Abstract
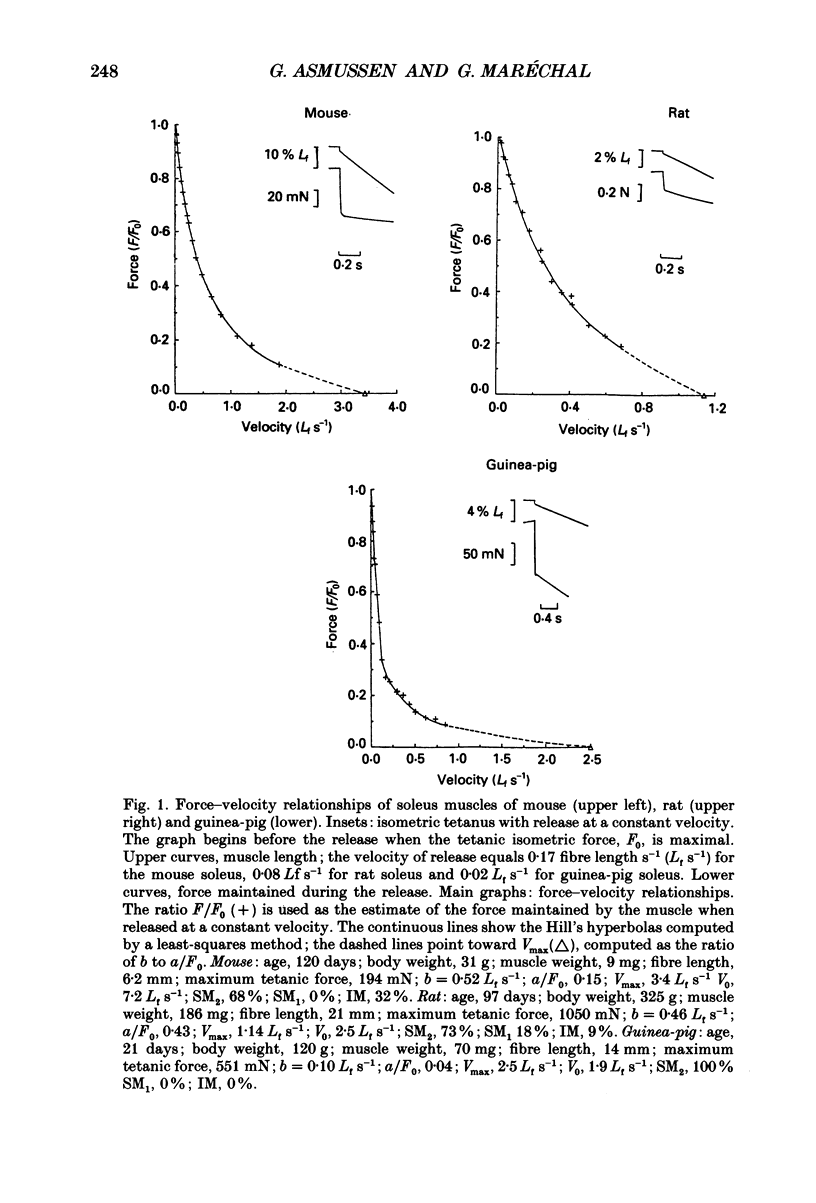
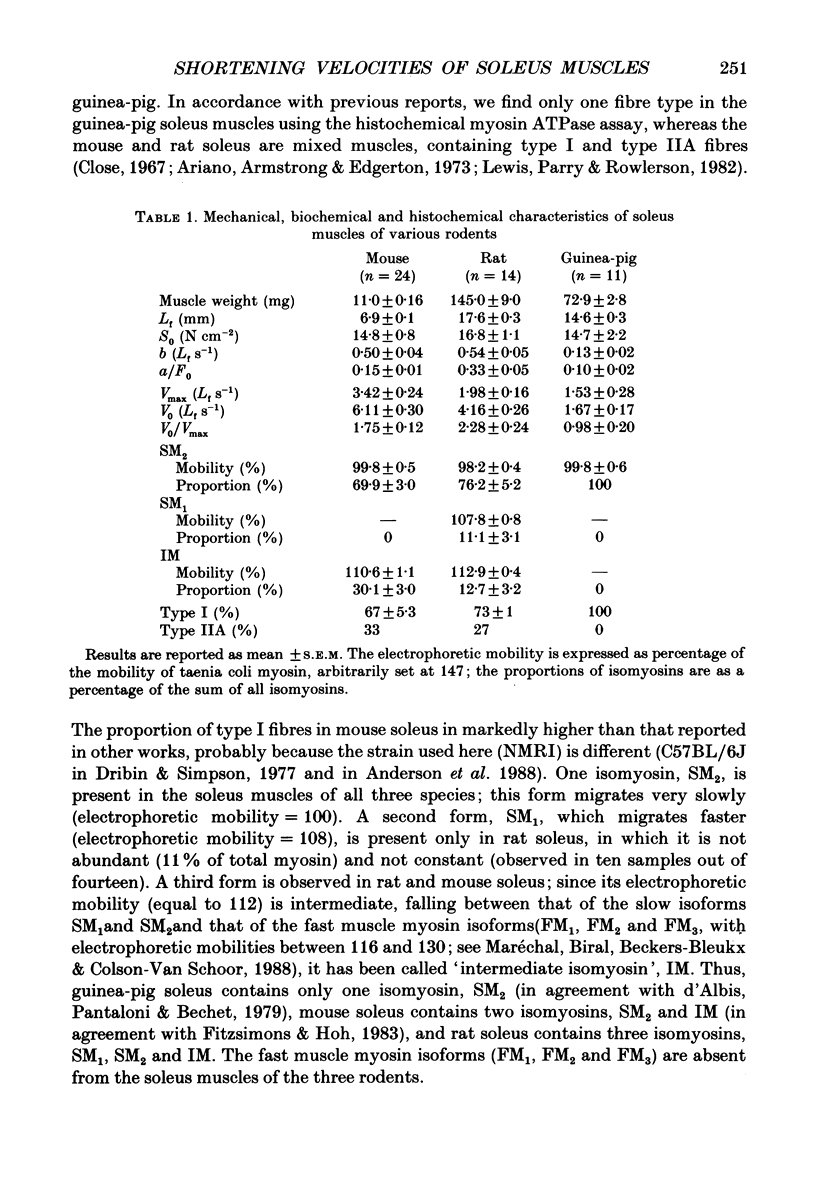
1. Guinea-pig soleus contains only type I fibres and slow isomyosin, SM2. Rat and mouse soleus contain about 70% of type I fibres and a mixture of isomyosins: slow, SM2 and intermediate, IM. Many rat soleus muscles contain a third isomyosin of a slow type, SM1. 2. The maximal velocity of unloaded shortening, V0, is largest in mouse soleus (6.11 Lf s-1), slowest in guinea-pig soleus (1.67 Lf s-1) and intermediate in rat soleus (4.16 Lf s-1) (Lf = fibre length). 3. In guinea-pig soleus, V0 is equal to the maximal velocity (Vmax) computed using the Hill force-velocity relationship; V0 is approximately twice as large as Vmax in mouse and rat soleus. 4. V0 measures the unloaded shortening velocity of the fastest fibres whereas Vmax is a function of the force-velocity characteristics of all the fibres contained in the muscle. 5. V0 increases according to the isomyosin composition of the fibres in the sequence SM2 less than SM1 + IM less than IM.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. E., Bressler B. H., Ovalle W. K. Functional regeneration in the hindlimb skeletal muscle of the mdx mouse. J Muscle Res Cell Motil. 1988 Dec;9(6):499–515. doi: 10.1007/BF01738755. [DOI] [PubMed] [Google Scholar]
- Ariano M. A., Armstrong R. B., Edgerton V. R. Hindlimb muscle fiber populations of five mammals. J Histochem Cytochem. 1973 Jan;21(1):51–55. doi: 10.1177/21.1.51. [DOI] [PubMed] [Google Scholar]
- Billeter R., Heizmann C. W., Howald H., Jenny E. Analysis of myosin light and heavy chain types in single human skeletal muscle fibers. Eur J Biochem. 1981 May 15;116(2):389–395. doi: 10.1111/j.1432-1033.1981.tb05347.x. [DOI] [PubMed] [Google Scholar]
- Biral D., Betto R., Danieli-Betto D., Salviati G. Myosin heavy chain composition of single fibres from normal human muscle. Biochem J. 1988 Feb 15;250(1):307–308. doi: 10.1042/bj2500307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooke M. H., Kaiser K. K. Muscle fiber types: how many and what kind? Arch Neurol. 1970 Oct;23(4):369–379. doi: 10.1001/archneur.1970.00480280083010. [DOI] [PubMed] [Google Scholar]
- Brooks S. V., Faulkner J. A. Contractile properties of skeletal muscles from young, adult and aged mice. J Physiol. 1988 Oct;404:71–82. doi: 10.1113/jphysiol.1988.sp017279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecchi G., Colomo F., Lombardi V. Force-velocity relation in normal and nitrate-treated frog single muscle fibres during rise of tension in an isometric tetanus. J Physiol. 