Abstract
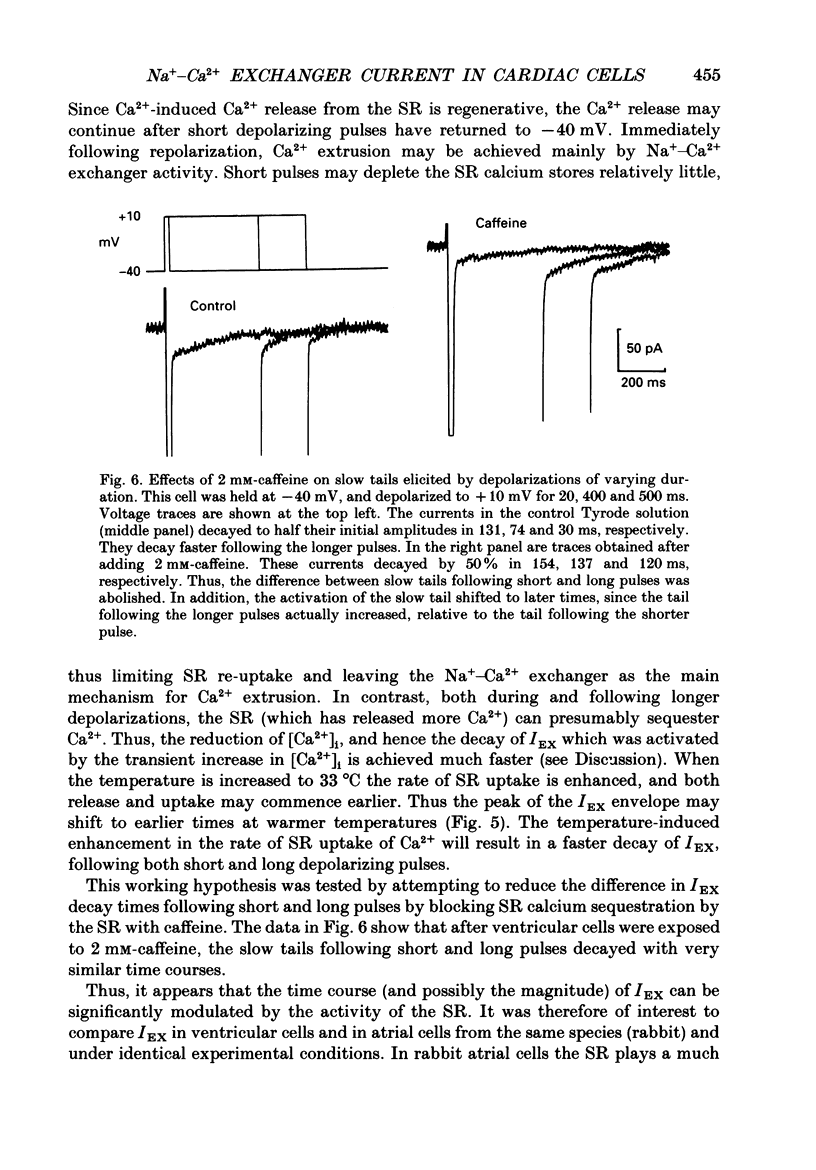
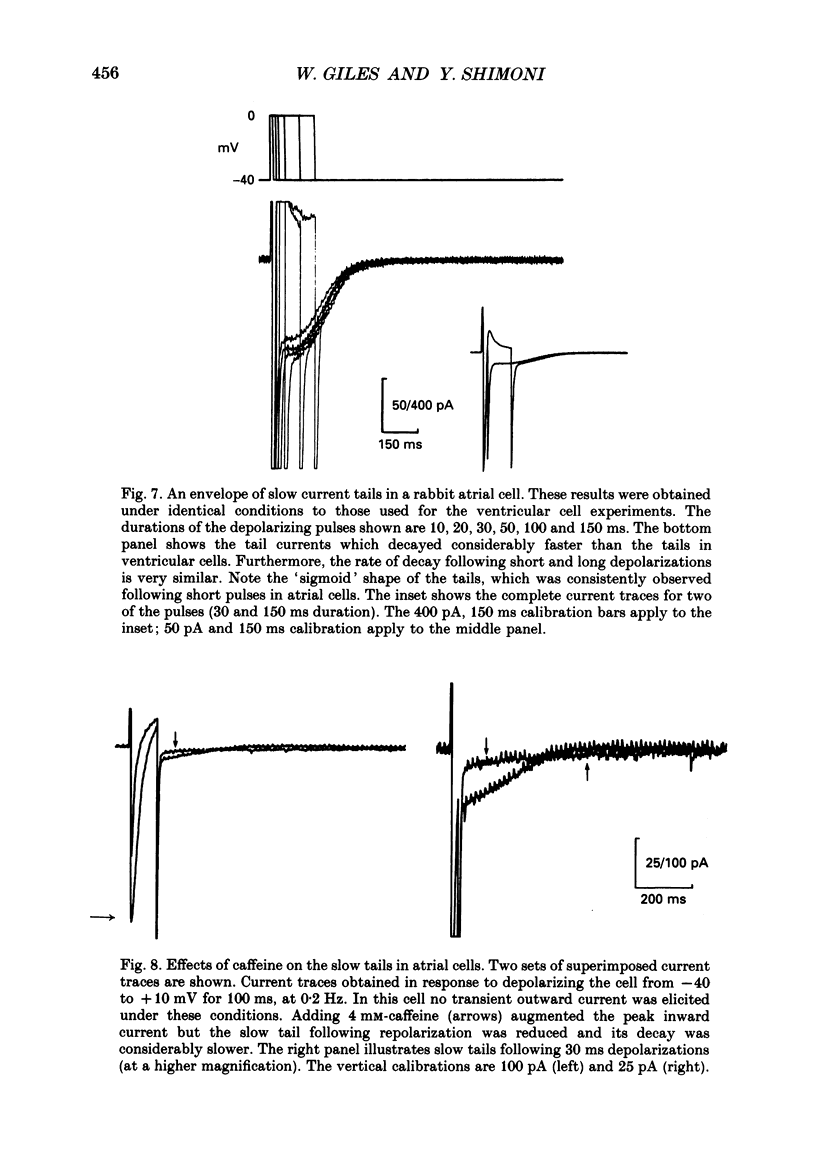
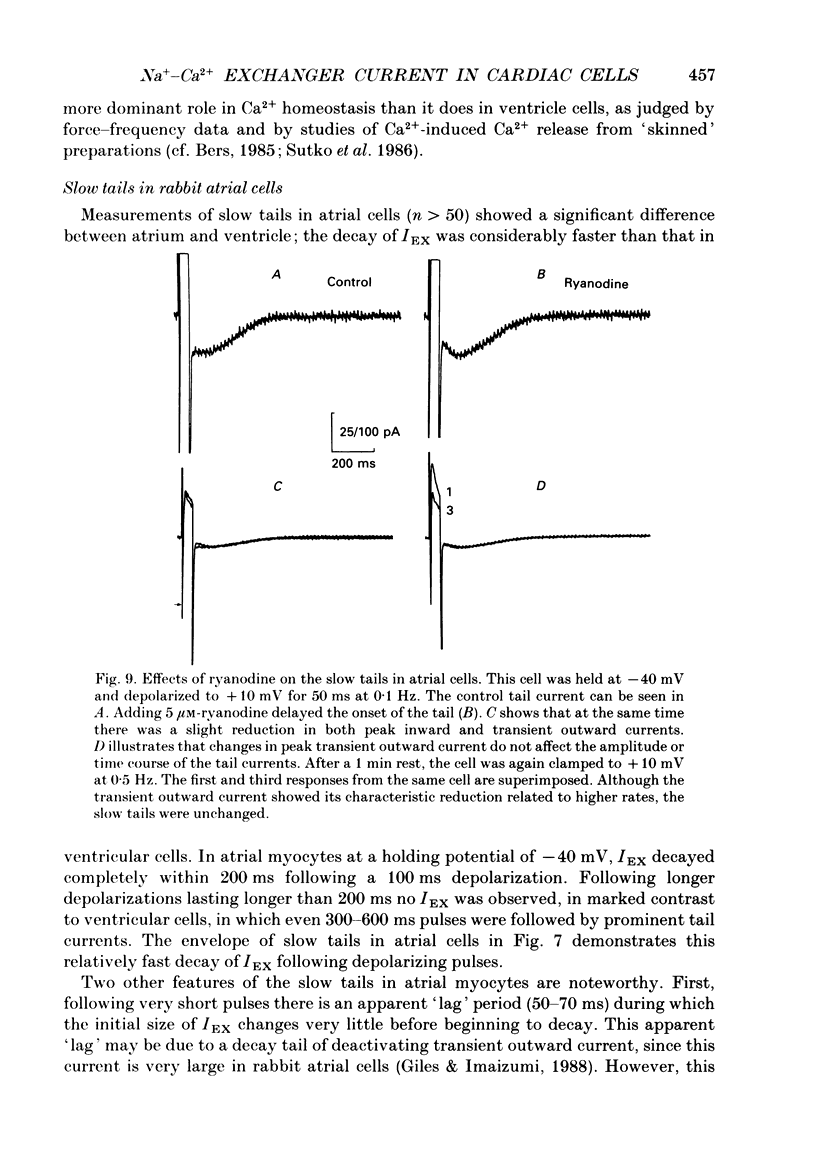
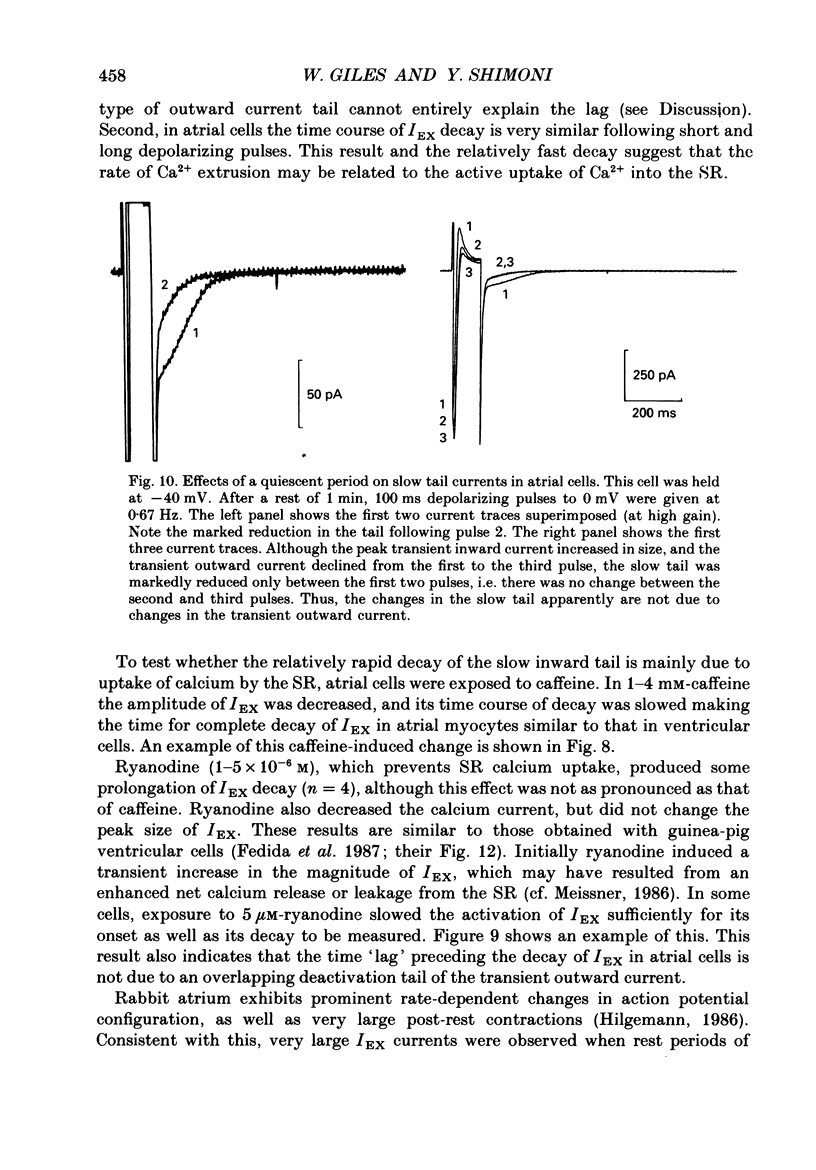
1. A whole-cell gigaseal suction microelectrode voltage-clamp technique has been used to study slow inward tail currents in single myocytes obtained by enzymatic dispersion of rabbit ventricle and atrium. A variety of stimulation protocols, Tyrode solutions and pharmacological agents have been used to test three hypotheses: (a) that the slow inward tail current is generated by an electrogenic Na(+)-Ca2+ exchanger; (b) that a rise