Abstract
Boron (B) is essential for plants but toxic when present in excess. Arabidopsis thaliana BOR1 is a B exporter for xylem loading and is essential for efficient B translocation from roots to shoots under B limitation. B translocation to shoots was enhanced under B limitation in WT but not in bor1-1 mutant plants. The enhanced translocation was suppressed upon resupply of high levels of B within several hours. Unlike a number of transporters for essential mineral nutrients, BOR1 mRNA accumulation was not strongly affected by B conditions. However, accumulation of a constitutively expressed BOR1-GFP fusion protein was elevated under conditions of limited B supply. Upon resupply of high levels of B, BOR1-GFP was degraded within several hours. These findings demonstrate that posttranscriptional mechanisms play a major role in regulation of BOR1 accumulation. Confocal laser scanning microscopy of root tip cells showed that BOR1-GFP is localized to the plasma membrane under B limitation. Shortly after B application, the protein was observed in dot-like structures in the cytoplasm before degradation. Colocalization studies of the fusion protein with an endocytic tracer FM4-64 and an endosomal Rab-GTPase Ara7 fused to monomeric red fluorescent protein suggested that BOR1 is transferred from the plasma membrane via the endosomes to the vacuole for degradation. These results establish that endocytosis and degradation of BOR1 are regulated by B availability, to avoid accumulation of toxic levels of B in shoots under high-B supply, while protecting the shoot from B deficiency under B limitation.
Keywords: endosome, FM4-64, Ara7
Boron (B) is an essential element for higher plants (1), and B deficiency is a widespread agricultural problem (2). The primary effect of B deficiency is reduction of cell enlargement in growing tissues (3), which has been explained mainly by the structural role of B in the cell wall. B was found to be located predominantly in cell walls in association with rhamnogalacturonan II (RG-II), a pectic polysaccharide, and the borate cross-linked RG-II was shown to be essential for plant growth (4). B has to be adequately supplied to growing portions of plants to facilitate formation of borate cross-linked RG-II for normal plant growth. Besides its essentiality, B can be toxic when present in excess. The concentration range between deficiency and toxicity is in particular narrow for B. For example, in wheat, B deficiency may express at tissue concentrations <10 μg/g, whereas B toxicity appears already at concentrations of 100 μg/g or above (1). In agricultural practice, fertilization of B has to be managed carefully to avoid deficiency as well as toxicity problems. Understanding of the mechanisms regulating B uptake and translocation in plants is thus an important subject in terms of physiology and agricultural practice.
B mainly exists as uncharged boric acid [B(OH)3], a very weak Lewis acid with a pKa of 9.24 [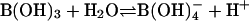 ; ref. 5), in solution of physiological pH and in the absence of interaction with bio-molecules. As an uncharged molecule, its permeability coefficient for transport across the lipid bilayer is several orders of magnitude higher than that of ions. It was long believed that passive diffusion of boric acid across the lipid bilayer was the major and possibly the only mechanism for membrane transport of B. However, recent studies provided evidence for channel- and/or transporter-mediated B transport systems (6, 7).
; ref. 5), in solution of physiological pH and in the absence of interaction with bio-molecules. As an uncharged molecule, its permeability coefficient for transport across the lipid bilayer is several orders of magnitude higher than that of ions. It was long believed that passive diffusion of boric acid across the lipid bilayer was the major and possibly the only mechanism for membrane transport of B. However, recent studies provided evidence for channel- and/or transporter-mediated B transport systems (6, 7).
The first isolation of a B transporter profited from the analysis of the bor1-1 mutant of Arabidopsis thaliana, which showed an enhanced susceptibility to B deficiency (8). bor1-1 mutant plants showed elevated sensitivity to B deficiency, especially in young growing organs in shoots, and the symptoms were associated with low-B contents in above-ground organs (8, 9). Physiological studies showed that WT A. thaliana plants concentrated more B in the xylem sap under B-limiting conditions relative to bor1-1 mutant plants (10, 11). Map-based cloning revealed that the BOR1 gene encodes a membrane protein that belongs to the bicarbonate transporter superfamily (11, 12). BOR1 was expressed in pericycle cells of the root stele and was localized to the plasma membrane (11). When expressed in yeast, BOR1 decreased cellular B concentrations, suggesting that BOR1 acts as a B exporter (11). These results demonstrated that BOR1-mediated B export from pericycle cells to the xylem represents a critical process under B-limiting growth conditions. It is reasonable to expect a tight regulation of BOR1 by B availability.
