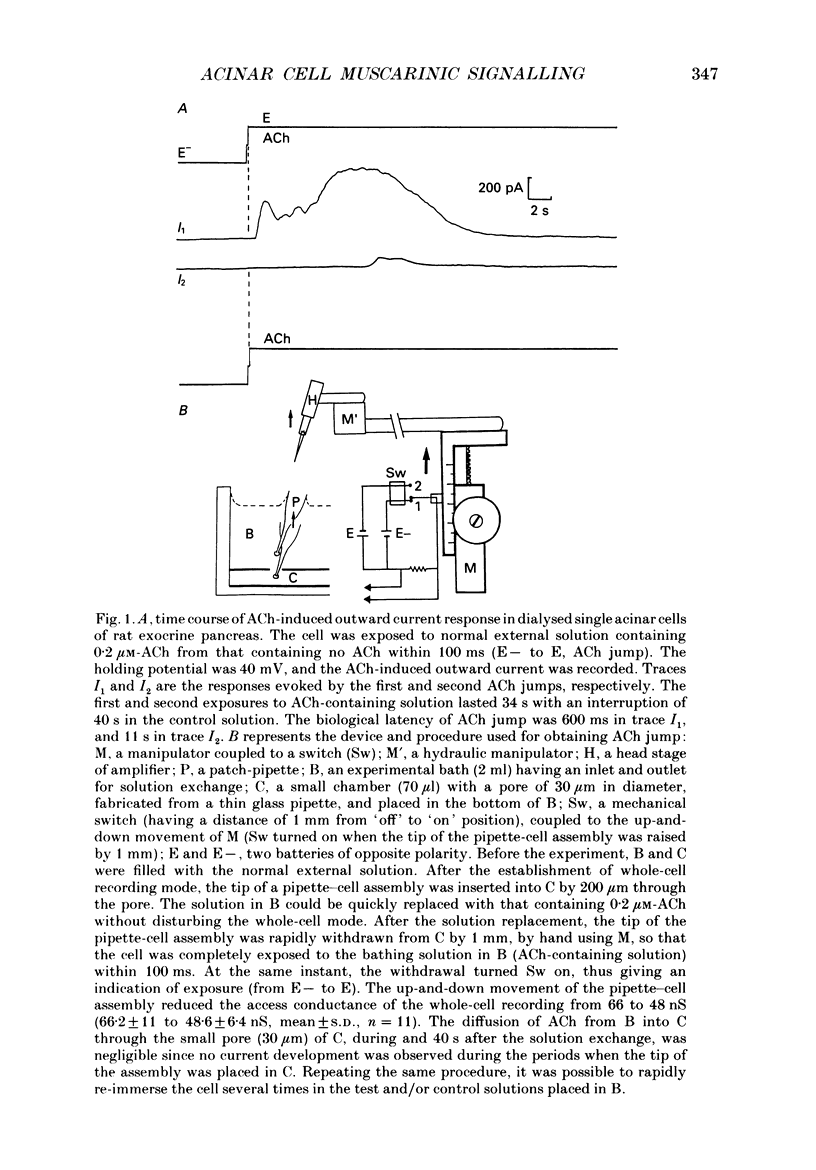
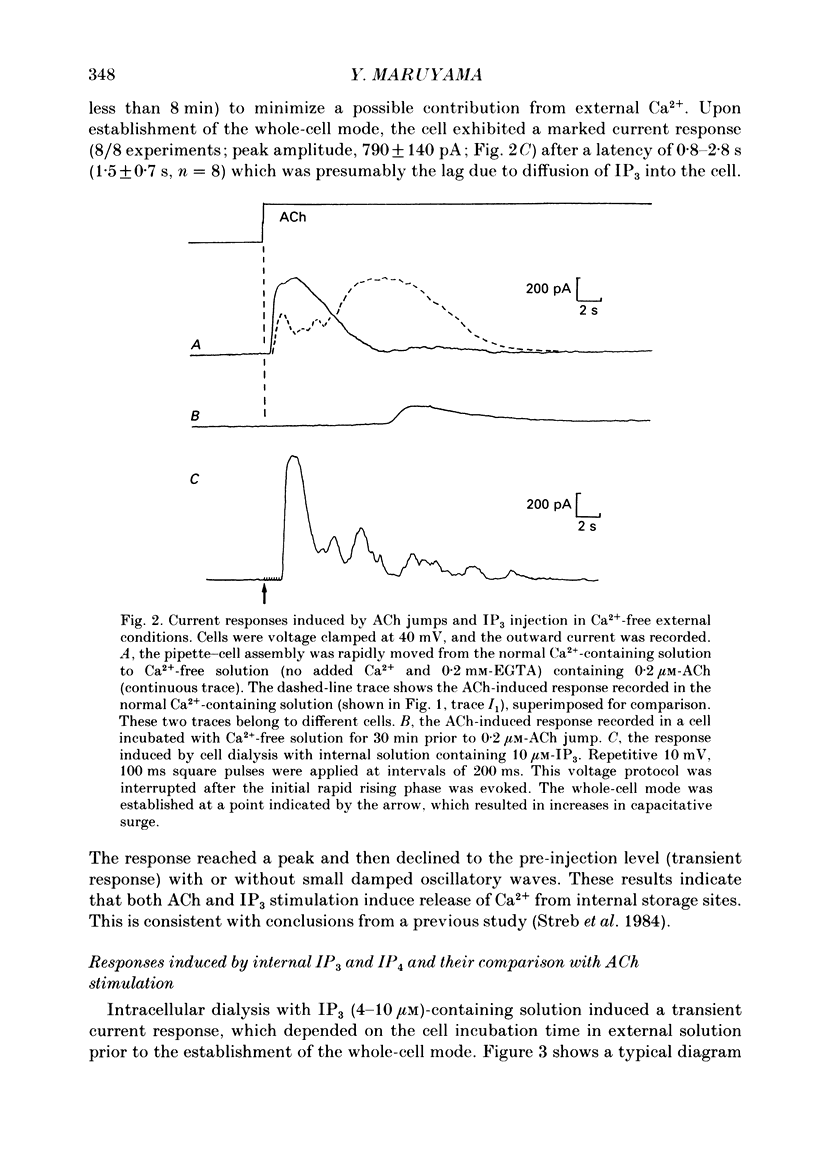
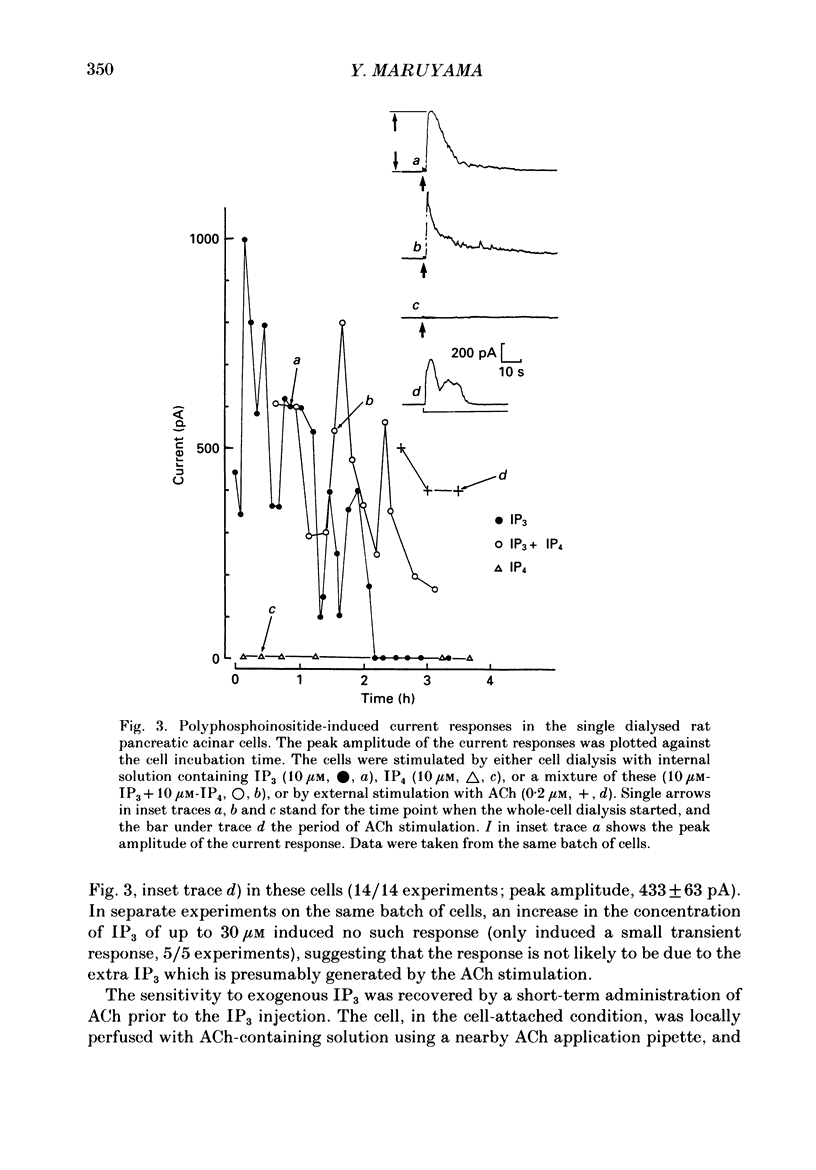
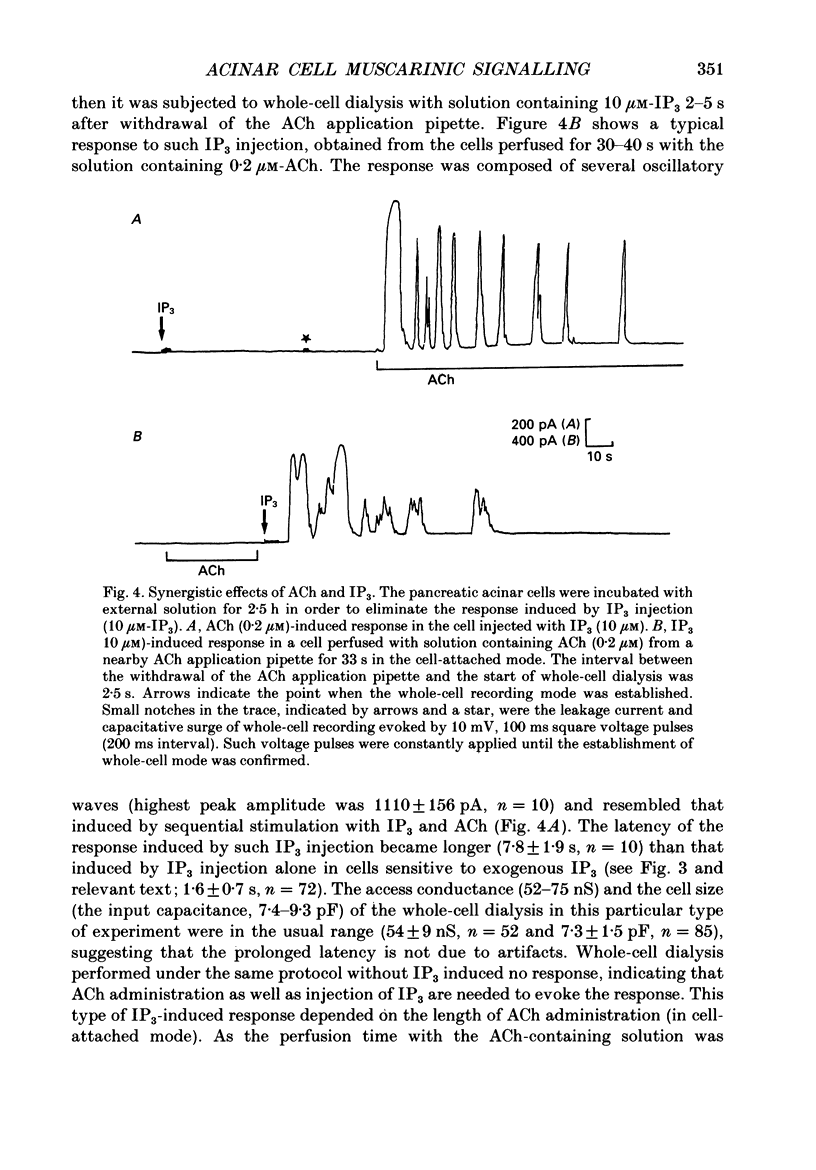
Abstract
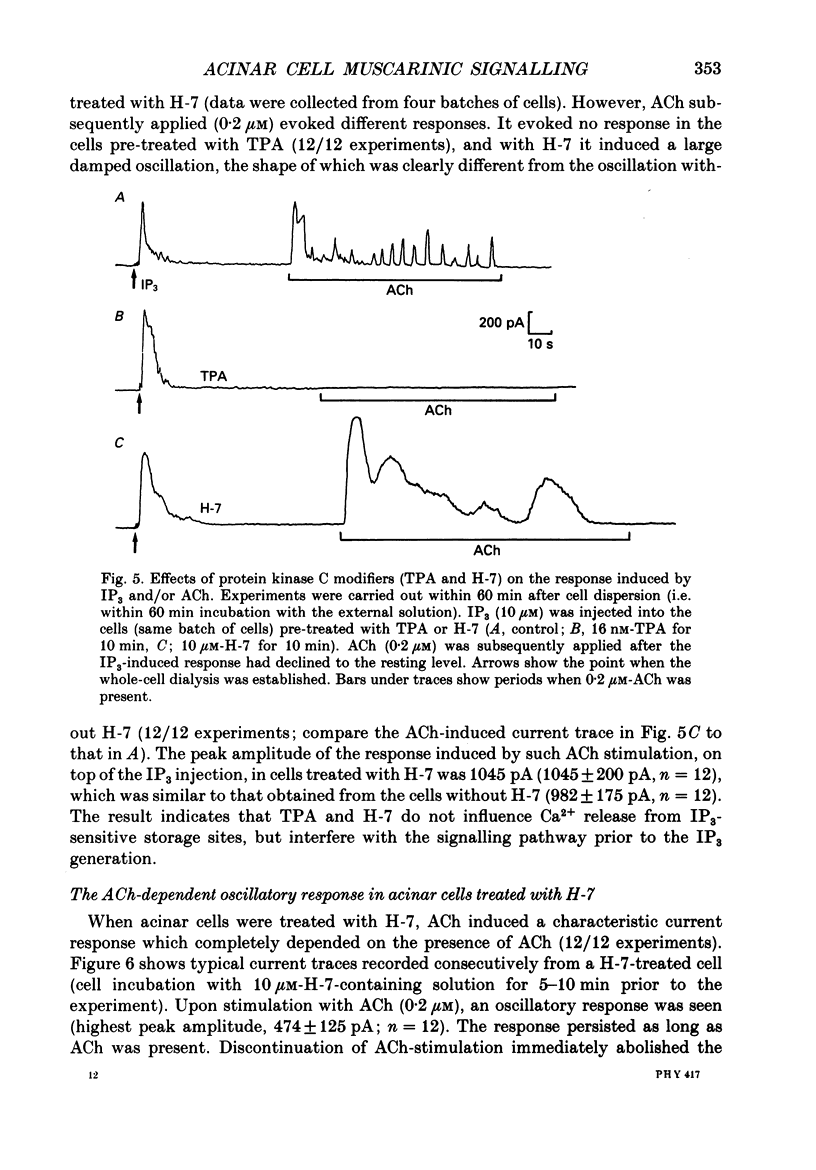
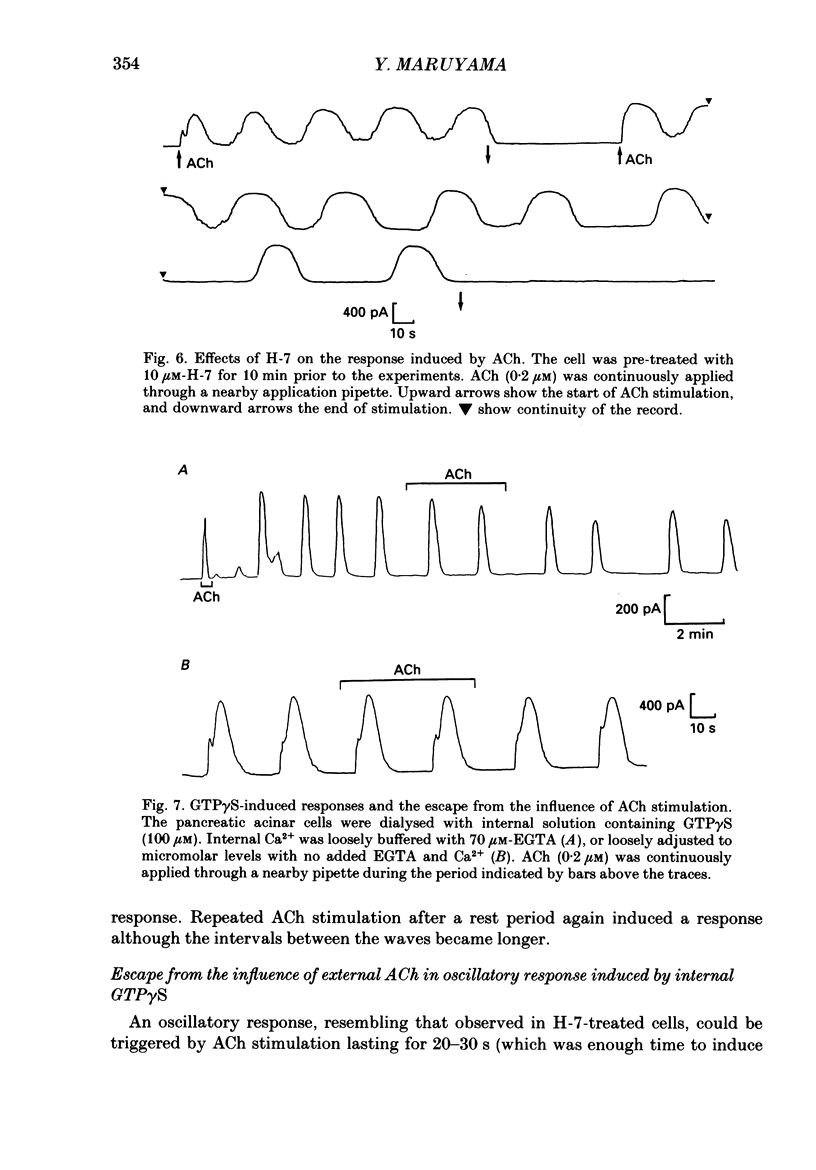
1. In single, enzymatically dissociated, rat pancreatic acinar cells both ACh stimulation and IP3 (inositol 1,4,5-trisphosphate) injection can evoke Ca(+)-dependent transient current responses. However, exogenously applied IP3 (10 microM) gradually loses its ability to induce the Ca2(+)-dependent response (an increase in [Ca2+]i) during cell incubation with a saline solution. 2. Administration of IP4 (inositol 1,3,4,5-tetrakisphosphate, 10 microM) together with the IP3 (the injection of IP3-IP4 mixture) allows partial recovery of the response, but not full replication of the response induced by ACh (0.2 microM). Injection of IP4 alone never induces the current response. 3. The sensitivity of IP3 recovers after short-term administration of ACh (0.2 microM), and in turn, the ACh-induced response is augmented by the presence of internal IP3. These results suggest that a synergism between IP3 and another ACh-induced substance plays an important role in muscarinic Ca2+ signalling. 