1978 Dec;285:257–273. doi: 10.1113/jphysiol.1978.sp012570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claflin D. R., Faulkner J. A. Shortening velocity extrapolated to zero load and unloaded shortening velocity of whole rat skeletal muscle. J Physiol. 1985 Feb;359:357–363. doi: 10.1113/jphysiol.1985.sp015589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Close R. I. Dynamic properties of mammalian skeletal muscles. Physiol Rev. 1972 Jan;52(1):129–197. doi: 10.1152/physrev.1972.52.1.129. [DOI] [PubMed] [Google Scholar]
- Close R. Properties of motor units in fast and slow skeletal muscles of the rat. J Physiol. 1967 Nov;193(1):45–55. doi: 10.1113/jphysiol.1967.sp008342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danieli Betto D., Zerbato E., Betto R. Type 1, 2A, and 2B myosin heavy chain electrophoretic analysis of rat muscle fibers. Biochem Biophys Res Commun. 1986 Jul 31;138(2):981–987. doi: 10.1016/s0006-291x(86)80592-7. [DOI] [PubMed] [Google Scholar]
- Dribin L. B., Simpson S. B., Jr Histochemical and morphological study of dystrophic (C57BL/6J dy2j/dy2j) and normal muscle. Exp Neurol. 1977 Sep;56(3):480–497. doi: 10.1016/0014-4886(77)90316-8. [DOI] [PubMed] [Google Scholar]
- Edman K. A. The velocity of unloaded shortening and its relation to sarcomere length and isometric force in vertebrate muscle fibres. J Physiol. 1979 Jun;291:143–159. doi: 10.1113/jphysiol.1979.sp012804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzsimons R. B., Hoh J. F. Myosin isoenzymes in fast-twitch and slow-twitch muscles of normal and dystrophic mice. J Physiol. 1983 Oct;343:539–550. doi: 10.1113/jphysiol.1983.sp014908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoh J. Y., McGrath P. A., White R. I. Electrophoretic analysis of multiple forms of myosin in fast-twitch and slow-twitch muscles of the chick. Biochem J. 1976 Jul 1;157(1):87–95. doi: 10.1042/bj1570087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julian F. J., Rome L. C., Stephenson D. G., Striz S. The maximum speed of shortening in living and skinned frog muscle fibres. J Physiol. 1986 Jan;370:181–199. doi: 10.1113/jphysiol.1986.sp015929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis D. M., Parry D. J., Rowlerson A. Isometric contractions of motor units and immunohistochemistry of mouse soleus muscle. J Physiol. 1982 Apr;325:393–401. doi: 10.1113/jphysiol.1982.sp014157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luff A. R. Dynamic properties of the inferior rectus, extensor digitorum longus, diaphragm and soleus muscles of the mouse. J Physiol. 