Regulating the activity of transport proteins is a critical process for organisms to respond to changing nutrient availability. In animal and yeast systems, posttranslational down-regulation of nutrient transporters in response to elevated nutrient availability has been well described. In yeast, endocytosis and degradation of the general amino acid permease Gap1, the zinc transporter Zrt1, the magnesium transporter Alr1, the sugar transporters Mal11, Mal61, Gal2, and Hxt6/7, and the uracil transporter Fur4 are all induced and/or accelerated in the presence of elevated substrate levels or metabolic regulators (13). For example, zinc uptake by the yeast Zrt1 transporter, a member of the ZIP (ZRT/IRT-like proteins) family, is inactivated upon exposure to high levels of zinc. The inactivation occurs through endocytosis of the protein, followed by degradation in the vacuole (14). Protein endocytosis and degradation ensure a rapid offset of zinc uptake in cells exposed to high zinc levels, thus preventing overaccumulation of this essential but potentially toxic metal. Recent evidences implied the similar mechanism of posttranslational down-regulation for plant members of the ZIP family. AtIRT1, the major plasma membrane transporter responsible for iron uptake from the soil, is regulated at both transcriptional and posttranscriptional levels. In transgenic plants constitutively expressing IRT1 mRNA, IRT1 protein accumulated in roots only under iron limitation (15). When the zinc transporter AtZIP1 was constitutively expressed in Hordeum vulgare, short-term zinc uptake rates were higher than in control lines only after zinc deprivation (16).
In this study, we demonstrated regulated endocytosis and degradation of BOR1, a plasma membrane transporter for B in plant. We monitored BOR1 activity and transcript and protein accumulations in response to various B supply and found posttranscriptional regulation as a major regulatory mechanism. Confocal microscopy of GFP-tagged BOR1 indicated that BOR1 endocytosis and degradation are regulated by B availability.
Materials and Methods
Plant Materials. Col-0 and bor1-1 mutant of A. thaliana (L.) Heynh. were from our laboratory stock (8). Cauliflower mosaic virus 35S RNA promoter (P35S):BOR1-GFP fusion was constructed as follows. The 5′-untranslated region (333 bp) and ORF of BOR1 cDNA, RZ119b07 provided by Kazusa DNA Research Institute (Chiba, Japan), were fused to the 5′ end of ORF of sGFP (kindly provided by Y. Niwa; ref. 17). In the construction process, a linker of five amino acids (Gly-Gly-Gly-Gly-Ala) was inserted between the BOR1 and sGFP ORFs. The BOR1-GFP fusion and the terminator of the nopaline synthase gene (Nos T) were subcloned into pBI221 (18) and then the P35S:BOR1-GFP-Nos T was subcloned into pBIN19 (19). A. thaliana ecotype Col-0 was transformed with the construct by the Agrobacterium-mediated floral dip method (20). Independent lines exhibiting 3:1 kanamycin (50 μg·ml-1) resistance segregation in the T2 generation were selected and homozygous T3 lines were established. The plasmid for stable expression of monomeric red fluorescent protein (mRFP)-Ara7 was constructed as follows. The coding sequence for mRFP-Ara7 was PCR-amplified from the plasmid for transient expression of mRFP-Ara7 (provided by T. Ueda; ref. 21). The PCR product was subcloned into pENTR/D-TOPO (Invitrogen) and then into pMDC32, which contains the dual P35S and Nos T (provided by M. Curtis; ref. 22), by using Gateway technology (Invitrogen). The construct was used for transformation of the homozygous P35S:BOR1-GFP transgenic line (line 18). Lines exhibiting hygromycin (20 μg·ml-1) resistance were selected, and T2 lines were used for experiments. Transgenic lines carrying P35S:GFP-PIP2;1 and P35S:GFP-Lti6b (23) were obtained from the Nottingham Arabidopsis Stock Centre (Nottingham, U.K.) and the Arabidopsis Biological Resource Center (Columbus, OH), respectively.
