C, Malik N, Anders DA, Ferrara KW, PET imaging of focused-ultrasound enhanced delivery of AAVs into the murine brain, Theranostics. 13 (2023) 5151–5169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Alonso A, Reinz E, Leuchs B, Kleinschmidt J, Fatar M, Geers B, Lentacker I, Hennerici MG, de Smedt SC, Meairs S, Focal Delivery of AAV2/1-transgenes Into the Rat Brain by Localized Ultrasound-induced BBB Opening, Mol. Ther. - Nucleic Acids 2 (2013) e73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Trinh D, Nash J, Goertz D, Hynynen K, Bulner S, Iqbal U, Keenan J, Microbubble drug conjugate and focused ultrasound blood brain barrier delivery of AAV-2 SIRT-3, Https://Doi.Org/10.1080/10717544.2022.2035855. 29 (2022) 1176–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Ye D, Yuan J, Yang Y, Yue Y, Hu Z, Fadera S, Chen H, Incisionless targeted adeno-associated viral vector delivery to the brain by focused ultrasound-mediated intranasal administration, EBioMedicine. 84 (2022) 104277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Parrini M, Naskar S, Alberti M, Colombi I, Morelli G, Rocchi A, Nanni M, Piccardi F, Charles S, Ronzitti G, Mingozzi F, Contestabile A, Cancedda L, Restoring neuronal chloride homeostasis with anti-NKCC1 gene therapy rescues cognitive deficits in a mouse model of Down syndrome, Mol. Ther 29 (2021) 3072–3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Queen NJ, Zou X, Anderson JM, Huang W, Appana B, Komatineni S, Wevrick R, Cao L, Hypothalamic AAV-BDNF gene therapy improves metabolic function and behavior in the Magel2-null mouse model of Prader-Willi syndrome, Mol. Ther. Methods Clin. Dev 27 (2022) 131–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Sánchez-Pernaute R, Harvey-White J, Cunningham J, Bankiewicz KS, Functional Effect of Adeno-associated Virus Mediated Gene Transfer of Aromatic L-Amino Acid Decarboxylase into the Striatum of 6-OHDA-Lesioned Rats, Mol. Ther 4 (2001) 324–330. [DOI] [PubMed] [Google Scholar]
- [165].Hadaczek P, Eberling JL, Pivirotto P, Bringas J, Forsayeth J, Bankiewicz KS, Eight years of clinical improvement in MPTP-lesioned primates after gene therapy with AAV2-hAADC, Mol. Ther 18 (2010) 1458–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Luo J, Kaplitt MG, Fitzsimons HL, Zuzga DS, Liu Y, Oshinsky ML, During MJ, Subthalamic GAD Gene Therapy in a Parkinson ‘ s Disease Rat Model, 298 (2002) 425–430. [DOI] [PubMed] [Google Scholar]
- [167].Emborg ME, Carbon M, Holden JE, During MJ, Ma Y, Tang C, Moirano J, Fitzsimons H, Roitberg BZ, Tuccar E, Roberts A, Kaplitt MG, Eidelberg D, Subthalamic glutamic acid decarboxylase gene therapy: Changes in motor function and cortical metabolism, J. Cereb. Blood Flow Metab 27 (2007) 501–509. [DOI] [PubMed] [Google Scholar]
- [168].Espinoza S, Scarpato M, Damiani D, Managò F, Mereu M, Contestabile A, Peruzzo O, Carninci P, Santoro C, Papaleo F, Mingozzi F, Ronzitti G, Zucchelli S, Gustincich S, SINEUP Non-coding RNA Targeting GDNF Rescues Motor Deficits and Neurodegeneration in a Mouse Model of Parkinson’s Disease, Mol. Ther 28 (2020) 642–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Maddalena A, Tereshchenko J, Bähr M, Kügler S, Adeno-associated virus-mediated, mifepristone-regulated transgene expression in the brain, Mol. Ther. - Nucleic Acids 2 (2013) e106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Back S, Peränen J, Galli E, Pulkkila P, Lonka-Nevalaita L, Tamminen T, Voutilainen MH, Raasmaja A, Saarma M, Männistö PT, Tuominen RK, Gene therapy with AAV2-CDNF provides functional benefits in a rat model of Parkinson’s disease, Brain Behav 3 (2013) 75–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Kells AP, Eberling J, Su X, Pivirotto P, Bringas J, Hadaczek P, Narrow WC, Bowers WJ, Federoff HJ, Forsayeth J, Bankiewicz KS, Regeneration of the MPTP-lesioned dopaminergic system after convection-enhanced delivery of AAV2-GDNF, J. Neurosci 30 (2010) 9567–9577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Johnston LC, Eberling J, Pivirotto P, Hadaczek P, Federoff HJ, Forsayeth J, Bankiewicz KS, Clinically relevant effects of convection-enhanced delivery of AAV2-GDNF on the dopaminergic nigrostriatal pathway in aged rhesus monkeys, Hum. Gene Ther 20 (2009) 497–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Herzog CD, Dass B, Holden JE, Stansell J, Gasmi M, Tuszynski MH, Bartus RT, Kordower JH, Striatal delivery of CERE-120, an AAV2 vector encoding human neurturin, enhances activity of the dopaminergic nigrostriatal system in aged monkeys, Mov. Disord 22 (2007) 1124–1132. [DOI] [PubMed] [Google Scholar]
