Abstract
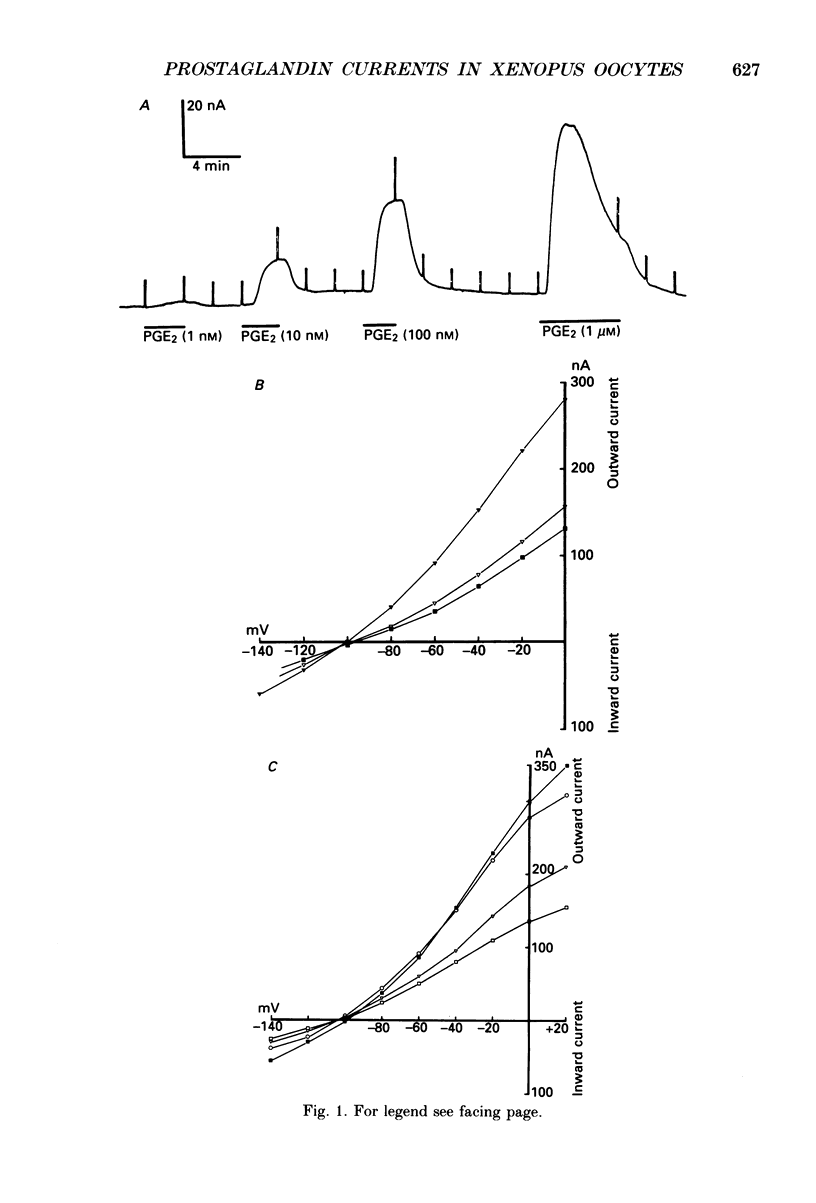
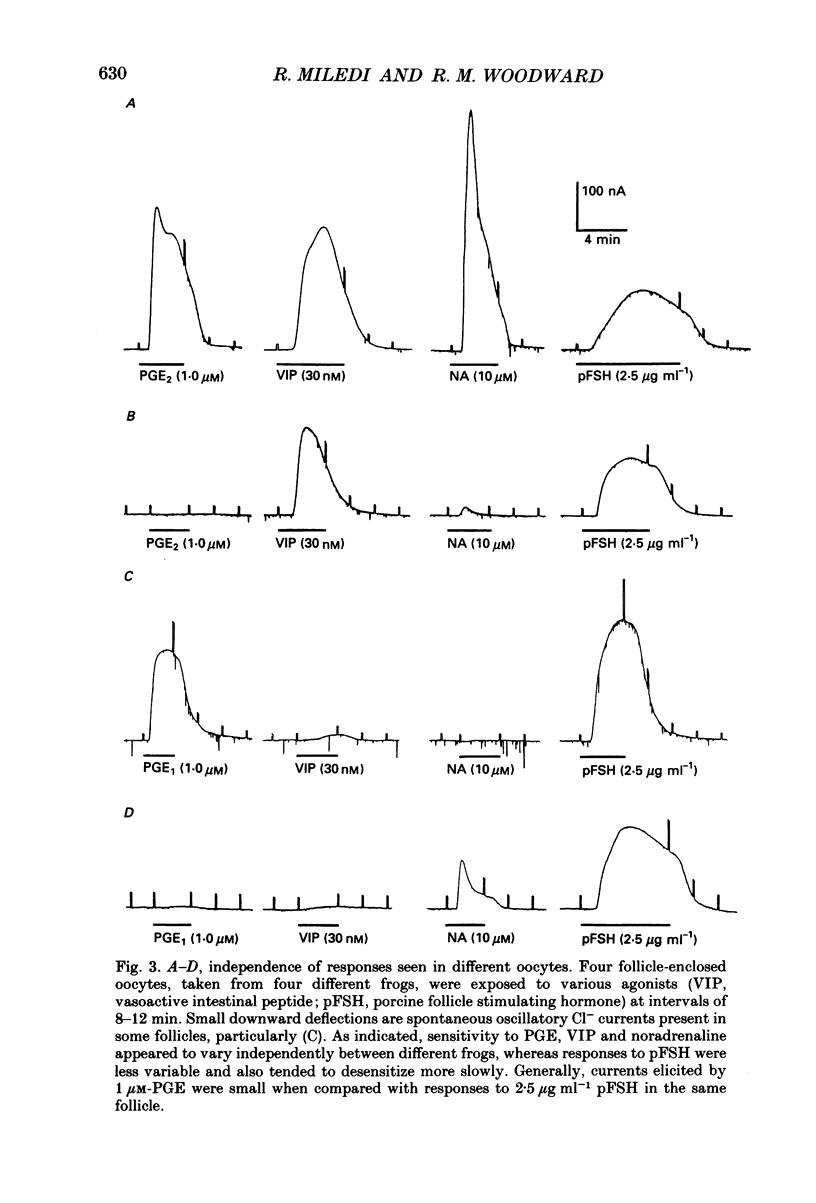
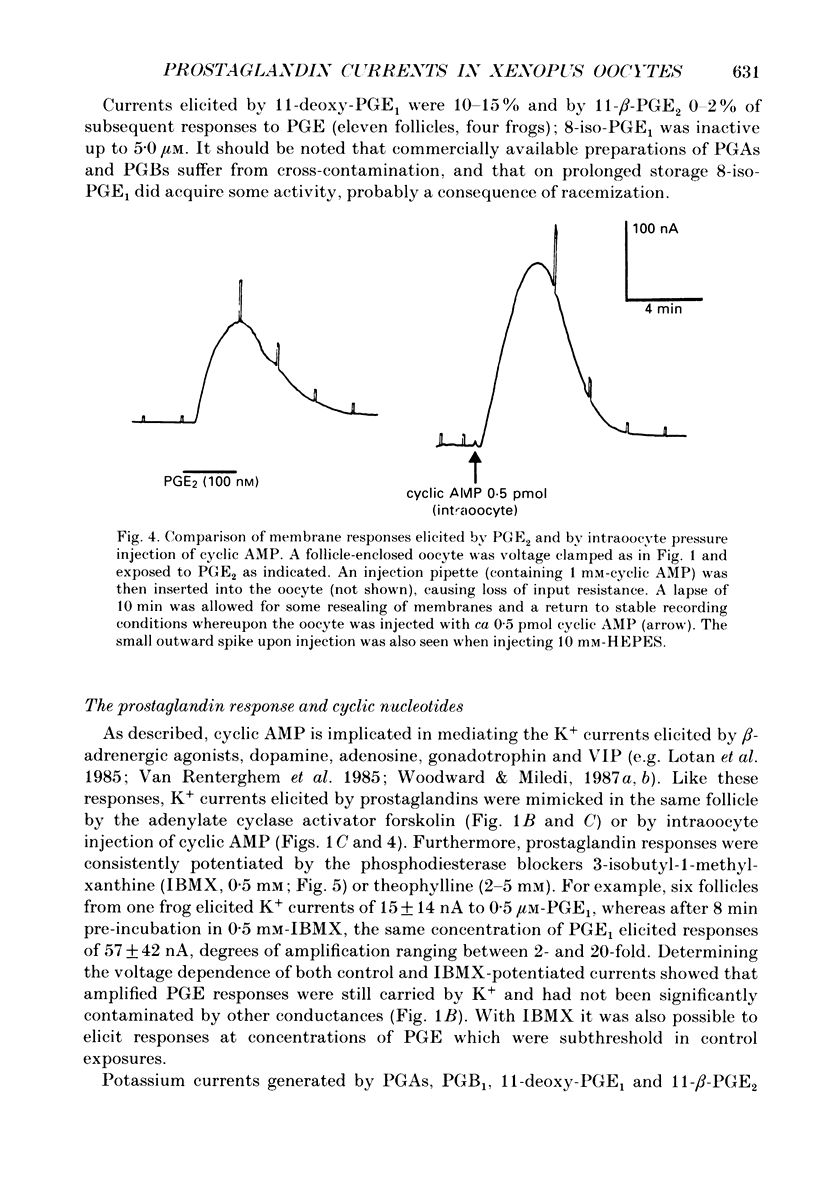
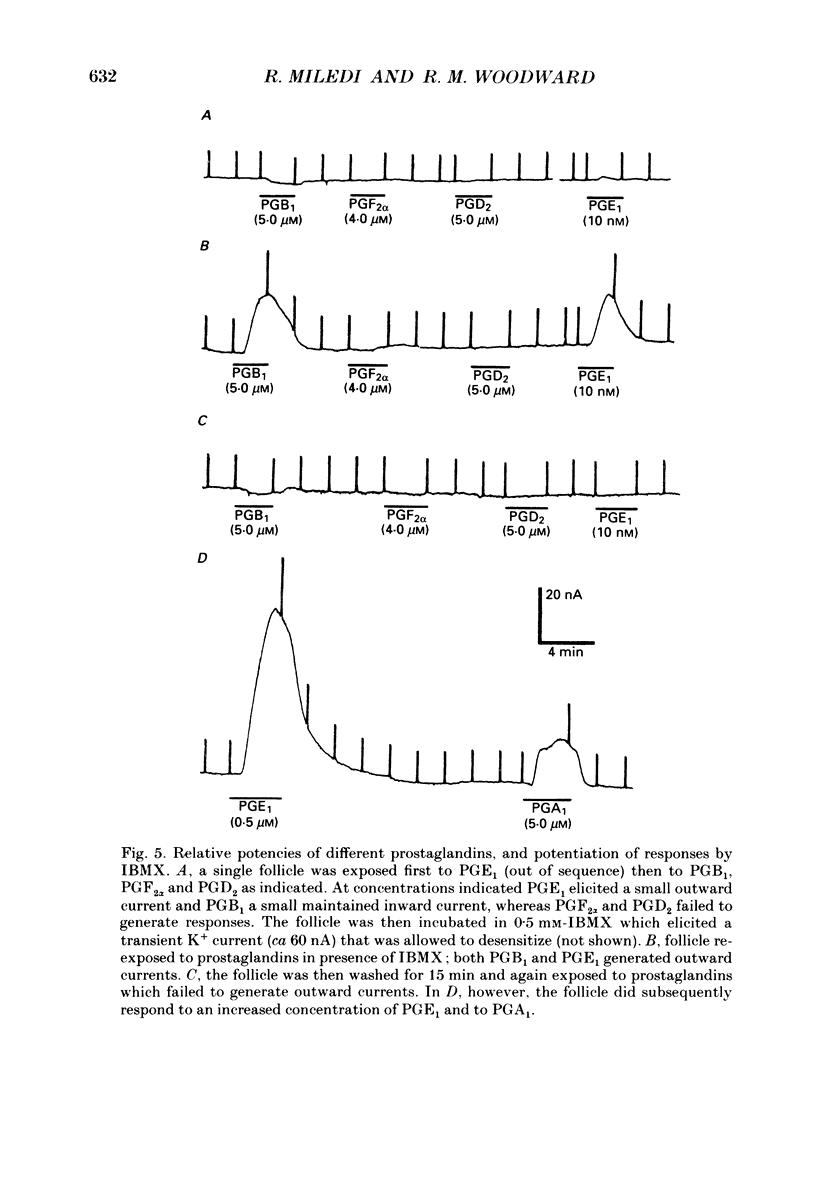
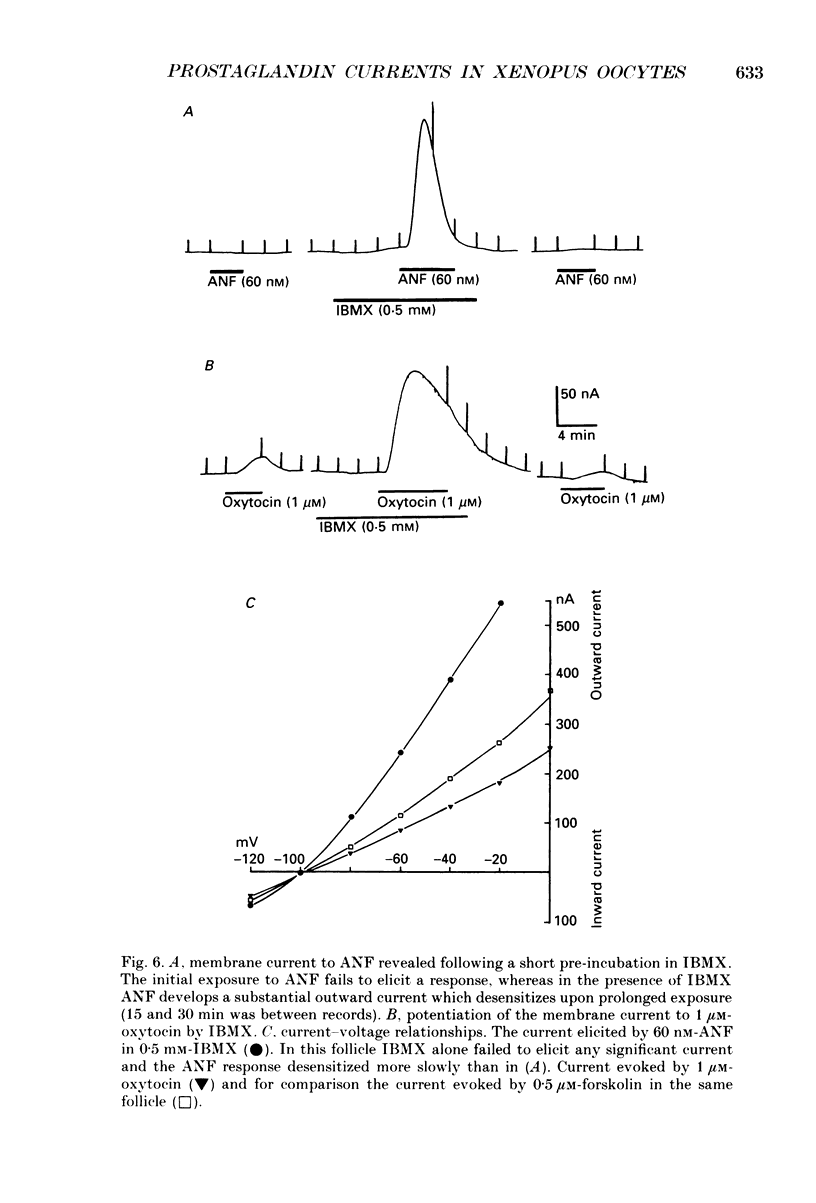
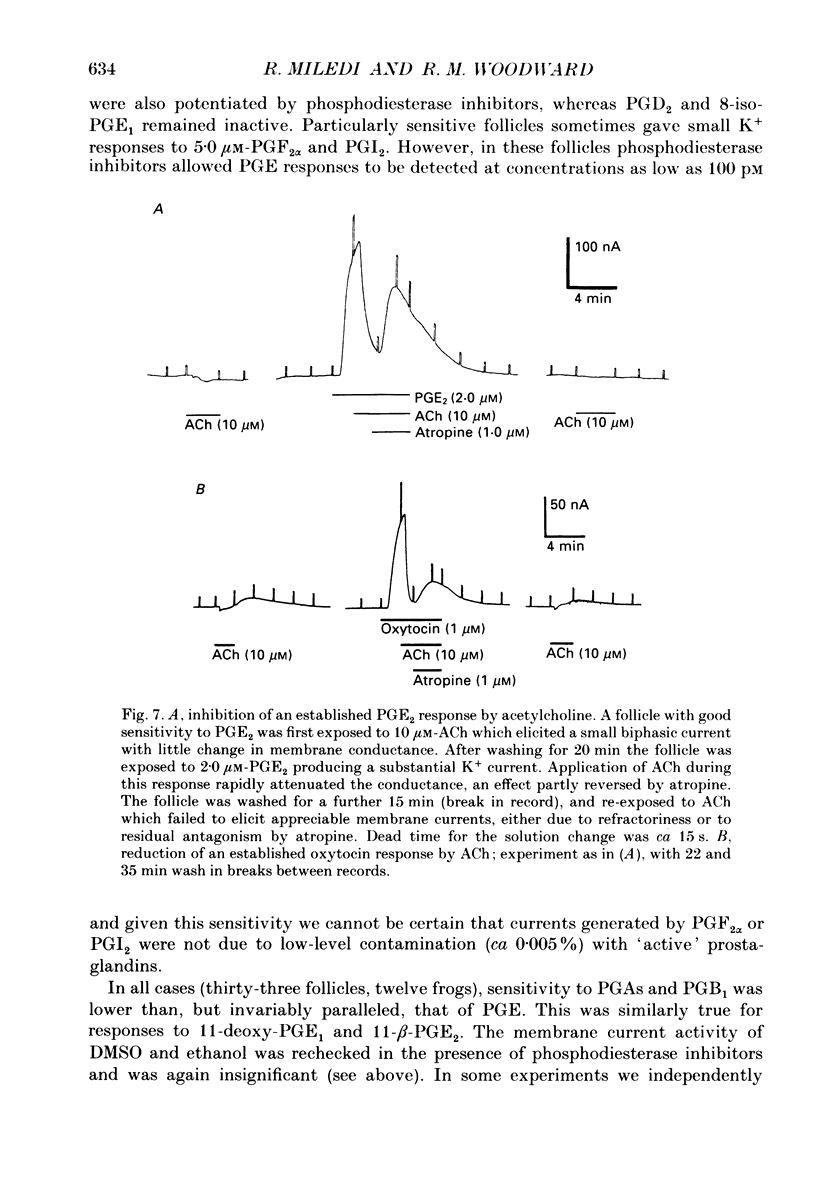
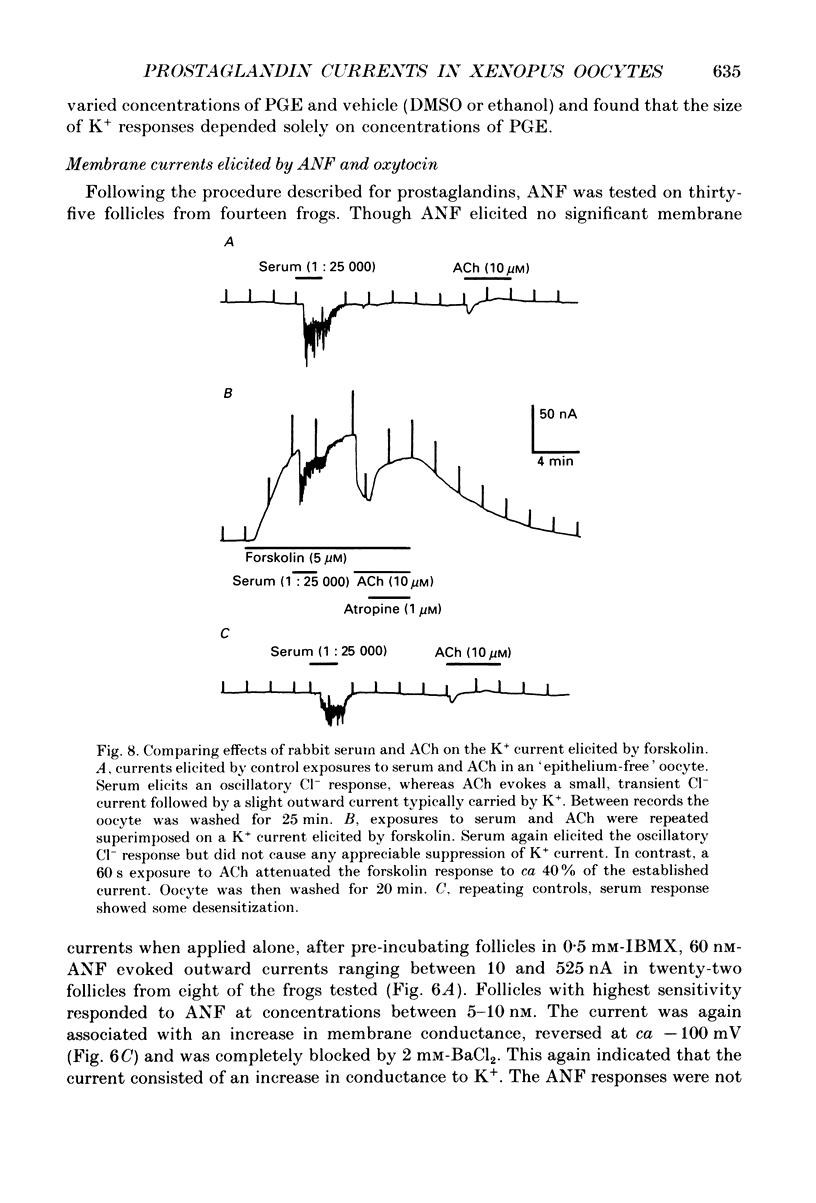
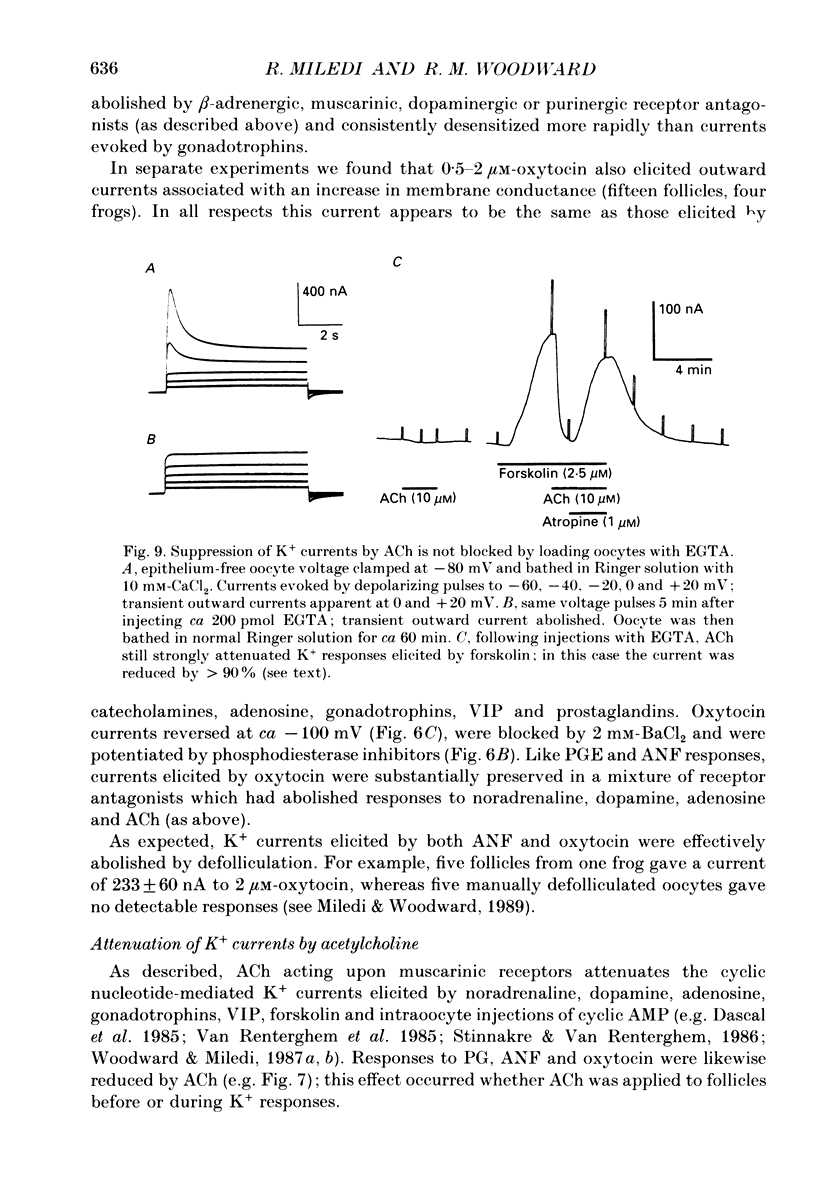
1. Membrane currents were recorded from voltage clamped Xenopus laevis oocytes, still surrounded by follicular cells, theca and enveloping inner ovarian epithelia (ovarian follicles). 