in [Ca2+]i, due to release from the sarcoplasmic reticulum can modulate the activity of this exchanger; and (c) that the uptake of calcium by the sarcoplasmic reticulum is a major determinant of the time course of the tail current. 2. As shown previously in amphibian atrium and guinea-pig ventricle, slow inward tail currents can be observed consistently under conditions in which action potentials and ionic currents are recorded using microelectrode constituents which only minimally disturb the intracellular milieu. 3. In ventricular cells, the envelope of these tail currents obtained by varying the duration of the preceding depolarizations shows that (a) the tail currents are activated by pulses as short as 10 ms, and reach a maximum for pulse durations of 100-200 ms, (b) the rate of decay of the tail current gradually increases as the activating depolarizations are prolonged, and (c) the tails cannot be due to deactivation of calcium currents, in agreement with other studies in frog heart. 4. When the mean level of [Ca2+]i is raised following inhibition of the Na(+)-K+ pump by strophanthidin (10(-5) M) or reductions in [K+]o (0.5 mM), the slow inward tail grows in size prior to the onset of a contracture or other signs of calcium-induced toxicity. 5. In a number of different preparations, replacement of [Ca2+]o with BaCl2 markedly or completely inhibits the Na(+)-Ca2+ exchanger, whereas Sr2+ replacement does not have this effect. In myocytes from rabbit ventricle the slow inward tails are reduced significantly and decay more slowly in 0.5-2.2 mM-BaCl2 Tyrode solution, while in 2.2 mM SrCl2 these tails are not altered. 6. The slow inward tail also shows a dependence on [K+]o, corresponding to previous data on Na(+)-Ca2+ exchange in other tissues. Increasing [K+]o in the Tyrode solution to