- [174].Kordower JH, Herzog CD, Dass B, Bakay RAE, Stansell J, Gasmi M, Bartus RT, Delivery of neurturin by AAV2 (CERE-120)-mediated gene transfer provides structural and functional neuroprotection and neurorestoration in MPTP-treated monkeys, Ann. Neurol 60 (2006) 706–715. [DOI] [PubMed] [Google Scholar]
- [175].Sucunza D, Rico AJ, Roda E, Gonz G, Rodr AI, Alfonso V, Glucocerebrosidase Gene Therapy Induces Alpha-Synuclein Clearance and Neuroprotection of Midbrain Dopaminergic Neurons in Mice and Macaques, (2021) 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Karakatsani ME, Wang S, Samiotaki G, Kugelman T, Olumolade OO, Acosta C, Sun T, Han Y, Kamimura HAS, Jackson-Lewis V, Przedborski S, Konofagou E, Amelioration of the nigrostriatal pathway facilitated by ultrasound-mediated neurotrophic delivery in early Parkinson’s disease, J. Control. Release 303 (2019) 289–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Kiyota T, Ingraham KL, Swan RJ, Jacobsen MT, Andrews SJ, Ikezu T, AAV serotype 2/1-mediated gene delivery of anti-inflammatory interleukin-10 enhances neurogenesis and cognitive function in APPPS1 mice, Gene Ther. 19 (2012) 724–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Kiyota T, Okuyama S, Swan RJ, Jacobsen MT, Gendelman HE, Ikezu T, CNS expression of anti‐inflammatory cytokine interleukin‐4 attenuates Alzheimer’s disease‐like pathogenesis in APP+PS1 bigenic mice, FASEB J. 24 (2010) 3093–3102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Kiyota T, Yamamoto M, Schroder B, Jacobsen MT, Swan RJ, Lambert MP, Klein WL, Gendelman HE, Ransohoff RM, Ikezu T, AAV1/2-mediated CNS gene delivery of dominant-negative CCL2 mutant suppresses gliosis, β-amyloidosis, and learning impairment of APP/ PS1 mice, Mol. Ther 17 (2009) 803–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Hudry E, Dashkoff J, Roe AD, Takeda S, Koffie RM, Hashimoto T, Scheel M, Spires-Jones T, Arbel-Ornath M, Betensky R, Davidson BL, Hyman BT, Gene transfer of human Apoe isoforms results in differential modulation of amyloid deposition and neurotoxicity in mouse brain, Sci. Transl. Med 5 (2013) 212ra161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Pascual‐Lucas M, Viana da Silva S, Di Scala M, Garcia‐Barroso C, González‐Aseguinolaza G, Mulle C, Alberini CM, Cuadrado‐Tejedor M, Garcia‐Osta A, Insulin‐like growth factor 2 reverses memory and synaptic deficits in APP transgenic mice , EMBO Mol. Med 6 (2014) 1246–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Iwata N, Sekiguchi M, Hattori Y, Takahashi A, Asai M, Ji B, Higuchi M, Staufenbiel M, Muramatsu SI, Saido TC, Global brain delivery of neprilysin gene by intravascular administration of AAV vector in mice, Sci. Rep 3 (2013) 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Levites Y, Jansen K, Smithson LA, Dakin R, Holloway VM, Das P, Golde TE, Intracranial adeno-associated virus-mediated delivery of anti-pan amyloid β, amyloid β40, and amyloid β42 single-chain variable fragments attenuates plaque pathology in amyloid precursor protein mice, J. Neurosci 26 (2006) 11923–11928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].McBride JL, During MJ, Wuu J, Chen E-Y, Leurgans SE, Kordower JH, Structural and functional neuroprotection in a rat model of Huntington’s disease by viral gene transfer of GDNF, Exp. Neurol 181 (2003) 213–223. [DOI] [PubMed] [Google Scholar]
- [185].Kells AP, Fong DM, Dragunow M, During MJ, Young D, Connor B, AAV-Mediated gene delivery of BDNF or GDNF is neuroprotective in a model of huntington disease, Mol. Ther 9 (2004) 682–688. [DOI] [PubMed] [Google Scholar]
- [186].Wu Z, Parry M, Hou XY, Liu MH, Wang H, Cain R, Pei ZF, Chen YC, Guo ZY, Abhijeet S, Chen G, Gene therapy conversion of striatal astrocytes into GABAergic neurons in mouse models of Huntington’s disease, Nat. Commun 11 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Connor B, Sun Y, Von Hieber D, Tang SK, Jones KS, Maucksch C, AAV 1/2-mediated BDNF gene therapy in a transgenic rat model of Huntington’s disease, Gene Ther. 23 (2016) 283–295. [DOI] [PubMed] [Google Scholar]