2. Superfusing follicles with frog Ringer solution containing E-series prostaglandins (PGE1 or PGE2) or oxytocin (0.5-2 microM) generated slow membrane currents arising from an increase in membrane conductance to K+. 3. Follicles taken from different frogs varied greatly in responsiveness to PGE and oxytocin. For example, enclosed oocytes with good sensitivity to prostaglandins responded to 1 nM-PGE, whereas follicles from some frogs failed to respond at 5 microM. 4. Oocytes with good responsiveness to PGE also produced K+ currents to PGA1, PGA2, PGB1, 11-deoxy-PGE1 and 11-beta-PGE2, whereas PGF2 alpha, PGI2, PGD2 and 8-iso-PGE1 generally failed to elicit membrane currents. 5. Responses to PGE and oxytocin were mimicked by the adenylate cyclase activator forskolin or by intraoocyte pressure injection of cyclic nucleotides. Responses were potentiated by the phosphodiesterase inhibitors theophylline and 3-isobutyl-1-methylxanthine (IBMX). In IBMX (0.5 mM), human atrial natriuretic factor (ANF) (10-60 nM) elicited a similar K+ conductance. This all implied that cyclic nucleotides played a role in the receptor-channel coupling mechanism of these responses. 6. Defolliculating oocytes effectively abolished responses to prostaglandins, oxytocin and ANF, suggesting that the currents arise in follicular cells. 7. The responses of PGE, oxytocin and ANF thus resembled currents elicited by catecholamines, adenosine, gonadotrophins and vasoactive intestinal peptide (VIP). However, PGE, oxytocin and ANF responses were not blocked by catecholaminergic or purinergic antagonists. Moreover, when comparing follicles isolated from different frogs, the sensitivity to PGE and oxytocin varied independently of that to gonadotrophin or VIP. These experiments suggest that Xenopus ovarian follicles contain specific and distinct receptors for PGE, oxytocin and ANF. 8. Acetylcholine attenuated the cyclic nucleotide-mediated K+ responses, including currents elicited by PGE, oxytocin and ANF. Attenuation was not dependent on, or mimicked by, activation of the inositol phosphate-diacylglycerol messenger pathways located in the oocyte itself, nor was it appreciably blocked by loading follicle-enclosed oocytes with 0.1-1.5 mM-EGTA.