a final concentration of 10-15 mM results in a marked inhibition of the slow tails. This effect cannot be accounted for by changes in the inwardly rectifying potassium current, IK1. 7. The slow tail currents were changed significantly by increasing the temperature of the superfusing Tyrode solution.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
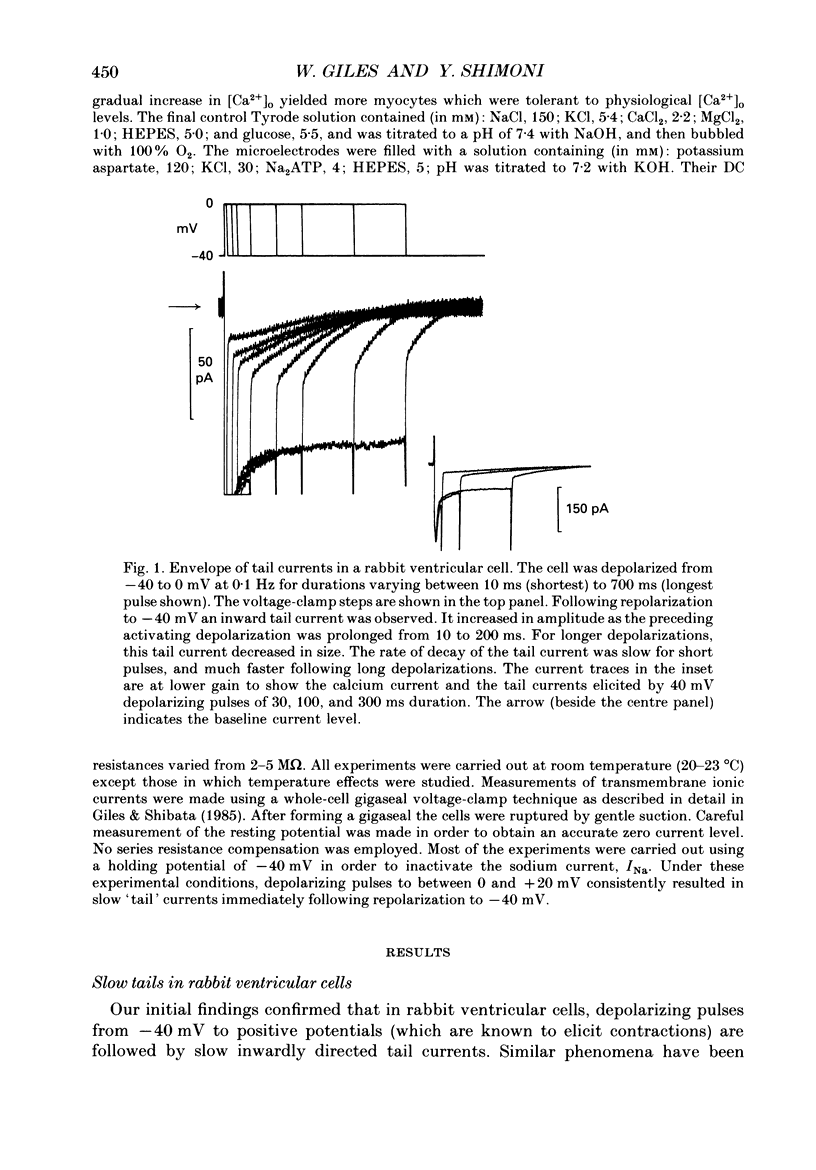
PDF


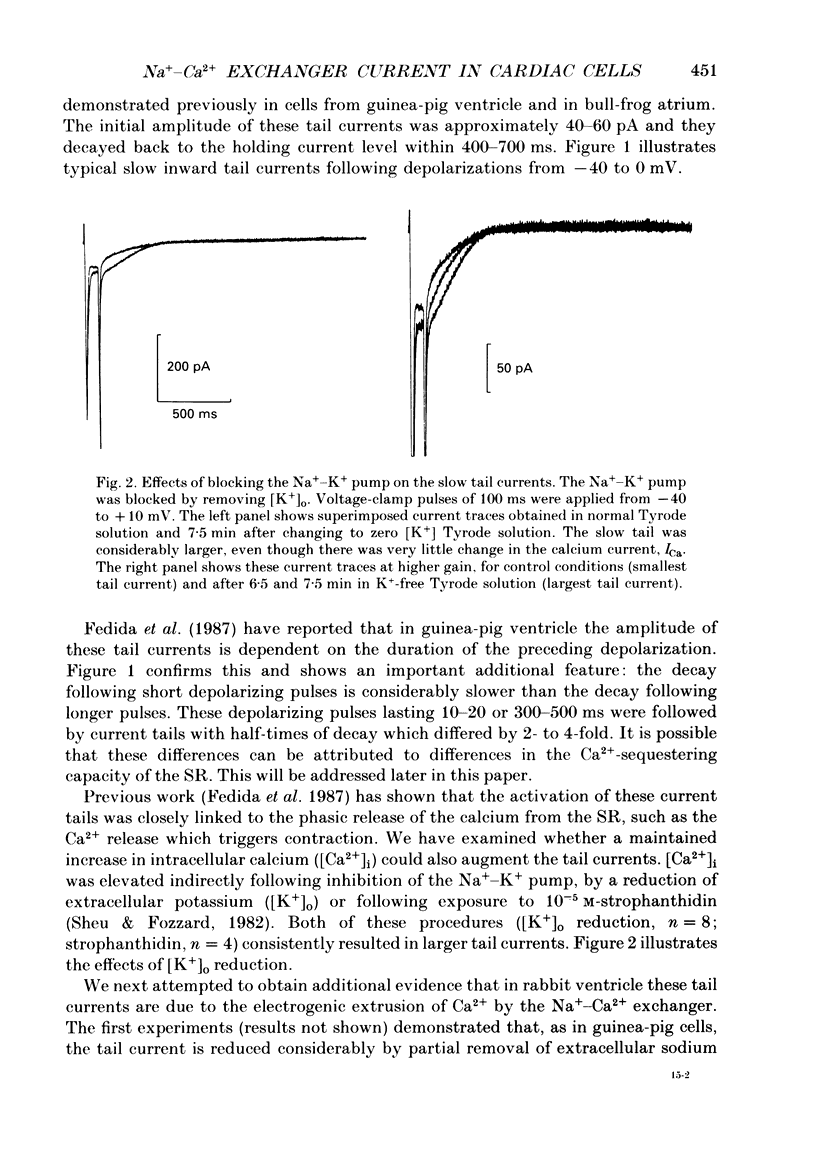






Selected References
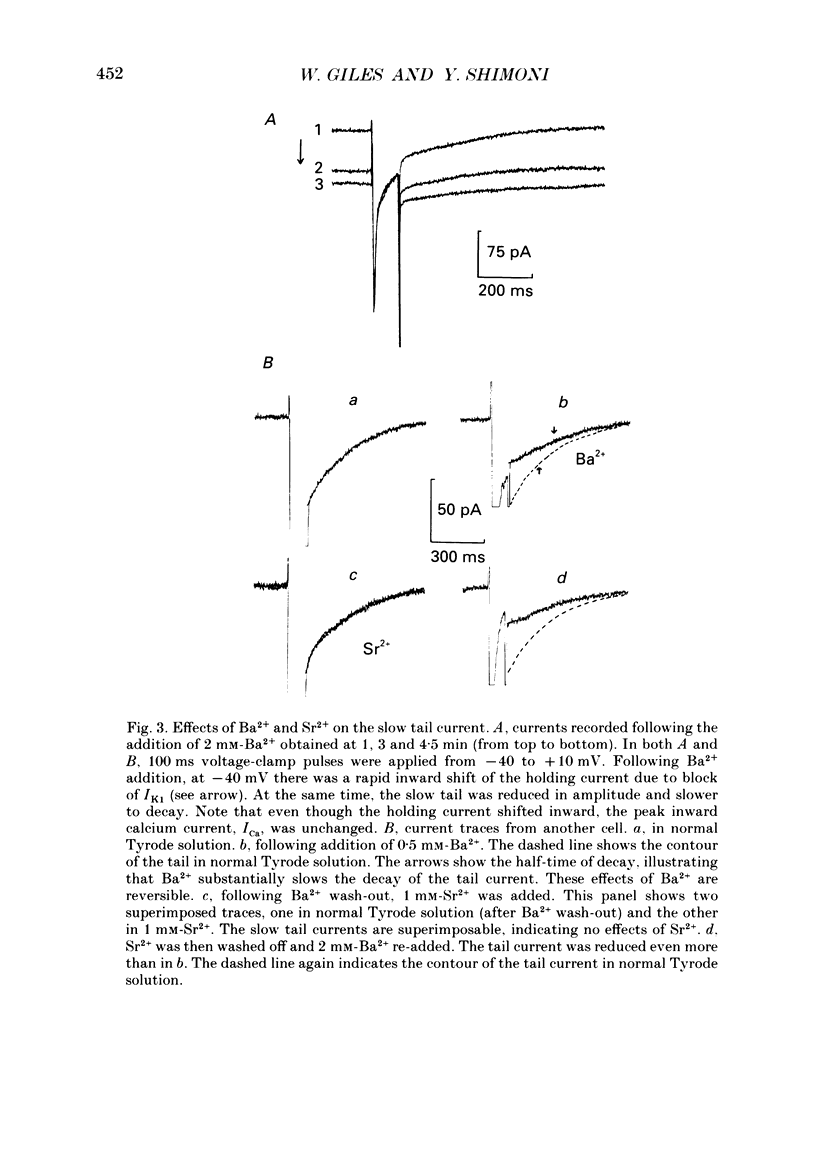
These references are in PubMed. This may not be the complete list of references from this article.