- [188].Evers MM, Miniarikova J, Juhas S, Vallès A, Bohuslavova B, Juhasova J, Skalnikova HK, Vodicka P, Valekova I, Brouwers C, Blits B, Lubelski J, Kovarova H, Ellederova Z, van Deventer SJ, Petry H, Motlik J, Konstantinova P, AAV5-miHTT Gene Therapy Demonstrates Broad Distribution and Strong Human Mutant Huntingtin Lowering in a Huntington’s Disease Minipig Model, Mol. Ther 26 (2018) 2163–2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Planques A, Boussicault L, Alves S, Lamazie A, Bolte S, Heck N, Moumne L, Galvan L, Piguet F, Aubourg P, Cartier N, Caboche J, Betuing S, CYP46A1 , the rate-limiting enzyme for cholesterol degradation , is neuroprotective in Huntington ‘ s disease, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Matalon R, Surendran S, Rady PL, Quast MJ, Campbell GA, Matalon KM, Tyring SK, Wei J, Peden CS, Ezell EL, Muzyczka N, Mandel RJ, Adeno-associated virus-mediated aspartoacylase gene transfer to the brain of knockout mouse for canavan disease, Mol. Ther 7 (2003) 580–587. [DOI] [PubMed] [Google Scholar]
- [191].McPhee SWJ, Francis J, Janson CG, Serikawa T, Hyland K, Ong EO, Raghavan SS, Freese A, Leone P, Effects of AAV-2-mediated aspartoacylase gene transfer in the tremor rat model of Canavan disease, Mol. Brain Res 135 (2005) 112–121. [DOI] [PubMed] [Google Scholar]
- [192].Ahmed SS, Li H, Cao C, Sikoglu EM, Denninger AR, Su Q, Eaton S, Liso Navarro AA, Xie J, Szucs S, Zhang H, Moore C, Kirschner DA, Seyfried TN, Flotte TR, Matalon R, Gao G, A single intravenous rAAV injection as late as P20 achieves efficacious and sustained CNS gene therapy in Canavan mice, Mol. Ther 21 (2013) 2136–2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Ahmed SS, Schattgen SA, Frakes AE, Sikoglu EM, Su Q, Li J, Hampton TG, Denninger AR, Kirschner DA, Kaspar B, Matalon R, Gao G, RAAV Gene Therapy in a Canavan’s Disease Mouse Model Reveals Immune Impairments and an Extended Pathology beyond the Central Nervous System, Mol. Ther 24 (2016) 1030–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Gessler DJ, Li D, Xu H, Su Q, Sanmiguel J, Tuncer S, Moore C, King J, Matalon R, Gao G, Redirecting N-acetylaspartate metabolism in the central nervous system normalizes myelination and rescues Canavan disease, JCI Insight. 2 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Sondhi D, Johnson L, Purpura K, Monette S, Souweidane MM, Kaplitt MG, Kosofsky B, Yohay K, Ballon D, Dyke J, Kaminksy SM, Hackett NR, Crystal RG, Long-term expression and safety of administration of AAVrh.10hCLN2 to the brain of rats and nonhuman primates for the treatment of late infantile neuronal ceroid lipofuscinosis, Hum. Gene Ther. Methods 23 (2012) 324–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Cabrera-Salazar MA, Roskelley EM, Bu J, Hodges BL, Yew N, Dodge JC, Shihabuddin LS, Sohar I, Sleat DE, Scheule RK, Davidson BL, Cheng SH, Lobel P, Passini MA, Timing of therapeutic intervention determines functional and survival outcomes in a mouse model of late infantile batten disease, Mol. Ther 15 (2007) 1782–1788. [DOI] [PubMed] [Google Scholar]
- [197].Sondhi D, Hackett NR, Peterson DA, Stratton J, Baad M, Travis KM, Wilson JM, Crystal RG, Enhanced survival of the LINCL mouse following CLN2 gene transfer using the rh.10 rhesus Macaque-derived Adeno-associated virus vector, Mol. Ther 15 (2007) 481–491. [DOI] [PubMed] [Google Scholar]
- [198].Passini MA, Dodge JC, Bu J, Yang W, Zhao Q, Sondhi D, Hackett NR, Kaminsky SM, Mao Q, Shihabuddin LS, Cheng SH, Sleat DE, Stewart GR, Davidson BL, Lobel P, Crystal RG, Intracranial delivery of CLN2 reduces brain pathology in a mouse model of classical late infantile neuronal ceroid lipofuscinosis, J. Neurosci 26 (2006) 1334–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].Haskell RE, Hughes SM, Chiorini JA, Alisky JM, Davidson BL, Viral-mediated delivery of the late-infantile neuronal ceroid lipofuscinosis gene, TPP-1 to the mouse central nervous system, Gene Ther. 10 (2003) 34–42. [DOI] [PubMed] [Google Scholar]
- [200].Wolf DA, Lenander AW, Nan Z, Belur LR, Whitley CB, Gupta P, Low WC, McIvor RS, Direct gene transfer to the CNS prevents emergence of neurologic disease in a murine model of mucopolysaccharidosis type I, Neurobiol. Dis 43 (2011) 123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Watson G, Bastacky J, Belichenko P, Buddhikot M, Jungles S, Vellard M, Mobley WC, Kakkis E, Intrathecal administration of AAV vectors for the treatment of lysosomal storage in the brains of MPS I mice, Gene Ther 13 (2006) 917–925. [DOI] [PubMed] [Google Scholar]