4. ACh-induced responses are inhibited by pre-incubation (10 min) with an activator of protein kinase C, TPA (12-O-tetradecanoylphorbol-13-acetate, 16 nM), or augmented by pre-incubation (10 min) with an inhibitor, H-7 (1-(5-isoquinoline-sulphonyl)-2-methylpiperazine, 10 microM), whilst IP3-induced responses are unaffected by that with both agents. These results indicate that protein kinase C acts negatively on the signalling elements prior to the formation of IP3. 5. The oscillatory responses, induced by cell dialysis with a nominally Ca2(+)-free (ca 1-10 microM) solution containing GTP gamma S (100 microM), are unaffected by the pre-treatment with TPA or H-7. In addition, these responses and/or those triggered by short-term stimulation with ACh and internal GTP gamma S are not influenced by external ACh. On the other hand, the oscillatory responses recorded in acinar cells pre-treated with H-7 are tightly controlled by external ACh. 6. Taken together these results suggest that activation of protein kinase C does not affect the activity of GTP-binding protein, but disconnects the link between the muscarinic ACh receptor and GTP-binding protein, or inhibits ACh binding to the receptor, in rat pancreatic acinar cells.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdelmoumene S., Gardner J. D. Cholecystokinin-induced desensitization of enzyme secretion in dispersed acini from guinea pig pancreas. Am J Physiol. 