Full text
PDF




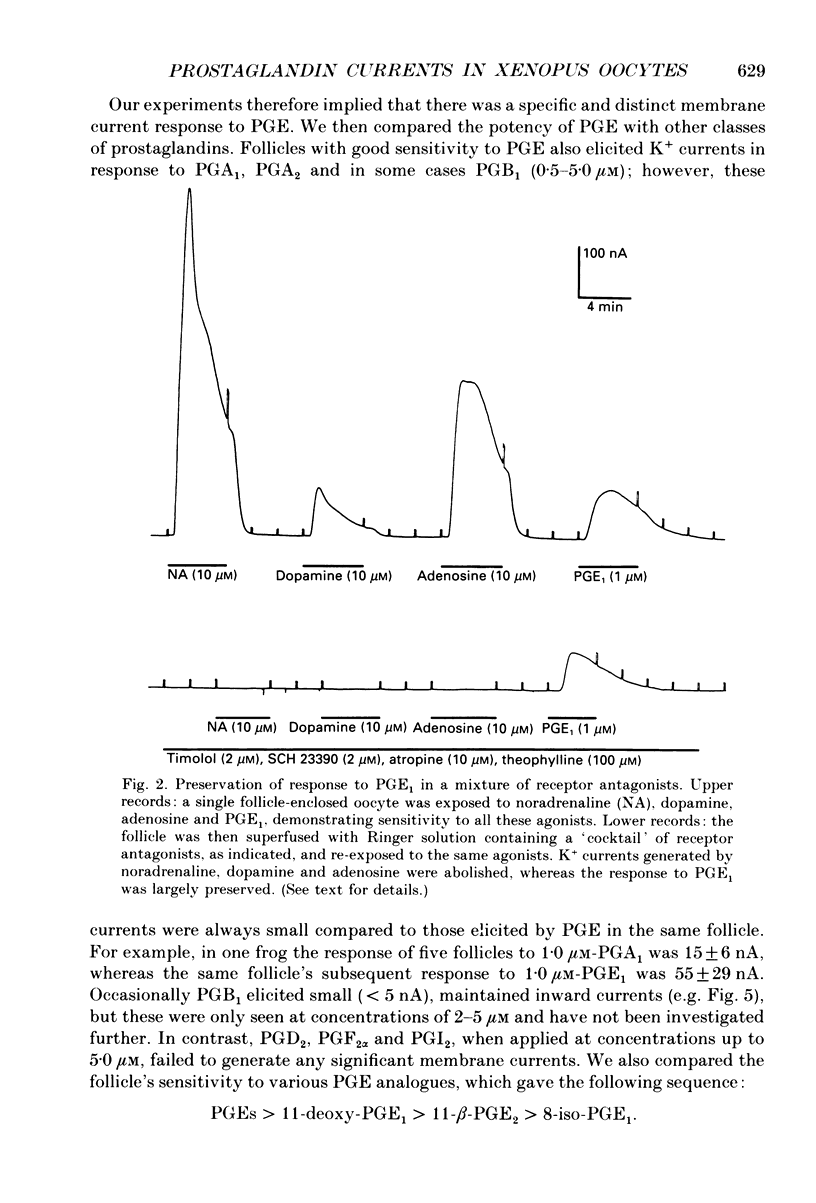







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adashi E. Y., Hsueh A. J. Stimulation of beta 2-adrenergic responsiveness by follicle-stimulating hormone in rat granulosa cells in vitro and in vivo. Endocrinology. 1981 Jun;108(6):2170–2178. doi: 10.1210/endo-108-6-2170. [DOI] [PubMed] [Google Scholar]
- Armstrong D. T. Prostaglandins and follicular functions. J Reprod Fertil. 1981 May;62(1):283–291. doi: 10.1530/jrf.0.0620283. [DOI] [PubMed] [Google Scholar]
- Behrman H. R. Prostaglandins in hypothalamo-pituitary and ovarian function. Annu Rev Physiol. 1979;41:685–700. doi: 10.1146/annurev.ph.41.030179.003345. [DOI] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol: two interacting second messengers. Annu Rev Biochem. 1987;56:159–193. doi: 10.1146/annurev.bi.56.070187.001111. [DOI] [PubMed] [Google Scholar]
- Browne C. L., Wiley H. S., Dumont J. N. Oocyte-follicle cell gap junctions in Xenopus laevis and the effects of gonadotropin on their permeability. Science. 1979 Jan 12;203(4376):182–183. doi: 10.1126/science.569364. [DOI] [PubMed] [Google Scholar]
- Budnik L. T., Brunswig B., Mukhopadhyay A. K. Atrial natriuretic factor stimulates luteal guanylate cyclase. Regul Pept. 1987 Oct;19(1-2):23–34. doi: 10.1016/0167-0115(87)90071-1. [DOI] [PubMed] [Google Scholar]
- Dascal N., Lotan I., Gillo B., Lester H. A., Lass Y. Acetylcholine and phorbol esters inhibit potassium currents evoked by adenosine and cAMP in Xenopus oocytes. Proc Natl Acad Sci U S A. 1985 Sep;82(17):6001–6005. doi: 10.1073/pnas.82.17.6001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dascal N., Lotan I., Lass Y. Dissociation of acetylcholine- and cyclic GMP-induced currents in Xenopus oocytes. Pflugers Arch. 1987 Aug;409(4-5):521–527. doi: 10.1007/BF00583810. [DOI] [PubMed] [Google Scholar]
- Dascal N., Yekuel R., Oron Y. Acetylcholine promotes progesterone-induced maturation of Xenopus oocytes. J Exp Zool. 1984 Apr;230(1):131–135. doi: 10.1002/jez.1402300117. [DOI] [PubMed] [Google Scholar]
- Davoren J. B., Hsueh A. J. Vasoactive intestinal peptide: a novel stimulator of steroidogenesis by cultured rat granulosa cells. Biol Reprod. 1985 Aug;33(1):37–52. doi: 10.1095/biolreprod33.1.37. [DOI] [PubMed] [Google Scholar]
- Dumont J. N. Oogenesis in Xenopus laevis (Daudin). I. Stages of oocyte development in laboratory maintained animals. J Morphol. 1972 Feb;136(2):153–179. doi: 10.1002/jmor.1051360203. [DOI] [PubMed] [Google Scholar]
- Eppig J. J., Downs S. M. Chemical signals that regulate mammalian oocyte maturation. Biol Reprod. 1984 Feb;30(1):1–11. doi: 10.1095/biolreprod30.1.1. [DOI] [PubMed] [Google Scholar]
- Espey L. L., Norris C., Saphire D. Effect of time and dose of indomethacin on follicular prostaglandins and ovulation in the rabbit. Endocrinology. 1986 Aug;119(2):746–754. doi: 10.1210/endo-119-2-746. [DOI] [PubMed] [Google Scholar]
- Gilula N. B., Epstein M. L., Beers W. H. Cell-to-cell communication and ovulation. A study of the cumulus-oocyte complex. J Cell Biol. 1978 Jul;78(1):58–75. doi: 10.1083/jcb.78.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goff A. K., Armstrong D. T. Changes in responsiveness of rat granulosa cells to prostaglandin E2 and follicle-stimulating hormone during culture. Can J Physiol Pharmacol. 1983 Jun;61(6):608–613. doi: 10.1139/y83-093. [DOI] [PubMed] [Google Scholar]