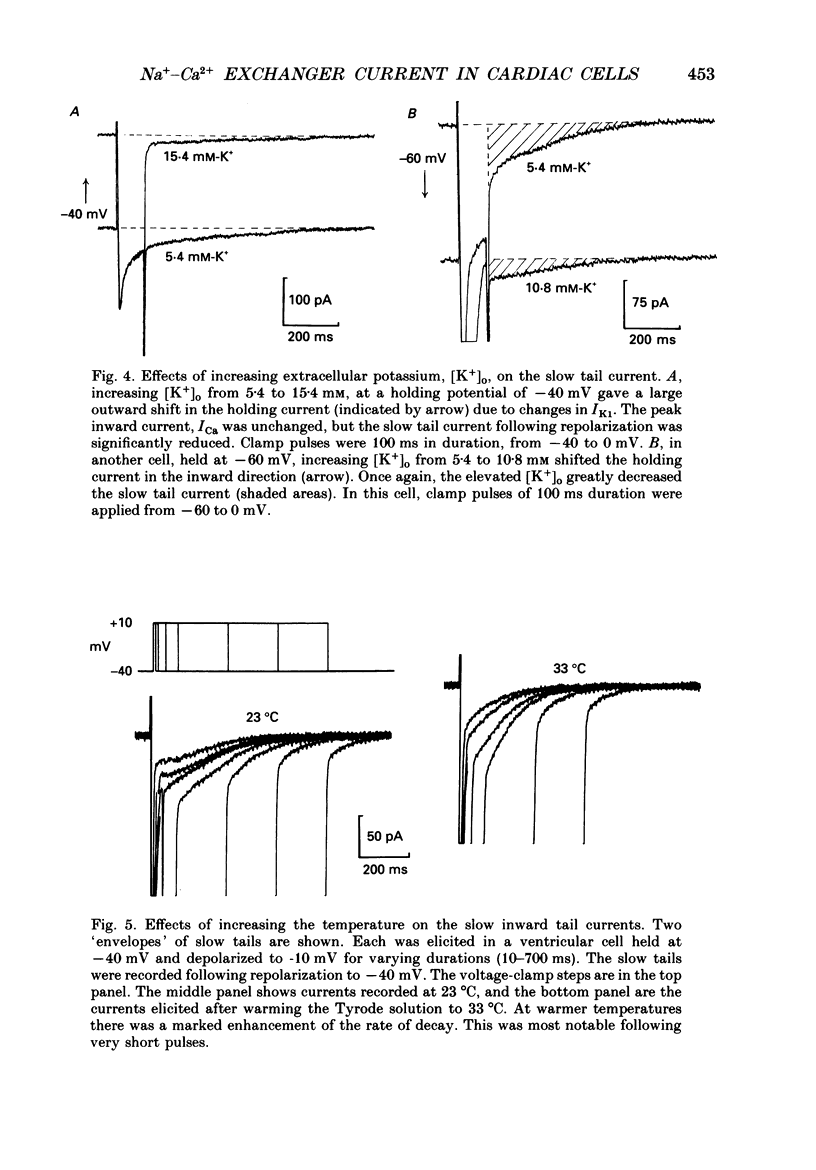
- Baker P. F., McNaughton P. A. Kinetics and energetics of calcium efflux from intact squid giant axons. J Physiol. 1976 Jul;259(1):103–144. doi: 10.1113/jphysiol.1976.sp011457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcenas-Ruiz L., Beuckelmann D. J., Wier W. G. Sodium-calcium exchange in heart: membrane currents and changes in [Ca2+]i. Science. 1987 Dec 18;238(4834):1720–1722. doi: 10.1126/science.3686010. [DOI] [PubMed] [Google Scholar]
- Barcenas-Ruiz L., Wier W. G. Voltage dependence of intracellular [Ca2+]i transients in guinea pig ventricular myocytes. Circ Res. 1987 Jul;61(1):148–154. doi: 10.1161/01.res.61.1.148. [DOI] [PubMed] [Google Scholar]
- Bers D. M. Ca influx and sarcoplasmic reticulum Ca release in cardiac muscle activation during postrest recovery. Am J Physiol. 1985 Mar;248(3 Pt 2):H366–H381. doi: 10.1152/ajpheart.1985.248.3.H366. [DOI] [PubMed] [Google Scholar]
- Bers D. M., Philipson K. D., Langer G. A. Cardiac contractility and sarcolemmal calcium binding in several cardiac muscle preparations. Am J Physiol. 1981 Apr;240(4):H576–H583. doi: 10.1152/ajpheart.1981.240.4.H576. [DOI] [PubMed] [Google Scholar]
- Bielefeld D. R., Hadley R. W., Vassilev P. M., Hume J. R. Membrane electrical properties of vesicular Na-Ca exchange inhibitors in single atrial myocytes. Circ Res. 1986 Oct;59(4):381–389. doi: 10.1161/01.res.59.4.381. [DOI] [PubMed] [Google Scholar]
- Blaustein M. P., Oborn C. J. The influence of sodium on calcium fluxes in pinched-off nerve terminals in vitro. J Physiol. 1975 Jun;247(3):657–686. doi: 10.1113/jphysiol.1975.sp010951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridge J. H. Relationships between the sarcoplasmic reticulum and sarcolemmal calcium transport revealed by rapidly cooling rabbit ventricular muscle. J Gen Physiol. 1986 Oct;88(4):437–473. doi: 10.1085/jgp.88.4.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell D. L., Giles W. R., Robinson K., Shibata E. F. Studies of the sodium-calcium exchanger in bull-frog atrial myocytes. J Physiol. 1988 Sep;403:317–340. doi: 10.1113/jphysiol.1988.sp017251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannell M. B., Berlin J. R., Lederer W. J. Effect of membrane potential changes on the calcium transient in single rat cardiac muscle cells. Science. 1987 Dec 4;238(4832):1419–1423. doi: 10.1126/science.2446391. [DOI] [PubMed] [Google Scholar]
- Capogrossi M. C., Kort A. A., Spurgeon H. A., Lakatta E. G. Single adult rabbit and rat cardiac myocytes retain the Ca2+- and species-dependent systolic and diastolic contractile properties of intact muscle. J Gen Physiol. 1986 Nov;88(5):589–613. doi: 10.1085/jgp.88.5.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFrancesco D., Noble D. A model of cardiac electrical activity incorporating ionic pumps and concentration changes. Philos Trans R Soc Lond B Biol Sci. 1985 Jan 10;307(1133):353–398. doi: 10.1098/rstb.1985.0001. [DOI] [PubMed] [Google Scholar]