Hydroponic Culture. Except for confocal imaging experiments, plants were grown hydroponically in growth chambers with the following environmental parameters: 10-h/14-h light/dark cycle, 22°C/19°C or 22°C under fluorescent lamps. The medium (24) containing 50 μM Fe-EDTA and 100 μM boric acid (high-B media) or 0.1 μM boric acid (low-B media) was used. Seeds were surface-sterilized and sown on 10 × 10-cm2 nylon meshes (300-μm pore size) placed on high-B media plates (15-cm diameter) containing 2% (wt/vol) sucrose and 0.2% (wt/vol) Gellan Gum (Wako Pure Chemical, Osaka). After 23 days, the lids of the plates were removed to expose plants to ambient air. On the next day, plants were removed carefully from the medium together with the nylon mesh, and the roots were rinsed in deionized water. The plants were transferred onto a 900-ml plastic container filled with liquid high-B medium and grown for 5 or 6 days before the plants were subjected to treatments with either high-B or low-B supply.
Tracer Experiments Using Stable Isotopes of B. Plants were grown hydroponically as described above (12 seeds per mesh) except that the plants were supplied with 11B-enriched boric acid (99%, Cambridge Isotope Laboratories, Andover, MA) instead of normal boric acid (10B:11B = 19.9:80.1). The plants grown and treated with the 11B-enriched boric acid were then transferred to medium with 30 μM 10B-enriched boric acid (99%, Cambridge Isotope Laboratories) and incubated for 60 min. Preparation of samples and B isotope determination by inductively coupled plasma MS (ICP-MS) have been described (11).
Quantitative RT-PCR. Plants were grown hydroponically as described above (16 seeds per mesh). Roots and shoots from a nylon mesh were separated into four samples, frozen immediately in liquid nitrogen, and stored at -80°C. Total RNA was extracted independently from the samples. Reverse transcription and real-time PCR analysis were carried out as described (25). The primers used for PCR were as follows: 5′-AATCTCGCAGCGGAAACG-3′ and 5′-TGGAGTCGAACTTGAACTTGTC-3′ for BOR1, 5′-CCTTGGTGTCAAGCAGATGA-3′ and 5′-TGAAGACACCTCCTTGATGATTT-3′ for Elongation Factor 1α, 5′-CACATGAAGCAGCACGACTT-3′ and 5′-AGTTCACCTTGATGCCGTTC-3′ for GFP, and 5′-GGAGGTGGAGAGTTCTGACA-3′ and 5′-AGACCAAGTGAAGTGTGGAC-3′ for Ubiquitin 10.
Preparation of Microsomal Proteins and Immunoblot Analysis. The transgenic plants were grown hydroponically as described above (25 seeds per mesh), and bulks of roots and shoots were harvested separately from each nylon mesh. All manipulations for preparation of proteins were conducted at 4°C. Tissues were homogenized with homogenization buffer (250 mM Tris, pH 8.5/290 mM sucrose/25 mM EDTA/75 mM 2-mercaptoethanol/1 mM PMSF) and centrifuged at 10,000 × g for 15 min at 4°C. The resultant supernatant was filtered through nylon mesh (58 μm) and centrifuged at 100,000 × g for 30 min at 4°C. The pellet, representing the microsomal fraction, was resuspended in homogenization buffer by pipetting. The protein concentration was estimated by a protein assay (Bio-Rad). Samples for SDS/PAGE were diluted with an equal volume of 2× sample buffer [125 mM Tris, pH 6.8/20% (vol/vol) glycerol/4% (wt/vol) SDS/5% (vol/vol) 2-mercaptoethanol/0.02% (wt/vol) bromophenol blue] and boiled for 3 min. Microsomal proteins (5 μg) were separated on 7.5% SDS polyacrylamide gels and transferred to polyvinylidene fluoride membranes by electroblotting. Blocking, incubation with antibodies, and detection were performed by using an ECL advance Western blotting detection kit according to the manufacturer's protocol (Amersham Pharmacia Biosciences). The anti-GFP antibody (Zymed Laboratories) was used at 2,000-fold dilution, and horseradish peroxidase-conjugated anti-mouse IgG antibody (Sigma-Aldrich) was used as the secondary antibody at 100,000-fold dilution.