- Hsueh A. J., Jones P. B., Adashi E. Y., Wang C., Zhuang L. Z., Welsh T. H., Jr Intraovarian mechanisms in the hormonal control of granulosa cell differentiation in rats. J Reprod Fertil. 1983 Sep;69(1):325–342. doi: 10.1530/jrf.0.0690325. [DOI] [PubMed] [Google Scholar]
- Jard S. Vasopressin: mechanisms of receptor activation. Prog Brain Res. 1983;60:383–394. doi: 10.1016/S0079-6123(08)64405-2. [DOI] [PubMed] [Google Scholar]
- Kasson B. G., Meidan R., Davoren J. B., Hsueh A. J. Identification of subpopulations of rat granulosa cells: sedimentation properties and hormonal responsiveness. Endocrinology. 1985 Sep;117(3):1027–1034. doi: 10.1210/endo-117-3-1027. [DOI] [PubMed] [Google Scholar]
- Kliachko S., Zor U. Increase in catecholamine-stimulated cyclic AMP and progesterone synthesis in rat granulosa cells during culture. Mol Cell Endocrinol. 1981 Jul;23(1):23–32. doi: 10.1016/0303-7207(81)90114-3. [DOI] [PubMed] [Google Scholar]
- Kolena J., Channing C. P. Stimulatory effects of LH, FSH and prostaglandins upon cyclic 3',5'-AMP levels in porcine granulosa cells. Endocrinology. 1972 Jun;90(6):1543–1550. doi: 10.1210/endo-90-6-1543. [DOI] [PubMed] [Google Scholar]
- Kusano K., Miledi R., Stinnakre J. Acetylcholine receptors in the oocyte membrane. Nature. 1977 Dec 22;270(5639):739–741. doi: 10.1038/270739a0. [DOI] [PubMed] [Google Scholar]
- Kusano K., Miledi R., Stinnakre J. Cholinergic and catecholaminergic receptors in the Xenopus oocyte membrane. J Physiol. 1982 Jul;328:143–170. doi: 10.1113/jphysiol.1982.sp014257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard J. P., Nargeot J., Snutch T. P., Davidson N., Lester H. A. Ca channels induced in Xenopus oocytes by rat brain mRNA. J Neurosci. 1987 Mar;7(3):875–881. doi: 10.1523/JNEUROSCI.07-03-00875.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotan I., Dascal N., Cohen S., Lass Y. Adenosine-induced slow ionic currents in the Xenopus oocyte. Nature. 1982 Aug 5;298(5874):572–574. doi: 10.1038/298572a0. [DOI] [PubMed] [Google Scholar]
- Lotan I., Dascal N., Oron Y., Cohen S., Lass Y. Adenosine-induced K+ current in Xenopus oocyte and the role of adenosine 3',5'-monophosphate. Mol Pharmacol. 1985 Aug;28(2):170–177. [PubMed] [Google Scholar]
- Miledi R. A calcium-dependent transient outward current in Xenopus laevis oocytes. Proc R Soc Lond B Biol Sci. 1982 Jul 22;215(1201):491–497. doi: 10.1098/rspb.1982.0056. [DOI] [PubMed] [Google Scholar]
- Miledi R., Parker I. Chloride current induced by injection of calcium into Xenopus oocytes. J Physiol. 1984 Dec;357:173–183. doi: 10.1113/jphysiol.1984.sp015495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R., Parker I., Woodward R. M. Membrane currents elicited by divalent cations in Xenopus oocytes. J Physiol. 1989 Oct;417:173–195. doi: 10.1113/jphysiol.1989.sp017796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R., Woodward R. M. Effects of defolliculation on membrane current responses of Xenopus oocytes. J Physiol. 1989 Sep;416:601–621. doi: 10.1113/jphysiol.1989.sp017780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulner O., Ozon R. The roles of follicular envelopes in the initiation of Xenopus oocyte maturation. Gen Comp Endocrinol. 1981 Jul;44(3):335–343. doi: 10.1016/0016-6480(81)90010-1. [DOI] [PubMed] [Google Scholar]