- Egan T. M., Noble D., Noble S. J., Powell T., Spindler A. J., Twist V. W. Sodium-calcium exchange during the action potential in guinea-pig ventricular cells. J Physiol. 1989 Apr;411:639–661. doi: 10.1113/jphysiol.1989.sp017596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A. Calcium release in skinned cardiac cells: variations with species, tissues, and development. Fed Proc. 1982 May;41(7):2238–2244. [PubMed] [Google Scholar]
- Fabiato A. Calcium-induced release of calcium from the cardiac sarcoplasmic reticulum. Am J Physiol. 1983 Jul;245(1):C1–14. doi: 10.1152/ajpcell.1983.245.1.C1. [DOI] [PubMed] [Google Scholar]
- Fabiato A. Myoplasmic free calcium concentration reached during the twitch of an intact isolated cardiac cell and during calcium-induced release of calcium from the sarcoplasmic reticulum of a skinned cardiac cell from the adult rat or rabbit ventricle. J Gen Physiol. 1981 Nov;78(5):457–497. doi: 10.1085/jgp.78.5.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedida D., Noble D., Shimoni Y., Spindler A. J. Inward current related to contraction in guinea-pig ventricular myocytes. J Physiol. 1987 Apr;385:565–589. doi: 10.1113/jphysiol.1987.sp016508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giles W. R., Imaizumi Y. Comparison of potassium currents in rabbit atrial and ventricular cells. J Physiol. 1988 Nov;405:123–145. doi: 10.1113/jphysiol.1988.sp017325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giles W. R., Shibata E. F. Voltage clamp of bull-frog cardiac pace-maker cells: a quantitative analysis of potassium currents. J Physiol. 1985 Nov;368:265–292. doi: 10.1113/jphysiol.1985.sp015857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giles W. R., van Ginneken A. C. A transient outward current in isolated cells from the crista terminalis of rabbit heart. J Physiol. 1985 Nov;368:243–264. doi: 10.1113/jphysiol.1985.sp015856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilgemann D. W. Extracellular calcium transients and action potential configuration changes related to post-stimulatory potentiation in rabbit atrium. J Gen Physiol. 1986 May;87(5):675–706. doi: 10.1085/jgp.87.5.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hume J. R., Uehara A. "Creep currents" in single frog atrial cells may be generated by electrogenic Na/Ca exchange. J Gen Physiol. 1986 Jun;87(6):857–884. doi: 10.1085/jgp.87.6.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hume J. R., Uehara A. Properties of "creep currents" in single frog atrial cells. J Gen Physiol. 1986 Jun;87(6):833–855. doi: 10.1085/jgp.87.6.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura J., Miyamae S., Noma A. Identification of sodium-calcium exchange current in single ventricular cells of guinea-pig. J Physiol. 1987 Mar;384:199–222. doi: 10.1113/jphysiol.1987.sp016450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura J., Noma A., Irisawa H. Na-Ca exchange current in mammalian heart cells. Nature. 1986 Feb 13;319(6054):596–597. doi: 10.1038/319596a0. [DOI] [PubMed] [Google Scholar]