Confocal Imaging. The transgenic plants were grown on vertically placed solid medium (24) containing 3 μMB,50 μM Fe-EDTA, 2% (wt/vol) sucrose, and 1.5% (wt/vol) Gellan Gum for 9-11 days. The plants were transferred to medium containing 0.1 μM B (low-B medium) and further grown for 2 days. Then, the roots of the plants were cut and transferred to liquid medium with or without inhibitor and incubated at room temperature. For FM4-64 staining, roots were incubated with 25 μM FM4-64 (Molecular Probes) in low-B medium for 2 min, washed twice with low-B medium, and treated as indicated. Brefeldin A (BFA, Sigma-Aldrich) and concanamycin A (Wako Pure Chemical) were prepared at 50 mM and 100 μM, respectively, in DMSO and used at 25 μM and 0.5 μM, respectively, in the liquid medium. Control treatments with 0.5% DMSO did not apparently affect the results (data not shown). Laser scanning confocal microscopy was performed by using an LSM 510 (Carl Zeiss, Jena, Germany) with the following wavelength for excitation and detection: 488 nm and 505-530 nm for GFP, 488 nm and >650 nm for FM4-64, and 543 nm and >585 nm for mRFP.
Results and Discussion
BOR1-Mediated Xylem Loading of B Is Regulated by B Availability at the Posttranscriptional Level. Based on the observations that BOR1 stimulates xylem loading under B limitation and that the phenotype of bor1-1 can be easily reversed by high levels of B supply (8, 9), it was expected that plants tightly control BOR1 activity with regard to external B availability.
To investigate the effect of B availability on BOR1-mediated xylem loading of B, plants were grown on 100 μM 11B-enriched B for 29 days before transfer to high-B (100 μM 11B-enriched B) or low-B medium (0.1 μM 11B-enriched) for 6 days. Then, the plants were exposed to 30 μM 10B-enriched B for 60 min, and 10B concentrations in shoots were determined (Fig. 1). Because the BOR1 cDNA carrying the bor1-1 mutation does not function for B export in yeast (data not shown), 10B accumulated in bor1-1 represented B being transported independent of the BOR1 transporter. Under high-B supply, 10B accumulation was similar between WT and bor1-1 mutant plants, suggesting little or no contribution of BOR1 to B translocation process under high-B conditions. Under low-B supply, however, a higher accumulation of 10B was observed in shoots of WT plants, confirming the contribution of BOR1 to root-to-shoot translocation of B. Interestingly, BOR1-mediated B translocation decreased within 6 h after resupply of B. These observations showed that BOR1-mediated xylem loading of B is tightly regulated by external B supply, most likely to protect the shoot from B toxicity under conditions of increasing B availability.
Fig. 1.
Translocation of B into shoots in response to B conditions. Col-0 (WT) and bor1-1 mutant plants were grown with media containing 11B-enriched B. The plants were treated with high-B (+B) or low-B (-B) media for 6 days, and a part of the plants with low-B media was resupplied with high-B media for 1, 3, and 6 h (re1h, re3h, re6h). Then, the plants were exposed to media containing 30 μM 10B-enriched B for 60 min, and 10B concentrations in shoots were determined. FW, fresh weight. Averages and standard deviations are shown (n = 3). Asterisks indicate significant difference from the WT plants at +B condition (P < 0.05, Student's t test).
To address the mechanisms of regulation, BOR1 mRNA accumulation was monitored by quantitative RT-PCR analysis. Surprisingly, BOR1 mRNA accumulation was not significantly affected by B limitation for 1, 3, or 6 days and resupply of B for 1, 3, or 6 h, either in roots or in shoots (Fig. 2). Therefore, it was concluded that posttranscriptional regulation mainly controls BOR1-dependent xylem loading (Fig. 1) in response to external B supply.
Fig. 2.