1980 Oct;239(4):G272–G279. doi: 10.1152/ajpgi.1980.239.4.G272. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Cuthbertson K. S., Cobbold P. H. Phorbol ester and sperm activate mouse oocytes by inducing sustained oscillations in cell Ca2+. Nature. 1985 Aug 8;316(6028):541–542. doi: 10.1038/316541a0. [DOI] [PubMed] [Google Scholar]
- Evans M. G., Marty A. Potentiation of muscarinic and alpha-adrenergic responses by an analogue of guanosine 5'-triphosphate. Proc Natl Acad Sci U S A. 1986 Jun;83(11):4099–4103. doi: 10.1073/pnas.83.11.4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray P. T. Oscillations of free cytosolic calcium evoked by cholinergic and catecholaminergic agonists in rat parotid acinar cells. J Physiol. 1988 Dec;406:35–53. doi: 10.1113/jphysiol.1988.sp017367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashida H., Brown D. A. Membrane current responses to intracellular injections of inositol 1,3,4,5-tetrakisphosphate and inositol 1,3,4-trisphosphate in NG108-15 hybrid cells. FEBS Lett. 1986 Nov 24;208(2):283–286. doi: 10.1016/0014-5793(86)81033-x. [DOI] [PubMed] [Google Scholar]