- [202].Hinderer C, Bell P, Gurda BL, Wang Q, Louboutin JP, Zhu Y, Bagel J, O’Donnell P, Sikora T, Ruane T, Wang P, Haskins ME, Wilson JM, Intrathecal gene therapy corrects cns pathology in a feline model of mucopolysaccharidosis i, Mol. Ther 22 (2014) 2018–2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [203].Hinderer C, Katz N, Louboutin JP, Bell P, Yu H, Nayal M, Kozarsky K, O’Brien WT, Goode T, Wilson JM, Delivery of an Adeno-associated virus vector into cerebrospinal fluid attenuates central nervous system disease in mucopolysaccharidosis type II mice, Hum. Gene Ther 27 (2016) 906–915. [DOI] [PubMed] [Google Scholar]
- [204].Bosch A, Perret E, Desmaris N, Heard JM, Long-Term and Significant Correction of Brain Lesions in Adult Mucopolysaccharidosis Type VII Mice Using Recombinant AAV Vectors, Mol. Ther 1 (2000) 63–70. [DOI] [PubMed] [Google Scholar]
- [205].Gurda BL, De Guilhem De Lataillade A, Bell P, Zhu Y, Yu H, Wang P, Bagel J, Vite CH, Sikora T, Hinderer C, Calcedo R, Yox AD, Steet RA, Ruane T, O’Donnell P, Gao G, Wilson JM, Casal M, Ponder KP, Haskins ME, Evaluation of AAV-mediated gene therapy for central nervous system disease in Canine Mucopolysaccharidosis VII, Mol. Ther 24 (2016) 206–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206].Chen YH, Claflin K, Geoghegan JC, Davidson BL, Sialic acid deposition impairs the utility of AAV9, but not peptide-modified AAVs for brain gene therapy in a mouse model of lysosomal storage disease, in: Mol. Ther, Nature Publishing Group, 2012: pp. 1393–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207].Marcó S, Haurigot V, Jaén ML, Ribera A, Sánchez V, Molas M, Garcia M, León X, Roca C, Sánchez X, Bertolin J, Pérez J, Elias G, Navarro M, Carretero A, Pumarola M, Andaluz A, Espada Y, Añor S, Bosch F, Seven-year follow-up of durability and safety of AAV CNS gene therapy for a lysosomal storage disorder in a large animal, Mol. Ther. - Methods Clin. Dev 23 (2021) 370–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [208].Haurigot V, Marcó S, Ribera A, Garcia M, Ruzo A, Villacampa P, Ayuso E, Añor S, Andaluz A, Pineda M, García-Fructuoso G, Molas M, Maggioni L, Muñoz S, Motas S, Ruberte J, Mingozzi F, Pumarola M, Bosch F, Whole body correction of mucopolysaccharidosis IIIA by intracerebrospinal fluid gene therapy, J. Clin. Invest 123 (2013) 3254–3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [209].Roca C, Motas S, Marco S, Ribera A, Sanchez V, Sanchez X, Bertolin J, Leon X, Perez J, Garcia M, Villacampa P, Ruberte J, Pujol A, Haurigot V, Bosch F, Disease correction by AAV-mediated gene therapy in a new mouse model of mucopolysaccharidosis type IIID, Hum. Mol. Genet 26 (2017) 1535–1551. [DOI] [PubMed] [Google Scholar]
- [210].Baek RC, Boekman MLD, Leroy SG, Tierney LA, Sandberg MA, D’Azzo A, Seyfried TN, Sena-Esteves M, AAV-mediated gene delivery in adult GM1-gangliosidosis mice corrects lysosomal storage in CNS and improves survival, PLoS One. 5 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [211].Weismann CM, Ferreira J, Keeler AM, Su Q, Qui L, Shaffer SA, Xu Z, Gao G, Sena-Esteves M, Systemic AAV9 gene transfer in adult GM1 gangliosidosis mice reduces lysosomal storage in CNS and extends lifespan., Hum. Mol. Genet 24 (2015) 4353–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [212].Golebiowski Di., Van Der Bom IMJ, Kwon CS, Miller AD, Petrosky K, Bradbury AM, Maitland S, Kühn AL, Bishop N, Curran E, Silva N, GuhaSarkar D, Westmoreland SV, Martin DR, Gounis MJ, Asaad WF, Sena-Esteves M, Direct Intracranial Injection of AAVrh8 Encoding Monkey β-N-Acetylhexosaminidase Causes Neurotoxicity in the Primate Brain, Hum. Gene Ther 28 (2017) 510–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [213].Dodge JC, Clarke J, Song A, Bu J, Yang W, Taksir TV, Griffiths D, Zhao MA, Schuchman EH, Cheng SH, O’Riordan CR, Shihabuddin LS, Passini MA, Stewart GR, Gene transfer of human acid sphingomyelinase corrects neuropathology and motor deficits in a mouse model of Niemann-Pick type A disease, Proc. Natl. Acad. Sci. U. S. A 102 (2005) 17822–17827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [214].Chandler RJ, Williams IM, Gibson AL, Davidson CD, Incao AA, Hubbard BT, Porter FD, Pavan WJ, Venditti CP, Systemic AAV9 gene therapy improves the lifespan of mice with Niemann-Pick disease, type C1, Hum. Mol. Genet 26 (2017) 52–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [215].Lioy DT, Garg SK, Monaghan CE, Raber J, Foust KD, Kaspar BK, Hirrlinger PG, Kirchhoff F, Bissonnette JM, Ballas N, Mandel G, A role for glia in the progression of Rett’s syndrome, Nature. 