BOR1 mRNA accumulation in response to B conditions quantified by reverse transcription-mediated real-time PCR. (Left) Col-0 plants were grown with high-B media (+B) and transferred to low-B media (-B) at 1, 3, or 6 days before harvested. (Right) The Col-0 plants incubated with low-B media for 6 days (-B) were resupplied with high-B media (Re+B) for 1, 3, and 6 h. Accumulation of the BOR1 mRNA relative to Elongation Factor 1α (EF) mRNA in roots and shoots was determined. Values are normalized with the means for the +B and -B samples in Left and Right, respectively. Averages and standard deviations from three to four batches of plant materials are shown. Relative mRNA levels were not significantly different from control conditions (+B and -Bin Left and Right panels, respectively, P > 0.1, Student's t test).
Posttranslational Regulation of BOR1-GFP Protein in P35S:BOR1-GFP Transgenic Plants. To further characterize BOR1 regulation, we generated transgenic plants expressing a BOR1-GFP translational fusion under the control of P35S, which is known to drive strong constitutive expression in plants with no tissue specificity (26). The BOR1-GFP fusion protein was still functional as verified by B export in yeast (data not shown). We selected two independent transgenic lines accumulating BOR1-GFP mRNA at high levels as determined by quantitative RT-PCR analysis. In both lines, BOR1-GFP mRNA levels were not affected by B limitation in both roots and shoots (Fig. 3). Immunoblot analysis of microsomal proteins with a GFP antibody detected a protein of ≈100 kDa, whereas a corresponding 100-kDa band was not detected in WT plants (Fig. 4A), indicating that the band represents the BOR1-GFP fusion protein. In both independent transgenic lines, BOR1-GFP fusion proteins accumulated to high levels in both roots and shoots under B limitation (-B, Fig. 4A). The BOR1-GFP levels increased within 24 h after transfer from high-B (+B) to low-B medium (-B, Fig. 4B) and decreased within 24 h after retransfer to high-B medium (Re +B, Fig. 4C) in both shoots and roots. These observations demonstrated that BOR1 protein accumulation was regulated in response to external B supply at the translational or posttranslational level.
Fig. 3.
BOR1-GFP mRNA accumulation in P35S:BOR1-GFP transgenic plants quantified by reverse transcription-mediated real-time PCR. The transgenic plants (line 15 and line 18) were supplied with high-B (+B) or low-B media (-B) for 6 days. Accumulation of the BOR1-GFP mRNA relative to ubiquitin10 (UBQ10) mRNA in roots and shoots was determined. Values are normalized with the mean for the root of line 15 plants (+B). Averages and standard deviations from three or four batches of plant materials are shown. Relative mRNA levels were not significantly different between the +B and -B conditions (P > 0.3, Student's t test).
Fig. 4.
BOR1-GFP protein accumulation in response to B conditions. (A) Col-0 (WT) and P35S:BOR1-GFP transgenic plants (lines 15 and 18) were supplied with high-B (+B) or low-B media (-B) for 6 days. (B) P35S:BOR1-GFP transgenic plants (line 18) were grown with +B and transferred to -B at 1, 3, or 6 days before harvest. (C) P35S:BOR1-GFP transgenic plants (line 18) supplied with low-B media for 6 days (-B) were retransferred to high-B media (Re +B) and harvested at times as indicated. Five micrograms of microsomal proteins were used to prepare protein gel blots. An anti-GFP antibody was used for the detection. Numbers at right indicate molecular mass (kDa).