- Irvine R. F., Moor R. M. Micro-injection of inositol 1,3,4,5-tetrakisphosphate activates sea urchin eggs by a mechanism dependent on external Ca2+. Biochem J. 1986 Dec 15;240(3):917–920. doi: 10.1042/bj2400917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob R., Merritt J. E., Hallam T. J., Rink T. J. Repetitive spikes in cytoplasmic calcium evoked by histamine in human endothelial cells. Nature. 1988 Sep 1;335(6185):40–45. doi: 10.1038/335040a0. [DOI] [PubMed] [Google Scholar]
- Kawamoto S., Hidaka H. 1-(5-Isoquinolinesulfonyl)-2-methylpiperazine (H-7) is a selective inhibitor of protein kinase C in rabbit platelets. Biochem Biophys Res Commun. 1984 Nov 30;125(1):258–264. doi: 10.1016/s0006-291x(84)80362-9. [DOI] [PubMed] [Google Scholar]
- Kimura T., Imamura K., Eckhardt L., Schulz I. Ca2+-, phorbol ester-, and cAMP-stimulated enzyme secretion from permeabilized rat pancreatic acini. Am J Physiol. 1986 May;250(5 Pt 1):G698–G708. doi: 10.1152/ajpgi.1986.250.5.G698. [DOI] [PubMed] [Google Scholar]
- Knight D. E., Koh E. Ca2+ and cyclic nucleotide dependence of amylase release from isolated rat pancreatic acinar cells rendered permeable by intense electric fields. Cell Calcium. 1984 Aug;5(4):401–418. doi: 10.1016/0143-4160(84)90007-1. [DOI] [PubMed] [Google Scholar]