475 (2011) 497–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [216].Gadalla KK, Bailey ME, Spike RC, Ross PD, Woodard KT, Kalburgi SN, Bachaboina L, Deng JV, West AE, Samulski RJ, Gray SJ, Cobb SR, Improved survival and reduced phenotypic severity following AAV9/MECP2 gene transfer to neonatal and juvenile male Mecp2 knockout mice, Mol. Ther 21 (2013) 18–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [217].Gadalla KKE, Vudhironarit T, Hector RD, Sinnett S, Bahey NG, Bailey MES, Gray SJ, Cobb SR, Development of a Novel AAV Gene Therapy Cassette with Improved Safety Features and Efficacy in a Mouse Model of Rett Syndrome, Mol. Ther. - Methods Clin. Dev 5 (2017) 180–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [218].Sinnett SE, Hector RD, Gadalla KKE, Heindel C, Chen D, Zaric V, Bailey MES, Cobb SR, Gray SJ, Improved MECP2 Gene Therapy Extends the Survival of MeCP2-Null Mice without Apparent Toxicity after Intracisternal Delivery, Mol. Ther. - Methods Clin. Dev 5 (2017) 106–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [219].Matagne V, Ehinger Y, Saidi L, Borges-Correia A, Barkats M, Bartoli M, Villard L, Roux J-C, A codon-optimized Mecp2 transgene corrects breathing deficits and improves survival in a mouse model of Rett syndrome, Neurobiol. Dis 99 (2017) 1–11. [DOI] [PubMed] [Google Scholar]
- [220].Luoni M, Giannelli S, Indrigo MT, Niro A, Massimino L, Iannielli A, Passeri L, Russo F, Morabito G, Calamita P, Gregori S, Deverman B, Broccoli V, Whole brain delivery of an instability-prone Mecp2 transgene improves behavioral and molecular pathological defects in mouse models of Rett syndrome, Elife. 9 (2020) 1–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [221].Sinnett SE, Boyle E, Lyons C, Gray SJ, Engineered microRNA-based regulatory element permits safe high-dose miniMECP2 gene therapy in Rett mice, Brain. 144 (2021) 3005–3019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [222].Matagne V, Borloz E, Ehinger Y, Saidi L, Villard L, Roux J-C, Severe offtarget effects following intravenous delivery of AAV9-MECP2 in a female mouse model of Rett syndrome, Neurobiol. Dis 149 (2021) 105235. [DOI] [PubMed] [Google Scholar]
- [223].Zeier Z, Kumar A, Bodhinathan K, Feller JA, Foster TC, Bloom DC, Fragile X mental retardation protein replacement restores hippocampal synaptic function in a mouse model of fragile X syndrome, Gene Ther. 16 (2009) 1122–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [224].Gholizadeh S, Arsenault J, on IC. Xuan Y, Pacey LK, Hampson DR, Reduced phenotypic severity following adeno-associated virus-mediated Fmr1 gene delivery in fragile X mice, Neuropsychopharmacology. 39 (2014) 3100–3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [225].Arsenault J, Gholizadeh S, Niibori Y, Pacey LK, Halder SK, Koxhioni E, Konno A, Hirai H, Hampson DR, FMRP Expression Levels in Mouse Central Nervous System Neurons Determine Behavioral Phenotype, Hum. Gene Ther 27 (2016) 982–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Jiang Y, Han L, Meng J, Wang Z, Zhou Y, Yuan H, Xu H, Zhang X, Zhao Y, Lu J, Xu H, Zhang C, wu Zhang Y, Gene therapy using human FMRP isoforms driven by the human FMR1 promoter rescues fragile X syndrome mouse deficits, Mol. Ther. - Methods Clin. Dev 27 (2022) 246–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [227].Hooper AWM, Wong H, Niibori Y, Abdoli R, Karumuthil-Melethil S, Qiao C, Danos O, Bruder JT, Hampson DR, Gene therapy using an ortholog of human fragile X mental retardation protein partially rescues behavioral abnormalities and EEG activity, Mol. Ther. - Methods Clin. Dev 22 (2021) 196–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [228].Habbas K, Cakil O, Zámbó B, Tabet R, Riet F, Dembele D, Mandel J, Hocquemiller M, Laufer R, Piguet F, Moine H, AAV‐delivered diacylglycerol kinase DGKk achieves long‐term rescue of fragile X syndrome mouse model, EMBO Mol. Med 14 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [229].Chadman KK, Adayev T, Udayan A, Ahmed R, Dai CL, Goodman JH, Meeker H, Dolzhanskaya N, Velinov M, Efficient Delivery of FMR1 across the Blood Brain Barrier Using AAVphp Construct in Adult FMR1 KO Mice Suggests the Feasibility of Gene Therapy for Fragile X Syndrome, Genes (Basel). 14 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [230].Wong H, Hooper AWM, Kang HR, Lee SJ, Zhao J, Sadhu C, Rawat S, Gray SJ, Hampson DR, CNS-dominant human FMRP isoform rescues seizures, fear, and sleep abnormalities in Fmr1-KO mice, JCI Insight. 