Next, we observed BOR1-dependent green fluorescence in root tip cells of the transgenic plants in response to B supply using confocal microscopy. The irregular shape of the root tip cells (Figs. 5, 6, 7) is a typical B-deficiency symptom (3). Under the B-limiting condition, BOR1-GFP was observed at the cell periphery, indicating plasma membrane localization (Fig. 5A and ref. 11). However, within 30 min after transfer to high-B medium, BOR1-dependent fluorescence was observed in rapidly moving dot-like structures in the cells from which the green fluorescence gradually disappeared over 120 min (Fig. 5A). Inhibition of protein synthesis by addition of cycloheximide before and during the incubation in high-B medium did not interfere with the localization of BOR1-GFP in the dot-like structures (data not shown). When transferred to low-B medium, BOR1-dependent fluorescence remained at the cell periphery during the whole experimental period of 120 min (Fig. 5A). To investigate whether B exerts a specific effect on BOR1 localization or a more general effect on protein trafficking, we followed the effect of B supply on localization of GFP-tagged plasma membrane proteins, which were also expressed under control of the P35S (23). In sharp contrast to BOR1, the GFP-PIP2;1 (Fig. 5B), GFP-Lti6b (Fig. 5C), and GFP-Lti6a (data not shown) fusion proteins remained localized at the cell periphery irrespective of external B supply. We therefore concluded that our B treatments did not affect the localization of plasma membrane proteins in general, but that BOR1 was specifically internalized and subsequently degraded in response to B supply.
Fig. 5.
Subcellular localization of BOR1-GFP in response to B conditions. GFP fluorescence in root tip cells of P35S:BOR1-GFP (line 18, A), P35S:GFP-PIP2;1 (B), and P35S:GFP-Lti6b (C) plants. The plants were grown on low-B media and treated with high-B (+B) or low-B media (-B) as indicated. (Scale bar, 10 μm.)
Fig. 6.
Colocalization of BOR1-GFP and endocytic markers upon high-B supply. (A) GFP (green) and FM4-64 (red) fluorescence in root tip cells of P35S:BOR1-GFP plants (line 18). The plants were grown on low-B media and treated with high-B media for 30 min with equal time of FM4-64 uptake. (B) GFP (green) and mRFP (red) fluorescence in root tip cells of the transgenic plants expressing BOR1-GFP and mRFP-Ara7. The plants were grown on low-B media and treated with high-B media for 30 min. The arrowheads indicate colabeled structures. (Scale bar, 10 μm.)
Fig. 7.
Inhibition of trafficking and degradation of BOR1-GFP. Shown is GFP (green) and FM4-64 (red) fluorescence in root tip cells of P35S:BOR1-GFP plants (line 18). The plants were grown on low-B media and treated as follows: high-B (+B) or low-B (-B) media with 25 μM BFA for 30 min (A), or high-B (+B) or low-B (-B) media with 0.5 μM concanamycin A for 180 min (B). (Scale bar, 10 μm.)
A question remains as to whether the endogenous BOR1 protein in pericycle will be similarly regulated by the B-dependent internalization and degradation as was observed in root tip cells of the P35S:BOR1-GFP transgenic plants. Although we cannot exclude possible artifacts due to strong expression, different nature of cell types, or fusion to GFP, the fact that the patterns of B-dependent internalization and degradation of BOR1 match closely the rapid down-regulation of B transport activity without significant change in mRNA levels in the WT plants (Figs. 1 and 2) suggests that the posttranslational down-regulation also occurs in pericycle cells.
Similar to the case of the mouse zinc transporters ZIP1 and ZIP3, which are regulated not significantly at their mRNA levels (27) but by means of endocytosis in dependence of the zinc status (28), it is most likely that posttranslational down-regulation is a major step in controlling BOR1 activity. Because B is essential and toxic for plant growth, our results support that plants favor posttranslational regulation for the fine tuning and rapid control of B homeostasis by BOR1.
Pathway of Endocytosis and Degradation of BOR1-GFP. In yeast and animal systems, turnover of most plasma membrane proteins is known to be mediated by endocytosis and subsequent degradation in vacuole/lysosome (13, 29, 30). Endocytosis of most plasma membrane proteins starts with internalization mediated by endocytic vesicles. The internalized proteins are transported to early endosomes and are either recycled to the plasma membrane or sorted further down the endocytic pathway for degradation. The proteins destined for the degradative pathway are sorted into internal vesicles of multivesicular bodies (MVBs), for delivery to the lumen of the hydrolytic vacuole/lysosome. In plant cells, endocytic recycling of plasma membrane proteins, pectins, and sterols has been demonstrated by using the fungal metabolite BFA, which inhibits exocytosis but allows the first steps of endocytosis to proceed (29-31). In addition, early studies using electron-dense markers, such as cationized ferritin, and recent studies using lipophilic styryl FM dyes and maker proteins such as Rab GTPase and SNAREs allowed us to visualize the endocytic route from the plasma membrane to the vacuole in plant cells (29, 30). FM4-64 confers red fluorescence upon insertion into the plasma membrane and then labels all other membranes in the endocytic pathway down to the vacuole in yeast (32) as well as in plant cells (33, 34). In A. thaliana cells, FM4-64 stained distinct populations of endosomes in which three members of Rab5-related GTPases were localized (21, 33). In tobacco BY-2 cells, FM4-64 stained MVBs/prevacuolar compartments (PVCs) known to be a part of the Golgi-vacuole transport pathway, indicating that the routes of endocytosis and biosynthetic vacuolar transport merge at the MVBs/PVCs (35). However, the pathway for endocytic degradation of plasma membrane proteins in plant cells is not yet fully understood.