- Llano I., Marty A. Protein kinase C activators inhibit the inositol trisphosphate-mediated muscarinic current responses in rat lacrimal cells. J Physiol. 1987 Dec;394:239–248. doi: 10.1113/jphysiol.1987.sp016868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llano I., Marty A., Tanguy J. Dependence of intracellular effects of GTP gamma S and inositoltrisphosphate on cell membrane potential and on external Ca ions. Pflugers Arch. 1987 Aug;409(4-5):499–506. doi: 10.1007/BF00583807. [DOI] [PubMed] [Google Scholar]
- Maruyama Y. Agonist-induced changes in cell membrane capacitance and conductance in dialysed pancreatic acinar cells of rats. J Physiol. 1988 Dec;406:299–313. doi: 10.1113/jphysiol.1988.sp017381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama Y., Petersen O. H. Cholecystokinin activation of single-channel currents is mediated by internal messenger in pancreatic acinar cells. Nature. 1982 Nov 4;300(5887):61–63. doi: 10.1038/300061a0. [DOI] [PubMed] [Google Scholar]
- Merritt J. E., Taylor C. W., Rubin R. P., Putney J. W., Jr Evidence suggesting that a novel guanine nucleotide regulatory protein couples receptors to phospholipase C in exocrine pancreas. Biochem J. 1986 Jun 1;236(2):337–343. doi: 10.1042/bj2360337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merritt J. E., Taylor C. W., Rubin R. P., Putney J. W., Jr Isomers of inositol trisphosphate in exocrine pancreas. Biochem J. 1986 Sep 15;238(3):825–829. doi: 