8 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [231].Niibori Y, Lee SJ, Minassian BA, Hampson DR, Sexually Divergent Mortality and Partial Phenotypic Rescue after Gene Therapy in a Mouse Model of Dravet Syndrome, Hum. Gene Ther 31 (2020) 339–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [232].Tanenhaus A, Stowe T, Young A, McLaughlin J, Aeran R, Lin IW, Li J, Hosur R, Chen M, Leedy J, Chou T, Pillay S, Vila MC, Kearney JA, Moorhead M, Belle A, Tagliatela S, Cell-Selective Adeno-Associated Virus-Mediated SCN1A Gene Regulation Therapy Rescues Mortality and Seizure Phenotypes in a Dravet Syndrome Mouse Model and Is Well Tolerated in Nonhuman Primates, Hum. Gene Ther 33 (2022) 579–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [233].Colasante G, Lignani G, Brusco S, Di Berardino C, Carpenter J, Giannelli S, Valassina N, Bido S, Ricci R, Castoldi V, Marenna S, Church T, Massimino L, Morabito G, Benfenati F, Schorge S, Leocani L, Kullmann DM, Broccoli V, dCas9-Based Scn1a Gene Activation Restores Inhibitory Interneuron Excitability and Attenuates Seizures in Dravet Syndrome Mice, Mol. Ther 28 (2020) 235–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [234].Yamagata T, Raveau M, Kobayashi K, Miyamoto H, Tatsukawa T, Ogiwara I, Itohara S, Hensch TK, Yamakawa K, CRISPR/dCas9-based Scn1a gene activation in inhibitory neurons ameliorates epileptic and behavioral phenotypes of Dravet syndrome model mice, Neurobiol. Dis 141 (2020) 104954. [DOI] [PubMed] [Google Scholar]
- [235].Daily JL, Nash K, Jinwal U, Golde T, Rogers J, Peters MM, Burdine RD, Dickey C, Banko JL, Weeber EJ, Adeno-associated virus-mediated rescue of the cognitive defects in a mouse model for Angelman syndrome, PLoS One. 6 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [236].Judson MC, Shyng C, Simon JM, Davis CR, Punt AM, Salmon MT, Miller NW, Ritola KD, Elgersma Y, Amaral DG, Gray SJ, Philpot BD, Dual-isoform hUBE3A gene transfer improves behavioral and seizure outcomes in Angelman syndrome model mice, JCI Insight. 6 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [237].Nenninger AW, Willman M, Willman J, Stewart E, Mesidor P, Novoa M, Morrill NK, Alvarez L, Joly-Amado A, Peters MM, Gulick D, Nash KR, Improving Gene Therapy for Angelman Syndrome with Secreted Human UBE3A, Neurotherapeutics. 19 (2022) 1329–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [238].Wolter JM, Mao H, Fragola G, Simon JM, Krantz JL, Bazick HO, Oztemiz B, Stein JL, Zylka MJ, Cas9 gene therapy for Angelman syndrome traps Ube3a-ATS long non-coding RNA, Nature. 587 (2020) 281–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [239].Schmid RS, Deng X, Panikker P, Msackyi M, Breton C, Wilson JM, CRISPR/Cas9 directed to the Ube3a antisense transcript improves Angelman syndrome phenotype in mice, J. Clin. Invest 131 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [240].O’Geen H, Beitnere U, Garcia MS, Adhikari A, Cameron DL, Fenton TA, Copping NA, Deng P, Lock S, Halmai JANM, Villegas IJ, Liu J, Wang D, Fink KD, Silverman JL, Segal DJ, Transcriptional reprogramming restores UBE3A brain-wide and rescues behavioral phenotypes in an Angelman syndrome mouse model, Mol. Ther 31 (2023) 1088–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [241].McPhee SWJ, Janson CG, Li C, Samulski RJ, Camp AS, Francis J, Shera D, Lioutermann L, Feely M, Freese A, Leone P, Immune responses to AAV in a phase I study for Canavan disease, J. Gene Med 8 (2006) 577–588. [DOI] [PubMed] [Google Scholar]
- [242].Leone P, Shera D, McPhee SWJ, Francis JS, Kolodny EH, Bilaniuk LT, Wang DJ, Assadi M, Goldfarb O, Goldman HW, Freese A, Young D, During MJ, Samulski RJ, Janson CG, Long-term follow-up after gene therapy for canavan disease, Sci. Transl. Med 4 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [243].Mao Q, zhi Qin W, Zhang A, Ye N, Recent advances in dopaminergic strategies for the treatment of Parkinson’s disease, Acta Pharmacol. Sin 41 (2020) 471–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [244].Christine CW, Starr PA, Larson PS, Eberling JL, Jagust WJ, Hawkins RA, Vanbrocklin HF, Wright JF, Bankiewicz KS, Aminoff MJ, Safety and tolerability of putaminal AADC gene therapy for Parkinson disease, Neurology. 73 (2009) 1662–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [245].Niethammer M, Tang CC, Vo A, Nguyen N, Spetsieris P, Dhawan V, Ma Y, Small M, Feigin A, During MJ, Kaplitt MG, Eidelberg D, Gene therapy reduces Parkinson’s disease symptoms by reorganizing functional brain connectivity, Sci. Transl. Med 10 (2018). [DOI] [PubMed] [Google Scholar]