1981;313:161–171. doi: 10.1113/jphysiol.1981.sp013656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maréchal G., Plaghki L. The deficit of the isometric tetanic tension redeveloped after a release of frog muscle at a constant velocity. J Gen Physiol. 1979 Apr;73(4):453–467. doi: 10.1085/jgp.73.4.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maréchal G., Schwartz K., Beckers-Bleukx G., Ghins E. Isozymes of myosin in growing and regenerating rat muscles. Eur J Biochem. 1984 Jan 16;138(2):421–428. doi: 10.1111/j.1432-1033.1984.tb07932.x. [DOI] [PubMed] [Google Scholar]
- Powell P. L., Roy R. R., Kanim P., Bello M. A., Edgerton V. R. Predictability of skeletal muscle tension from architectural determinations in guinea pig hindlimbs. J Appl Physiol Respir Environ Exerc Physiol. 1984 Dec;57(6):1715–1721. doi: 10.1152/jappl.1984.57.6.1715. [DOI] [PubMed] [Google Scholar]
- Reiser P. J., Moss R. L., Giulian G. G., Greaser M. L. Shortening velocity in single fibers from adult rabbit soleus muscles is correlated with myosin heavy chain composition. J Biol Chem. 1985 Aug 5;260(16):9077–9080. [PubMed] [Google Scholar]
- Rowe R. W., Goldspink G. Surgically induced hypertrophy in skeletal muscles of the laboratory mouse. Anat Rec. 1968 May;161(1):69–75. doi: 10.1002/ar.1091610107. [DOI] [PubMed] [Google Scholar]
- Staron R. S., Pette D. The multiplicity of combinations of myosin light chains and heavy chains in histochemically typed single fibres. Rabbit soleus muscle. Biochem J. 1987 May 1;243(3):687–693. doi: 10.1042/bj2430687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney H. L., Kushmerick M. J., Mabuchi K., Sréter F. A., Gergely J. Myosin alkali light chain and heavy chain variations correlate with altered shortening velocity of isolated skeletal muscle fibers. J Biol Chem. 1988 Jun 25;263(18):9034–9039. [PubMed] [Google Scholar]
- Witzmann F. A., Kim D. H., Fitts R. H. Effect of hindlimb immobilization on the fatigability of skeletal muscle. J Appl Physiol Respir Environ Exerc Physiol. 1983 May;54(5):1242–1248. doi: 10.1152/jappl.1983.54.5.1242. [DOI] [PubMed] [Google Scholar]
- Yamaoka K., Tanigawara Y., Nakagawa T., Uno T. A pharmacokinetic analysis program (multi) for microcomputer. J Pharmacobiodyn. 1981 Nov;4(11):879–885. doi: 10.1248/bpb1978.4.879. [DOI] [PubMed] [Google Scholar]
- d'Albis A., Pantaloni C., Bechet J. J. An electrophoretic study of native myosin isozymes and of their subunit content. Eur J Biochem. 1979 Sep;99(2):261–272. doi: 10.1111/j.1432-1033.1979.tb13253.x. [DOI] [PubMed] [Google Scholar]