To confirm that BOR1-GFP undergoes endocytosis upon B supply, we performed double-labeling experiments employing the endocytic tracer FM4-64. After incubating the roots in high-B medium for 30 min with equal time for FM4-64 uptake, BOR1-derived green and FM4-64-derived red fluorescence colocalized in the plasma membrane and in the dot-like structures (Fig. 6A), indicating that these dot-like structures belong to the endocytic pathway. To further investigate the localization of BOR1-GFP in the endocytic pathway, we generated transgenic lines coexpressing BOR1-GFP and mRFP-tagged Ara7 under the control of the P35S. Ara7 is a Rab5-related GTPase and has been shown to localize to endosomes in A. thaliana cells (21, 33). After incubating the roots of the transgenic plants in high-B medium for 30 min, BOR1-derived green and Ara7-derived red fluorescence colocalized in the dot-like structures (Fig. 6B), indicating that BOR1-GFP localizes to Ara7-positive endosomes upon B supply.
To characterize the pathway of BOR1 endocytosis and degradation in more detail, we tested the effects of inhibitors on B-dependent BOR1-GFP trafficking. Concentrations of 25-50 μM BFA were shown to cause endosomal aggregation that can be distinguished from endoplasmatic reticulum (ER) and Golgi, as indicated by ultrastructural analysis and double labeling with ER, Golgi, and endosomal markers in A. thaliana root cells (36-38). Upon treatment with 25 μM BFA, BOR1-GFP fluorescence accumulated in BFA-induced patches irrespective of B supply (Fig. 7A). These patches were intensively stained with FM4-64 as well (Fig. 7A), and inhibition of protein synthesis by addition of cycloheximide before and during the BFA treatment did not interfere with the accumulation of BOR1-GFP in the BFA-induced compartments (data not shown). These results suggested a continuous internalization of BOR1 proteins from the plasma membrane into endosomes. It should be noted that BOR1-GFP fluorescence also appeared as rapidly moving dot-like structures in high-B medium but not in low-B medium in the presence of BFA (Fig. 7A). Under high-B supply, a part of the BOR1-GFP was most likely transported from BFA-sensitive early endosomes to MVBs, which were shown not to be affected by BFA (35, 38). Interestingly, in maize root tip cells under B limitation, the abundance of pectins in the cell wall increased, and the accumulation of pectins in BFA-induced compartments was effectively inhibited (39). These findings suggested that pectin recycling is regulated at the level of internalization in response to B supply. By contrast, our studies show that, in response to B supply, BOR1 is most likely to be regulated at the level of protein sorting from early endosomes to either later endosomal compartments for degradation or to the plasma membrane for recycling.
We then examined the effect of concanamycin A, a specific inhibitor of V-ATPases (40, 41), which was previously shown to inhibit GFP degradation in the vacuoles of A. thaliana root (42). GFP fluorescence accumulated in dot-like structures and vacuoles after 180 min of incubation in high-B medium in the presence of concanamycin A (+B, Fig. 7B), although GFP fluorescence was hardly detectable after 120 min of incubation in high-B medium in the absence of concanamycin A (Fig. 5A). Concanamycin A did not significantly affect localization of BOR1-GFP in the cell periphery under low-B supply (-B, Fig. 7B). These results suggest that BOR1-GFP is transported to and degraded in the vacuole upon high-B supply. In yeast cells lacking major vacuolar proteases, the immunolocalization study of the Zrt1 zinc transporter yielded vacuolar staining in response to zinc (14). Similarly, we presumed that concanamycin A raised the pH in vacuolar lumens, thus preventing vacuolar protease activities. Alternatively, concanamycin A, which has been shown to strongly affect the morphology of the Golgi apparatus (43, 44), may inhibit transport of vacuolar proteases necessary for GFP degradation.