- [246].Kaplitt MG, Feigin A, Tang C, Fitzsimons HL, Mattis P, Lawlor PA, Bland RJ, Young D, Strybing K, Eidelberg D, During MJ, Safety and tolerability of gene therapy with an adeno-associated virus (AAV) borne GAD gene for Parkinson’s disease: an open label, phase I trial, Lancet. 369 (2007) 2097–2105. [DOI] [PubMed] [Google Scholar]
- [247].Feigin A, Kaplitt MG, Tang C, Lin T, Mattis P, Dhawan V, During MJ, Eidelberg D, Modulation of metabolic brain networks after subthalamic gene therapy for Parkinson’s disease, Proc. Natl. Acad. Sci. U. S. A 104 (2007) 19559–19564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [248].Björklund A, Kirik D, Rosenblad C, Georgievska B, Lundberg C, Mandel RJ, Towards a neuroprotective gene therapy for Parkinson’s disease: Use of adenovirus, AAV and lentivirus vectors for gene transfer of GDNF to the nigrostriatal system in the rat Parkinson model, Brain Res. 886 (2000) 82–98. [DOI] [PubMed] [Google Scholar]
- [249].Warren Olanow C, Bartus RT, Baumann TL, Factor S, Boulis N, Stacy M, Turner DA, Marks W, Larson P, Starr PA, Jankovic J, Simpson R, Watts R, Guthrie B, Poston K, Henderson JM, Stern M, Baltuch G, Goetz CG, Herzog C, Jeffrey H, Alterman R, Lozano AM, Lang AE, Gene delivery of neurturin to putamen and substantia nigra in Parkinson disease: A double-blind, randomized, controlled trial, Ann. Neurol 78 (2015) 248–257. [DOI] [PubMed] [Google Scholar]
- [250].Zheng W, Fan D, Glucocerebrosidase Mutations Cause Mitochondrial and Lysosomal Dysfunction in Parkinson ‘ s Disease : Pathogenesis and Therapeutic Implications, 14 (2022) 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [251].Martínez-Serrano A, Fischer W, Söderström S, Ebendal T, Björklund A, Long-term functional recovery from age-induced spatial memory impairments by nerve growth factor gene transfer to the rat basal forebrain, Proc. Natl. Acad. Sci. U. S. A 93 (1996) 6355–6360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [252].Rafii MS, Tuszynski MH, Thomas RG, Barba D, Brewer JB, Rissman RA, Siffert J, Aisen PS, Mintzer J, Lerner A, Levey A, Burke J, Sano M, Turner S, Zamrini E, Grill J, Marson D, Adeno-associated viral vector (serotype 2)-nerve growth factor for patients with Alzheimer disease a randomized clinical trial, JAMA Neurol. 75 (2018) 834–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [253].Rafii MS, Baumann TL, Bakay RAE, Ostrove JM, Siffert J, Fleisher AS, Herzog CD, Barba D, Pay M, Salmon DP, Chu Y, Kordower JH, Bishop K, Keator D, Potkin S, Bartus RT, A phase1 study of stereotactic gene delivery of AAV2-NGF for Alzheimer’s disease, Alzheimer’s Dement. 10 (2014) 571–581. [DOI] [PubMed] [Google Scholar]
- [254].Zhao L, Gottesdiener AJ, Parmar M, Li M, Kaminsky SM, Chiuchiolo MJ, Sondhi D, Sullivan PM, Holtzman DM, Crystal RG, Paul SM, Intracerebral adeno-associated virus gene delivery of apolipoprotein E2 markedly reduces brain amyloid pathology in Alzheimer’s disease mouse models, Neurobiol. Aging 44 (2016) 159–172. [DOI] [PubMed] [Google Scholar]
- [255].Bernardes de Jesus B, Vera E, Schneeberger K, Tejera AM, Ayuso E, Bosch F, Blasco MA, Telomerase gene therapy in adult and old mice delays aging and increases longevity without increasing cancer, EMBO Mol. Med 4 (2012) 691–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [256].Zhang J, Wu X, Qin C, Qi J, Ma S, Zhang H, Kong Q, Chen D, Ba D, He W, A novel recombinant adeno-associated virus vaccine reduces behavioral impairment and β-amyloid plaques in a mouse model of Alzheimer’s disease, Neurobiol. Dis 14 (2003) 365–379. [DOI] [PubMed] [Google Scholar]