- Neyton J., Trautmann A. Acetylcholine modulation of the conductance of intercellular junctions between rat lacrimal cells. J Physiol. 1986 Aug;377:283–295. doi: 10.1113/jphysiol.1986.sp016187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oron Y., Dascal N., Nadler E., Lupu M. Inositol 1,4,5-trisphosphate mimics muscarinic response in Xenopus oocytes. Nature. 1985 Jan 10;313(5998):141–143. doi: 10.1038/313141a0. [DOI] [PubMed] [Google Scholar]
- Pandey K. N., Osteen K. G., Inagami T. Specific receptor-mediated stimulation of progesterone secretion and cGMP accumulation by rat atrial natriuretic factor in cultured human granulosa-lutein (G-L) cells. Endocrinology. 1987 Sep;121(3):1195–1197. doi: 10.1210/endo-121-3-1195. [DOI] [PubMed] [Google Scholar]
- Parker I., Miledi R. Changes in intracellular calcium and in membrane currents evoked by injection of inositol trisphosphate into Xenopus oocytes. Proc R Soc Lond B Biol Sci. 1986 Aug 22;228(1252):307–315. doi: 10.1098/rspb.1986.0057. [DOI] [PubMed] [Google Scholar]
- Pfaffinger P. J., Leibowitz M. D., Subers E. M., Nathanson N. M., Almers W., Hille B. Agonists that suppress M-current elicit phosphoinositide turnover and Ca2+ transients, but these events do not explain M-current suppression. Neuron. 1988 Aug;1(6):477–484. doi: 10.1016/0896-6273(88)90178-x. [DOI] [PubMed] [Google Scholar]
- Robertson R. P. Characterization and regulation of prostaglandin and leukotriene receptors: an overview. Prostaglandins. 1986 Mar;31(3):395–411. doi: 10.1016/0090-6980(86)90105-x. [DOI] [PubMed] [Google Scholar]
- Smith A. A., Brooker T., Brooker G. Expression of rat mRNA coding for hormone-stimulated adenylate cyclase in Xenopus oocytes. FASEB J. 1987 Nov;1(5):380–387. doi: 10.1096/fasebj.1.5.2824269. [DOI] [PubMed] [Google Scholar]
- Stinnakre J., Van Renterghem C. Cyclic adenosine monophosphate, calcium, acetylcholine and the current induced by adenosine in the Xenopus oocyte. J Physiol. 1986 May;374:551–569. doi: 10.1113/jphysiol.1986.sp016097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Renterghem C., Renit-Soria J., Stinnakre J. beta-Adrenergic induced K+ current in Xenopus oocytes: role of cAMP, inhibition by muscarinic agents. Proc R Soc Lond B Biol Sci. 1985 Jan 22;223(1232):389–402. doi: 10.1098/rspb.1985.0008. [DOI] [PubMed] [Google Scholar]
- Woodward R. M., Miledi R. Hormonal activation of ionic currents in follicle-enclosed Xenopus oocytes. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4135–4139. doi: 10.1073/pnas.84.12.4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward R. M., Miledi R. Membrane currents elicited by porcine vasoactive intestinal peptide (VIP) in follicle-enclosed Xenopus oocytes. Proc R Soc Lond B Biol Sci. 1987 Sep 22;231(1265):489–497. doi: 10.1098/rspb.1987.0057. [DOI] [PubMed] [Google Scholar]
- van den Hoef M. H., Dictus W. J., Hage W. J., Bluemink J. G. The ultrastructural organization of gap junctions between follicle cells and the oocyte in Xenopus laevis. Eur J Cell Biol. 1984 Mar;33(2):242–247. [PubMed] [Google Scholar]