- Lewartowski B., Pytkowski B. Cellular mechanism of the relationship between myocardial force and frequency of contractions. Prog Biophys Mol Biol. 1987;50(2):97–120. doi: 10.1016/0079-6107(87)90005-8. [DOI] [PubMed] [Google Scholar]
- Lipp P., Pott L. Transient inward current in guinea-pig atrial myocytes reflects a change of sodium-calcium exchange current. J Physiol. 1988 Mar;397:601–630. doi: 10.1113/jphysiol.1988.sp017021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipp P., Pott L. Voltage dependence of sodium-calcium exchange current in guinea-pig atrial myocytes determined by means of an inhibitor. J Physiol. 1988 Sep;403:355–366. doi: 10.1113/jphysiol.1988.sp017253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London B., Krueger J. W. Contraction in voltage-clamped, internally perfused single heart cells. J Gen Physiol. 1986 Oct;88(4):475–505. doi: 10.1085/jgp.88.4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechmann S., Pott L. Identification of Na-Ca exchange current in single cardiac myocytes. Nature. 1986 Feb 13;319(6054):597–599. doi: 10.1038/319597a0. [DOI] [PubMed] [Google Scholar]
- Meissner G. Ryanodine activation and inhibition of the Ca2+ release channel of sarcoplasmic reticulum. J Biol Chem. 1986 May 15;261(14):6300–6306. [PubMed] [Google Scholar]
- Mentrard D., Vassort G., Fischmeister R. Calcium-mediated inactivation of the calcium conductance in cesium-loaded frog heart cells. J Gen Physiol. 1984 Jan;83(1):105–131. doi: 10.1085/jgp.83.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins L. J. The generation of electric currents in cardiac fibers by Na/Ca exchange. Am J Physiol. 1979 Mar;236(3):C103–C110. doi: 10.1152/ajpcell.1979.236.3.C103. [DOI] [PubMed] [Google Scholar]
- Schnetkamp P. P., Bownds M. D. Na+- and cGMP-induced Ca2+ fluxes in frog rod photoreceptors. J Gen Physiol. 1987 Mar;89(3):481–500. doi: 10.1085/jgp.89.3.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheu S. S., Fozzard H. A. Transmembrane Na+ and Ca2+ electrochemical gradients in cardiac muscle and their relationship to force development. J Gen Physiol. 1982 Sep;80(3):325–351. doi: 10.1085/jgp.80.3.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutko J. L., Bers D. M., Reeves J. P. Postrest inotropy in rabbit ventricle: Na+-Ca2+ exchange determines sarcoplasmic reticulum Ca2+ content. Am J Physiol. 1986 Apr;250(4 Pt 2):H654–H661. doi: 10.1152/ajpheart.1986.250.4.H654. [DOI] [PubMed] [Google Scholar]
- Wier W. G., Cannell M. B., Berlin J. R., Marban E., Lederer W. J. Cellular and subcellular heterogeneity of [Ca2+]i in single heart cells revealed by fura-2. Science. 1987 Jan 16;235(4786):325–328. doi: 10.1126/science.3798114. [DOI] [PubMed] [Google Scholar]
- Yau K. W., Nakatani K. Electrogenic Na-Ca exchange in retinal rod outer segment. Nature. 1984 Oct 18;311(5987):661–663. doi: 10.1038/311661a0. [DOI] [PubMed] [Google Scholar]