- [257].Miniarikova J, Zimmer V, Martier R, Brouwers CC, Pythoud C, Richetin K, Rey M, Lubelski J, Evers MM, Van Deventer SJ, Petry H, Déglon N, Konstantinova P, AAV5-miHTT gene therapy demonstrates suppression of mutant huntingtin aggregation and neuronal dysfunction in a rat model of Huntington’s disease, Gene Ther. 24 (2017) 630–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [258].Miniarikova J, Zanella I, Huseinovic A, van der Zon T, Hanemaaijer E, Martier R, Koornneef A, Southwell AL, Hayden MR, van Deventer SJ, Petry H, Konstantinova P, Design, Characterization, and Lead Selection of Therapeutic miRNAs Targeting Huntingtin for Development of Gene Therapy for Huntington’s Disease, Mol. Ther. - Nucleic Acids 5 (2016) e297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [259].Spronck EA, Brouwers CC, Vallès A, de Haan M, Petry H, van Deventer SJ, Konstantinova P, Evers MM, AAV5-miHTT Gene Therapy Demonstrates Sustained Huntingtin Lowering and Functional Improvement in Huntington Disease Mouse Models, Mol. Ther. - Methods Clin. Dev 13 (2019) 334–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [260].Bridget M, Peplow P, Altered microRNA expression in animal models of Huntington’s disease and potential therapeutic strategies, Neural Regen. Res 16 (2021) 2159–2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [261].Keskin S, Brouwers CC, Sogorb-gonzalez M, Martier R, Depla JA, Vallès A, Van Deventer SJ, Konstantinova P, Evers MM, AAV5-miHTT Lowers Huntingtin mRNA and Protein without Off-Target Effects in Patient-Derived Neuronal Cultures and Astrocytes, Mol. Ther. Methods Clin. Dev 15 (2019) 275–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [262].Corti M, Byrne BJ, Gessler DJ, Thompson G, Norman S, Lammers J, Coleman KE, Liberati C, Elder ME, Escolar ML, Tuna IS, Mesaros C, Kleiner GI, Barbouth DS, Gray-Edwards HL, Clement N, Cleaver BD, Gao G, Adeno-associated virus-mediated gene therapy in a patient with Canavan disease using dual routes of administration and immune modulation, Mol. Ther. - Methods Clin. Dev 30 (2023) 303–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [263].Worgall S, Sondhi D, Hackett NR, Kosofsky B, V Kekatpure M, Neyzi N, Dyke JP, Ballon D, Heier L, Greenwald BM, Christos P, Mazumdar M, Souweidane MM, Kaplitt MG, Crystal RG, Treatment of Late Infantile Neuronal Ceroid Lipofuscinosis by CNS Administration of a Serotype 2 Adeno-Associated Virus Expressing CLN2 cDNA, Hum. Gene Ther 19 (2008) 463–474. [DOI] [PubMed] [Google Scholar]
- [264].Sondhi D, Kaminsky SM, Hackett NR, Pagovich OE, Rosenberg JB, De BP, Chen A, Van de Graaf B, Mezey JG, Mammen GW, Mancenido D, Xu F, Kosofsky B, Yohay K, Worgall S, Kaner RJ, Souwedaine M, Greenwald BM, Kaplitt M, Dyke JP, Ballon DJ, Heier LA, Kiss S, Crystal RG, Slowing late infantile Batten disease by direct brain parenchymal administration of a rh.10 adeno-associated virus expressing CLN2, Sci. Transl. Med 12 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [265].McBride KL, Smith NJC, Couce ML, Flanigan KM, V Truxal K, Simmons TR, de Castro MJ, Rinaldi F, Oreiro MT, Jaensch L, Fuller M, Ruiz J, Safety, Tolerability, and Preliminary Evidence of Biopotency in Transpher B, a Multicenter, Singledose, Phase 1/2 Clinical Trial of ABO-101 Gene Therapy for Sanfilippo Syndrome Type B (Mucopolysaccharidosis IIIB) (5175), Neurology. 94 (2020) 5175. http://n.neurology.org/content/94/15_Supplement/5175.abstract. [Google Scholar]
- [266].Panayotis N, Ehinger Y, Felix MS, Roux JC, State-of-the-art therapies for Rett syndrome, Dev. Med. Child Neurol 65 (2023) 162–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [267].Palmieri M, Pozzer D, Landsberger N, Advanced genetic therapies for the treatment of Rett syndrome: state of the art and future perspectives, Front. Neurosci 17 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [268].Yang K, Cheng C, Yuan Y, Zhang Y, Shan S, Qiu Z, Extension of the Lifespan of a Mouse Model of Rett Syndrome by Intracerebroventricular Delivery of MECP2, Neurosci. Bull 39 (2023) 297–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [269].Hampson DR, Hooper AWM, Niibori Y, The application of adeno-associated viral vector gene therapy to the treatment of fragile X syndrome, Brain Sci. 9 (2019) 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [270].Gholizadeh S, Tharmalingam S, MacAldaz ME, Hampson DR, Transduction of the central nervous system after intracerebroventricular injection of adeno-associated viral vectors in neonatal and juvenile mice, Hum. Gene Ther. Methods 24 (2013) 205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [271].Chilcott E, Díaz JA, Bertram C, Berti M, Karda R, Genetic therapeutic advancements for Dravet Syndrome, Epilepsy Behav. 132 (2022) 108741. [DOI] [PubMed] [Google Scholar]
- [272].Smith SEP, Zhou YD, Zhang G, Jin Z, Stoppel DC, Anderson MP, Increased gene dosage of Ube3a results in autism traits and decreased glutamate synaptic transmission in mice, Sci. Transl. Med 3 (2011) 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]