10.1042/bj2380825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris A. P., Gallacher D. V., Irvine R. F., Petersen O. H. Synergism of inositol trisphosphate and tetrakisphosphate in activating Ca2+-dependent K+ channels. Nature. 1987 Dec 17;330(6149):653–655. doi: 10.1038/330653a0. [DOI] [PubMed] [Google Scholar]
- Nishiyama A., Petersen O. H. Pancreatic acinar cells: ionic dependence of acetylcholine-induced membrane potential and resistance change. J Physiol. 1975 Jan;244(2):431–465. doi: 10.1113/jphysiol.1975.sp010807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizuka Y. The molecular heterogeneity of protein kinase C and its implications for cellular regulation. Nature. 1988 Aug 25;334(6184):661–665. doi: 10.1038/334661a0. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Ohya Y., Terada K., Yamaguchi K., Inoue R., Okabe K., Kitamura K., Hirata M., Kuriyama H. Effects of inositol phosphates on the membrane activity of smooth muscle cells of the rabbit portal vein. Pflugers Arch. 1988 Sep;412(4):382–389. doi: 10.1007/BF01907556. [DOI] [PubMed] [Google Scholar]
- Pandol S. J., Schoeffield M. S. 1,2-Diacylglycerol, protein kinase C, and pancreatic enzyme secretion. J Biol Chem. 1986 Apr 5;261(10):4438–4444. [PubMed] [Google Scholar]
- Putney J. W., Jr Formation and actions of calcium-mobilizing messenger, inositol 1,4,5-trisphosphate. Am J Physiol. 1987 Feb;252(2 Pt 1):G149–G157. doi: 10.1152/ajpgi.1987.252.2.G149. [DOI] [PubMed] [Google Scholar]
- Randriamampita C., Chanson M., Trautmann A. Calcium and secretagogues-induced conductances in rat exocrine pancreas. Pflugers Arch. 1988 Jan;411(1):53–57. doi: 10.1007/BF00581646. [DOI] [PubMed] [Google Scholar]
- Streb H., Heslop J. P., Irvine R. F., Schulz I., Berridge M. J. Relationship between secretagogue-induced Ca2+ release and inositol polyphosphate production in permeabilized pancreatic acinar cells. J Biol Chem. 1985 Jun 25;260(12):7309–7315. [PubMed] [Google Scholar]
- Streb H., Irvine R. F., Berridge M. J., Schulz I. Release of Ca2+ from a nonmitochondrial intracellular store in pancreatic acinar cells by inositol-1,4,5-trisphosphate. Nature. 1983 Nov 3;306(5938):67–69. doi: 10.1038/306067a0. [DOI] [PubMed] [Google Scholar]
- Ueda S., Oiki S., Okada Y. Oscillations of cytoplasmic concentrations of Ca2+ and K+ in fused L cells. J Membr Biol. 1986;91(1):65–72. doi: 10.1007/BF01870215. [DOI] [PubMed] [Google Scholar]
- Woods N. M., Cuthbertson K. S., Cobbold P. H. Repetitive transient rises in cytoplasmic free calcium in hormone-stimulated hepatocytes. Nature. 1986 Feb 13;319(6054):600–602. doi: 10.1038/319600a0. [DOI] [PubMed] [Google Scholar]