Taken together, we conclude that BOR1 is internalized continuously from the plasma membrane into early endosomes for recycling to the plasma membrane under B limitation, although it proceeds to later endosomal compartments and finally is degraded in vacuole upon exposure to high B. B-dependent regulation of BOR1 endocytosis and degradation provides a fast and efficient way to control BOR1 activity necessary under B deficiency but detrimental under high-B supply.
Acknowledgments
We thank K. Mukaikawato for the selection of transgenic lines. We acknowledge Drs. Y. Niwa, T. Ueda, and M. Curtis for providing plasmids. We appreciate Drs. K. Schumacher, T. Ueda, A. Nozawa, and H. Hanaoka for critical reading. This work was supported in part by grants from the Japanese Society for the Promotion of Science (to J.T.) and from the University of Tokyo, for Scientific Research on Priority Areas from the Ministry of Education, Culture, Sports, Science and Technology of Japan, from a 21st Century Center of Excellence project and from Rice Genome Research Project (to T.F.).
Author contributions: J.T., N.v.W., and T.F. designed research; J.T., K.M., L.Y., and T.F. performed research; J.T. analyzed data; and J.T., N.v.W., and T.F. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: BFA, brefeldin A; P35S, cauliflower mosaic virus 35S RNA promoter; mRFP, monomeric red fluorescent protein; MVB, multivesicular body.
References
- 1.Marschner, H. (1995) Mineral Nutrition of Higher Plants (Academic, San Diego), 2nd Ed.
- 2.Shorrocks, V. M. (1997) Plant Soil 193, 121-148. [Google Scholar]
- 3.Dell, B. & Huang, L. (1997) Plant Soil 193, 103-120. [Google Scholar]
- 4.O'Neill, M. A., Ishii, T., Albersheim, P. & Darvill, A. G. (2004) Annu. Rev. Plant Biol. 55, 109-139. [DOI] [PubMed] [Google Scholar]
- 5.Woods, W. G. (1996) J. Trace Elem. Exp. Med. 9, 153-163. [Google Scholar]
- 6.Brown, P. H., Bellaloui, N., Wimmer, M. A., Bassil, E. S., Ruiz, J., Hu, H., Pfeffer, H., Dannel, F. & Römheld, V. (2002) Plant Biol. 4, 205-223. [Google Scholar]
- 7.Dannel, F., Pfeffer, H. & Römheld, V. (2002) Plant Biol. 4, 193-204. [Google Scholar]
- 8.Noguchi, K., Yasumori, M., Imai, T., Naito, S., Matsunaga, T., Oda, H., Hayashi, H., Chino, M. & Fujiwara, T. (1997) Plant Physiol. 115, 901-906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takano, J., Yamagami, M., Noguchi, K., Hayashi, H. & Fujiwara, T. (2001) Soil Sci. Plant Nutr. 47, 345-357. [Google Scholar]
- 10.Noguchi, K., Dannel, F., Pfeffer, H., Römheld, V., Hayashi, H. & Fujiwara, T. (2000) J. Plant Physiol. 156, 751-755. [Google Scholar]
- 11.Takano, J., Noguchi, K., Yasumori, M., Kobayashi, M., Gajdos, Z., Miwa, K., Hayashi, H., Yoneyama, T. & Fujiwara, T. (2002) Nature 420, 337-340. [DOI] [PubMed] [Google Scholar]
- 12.Frommer, W. B. & von Wirén, N. (2002) Nature 420, 282-283. [DOI] [PubMed] [Google Scholar]
- 13.Dupre, S., Urban-Grimal, D. & Haguenauer-Tsapis, R. (2004) Biochim. Biophys. Acta 1965, 89-111. [DOI] [PubMed] [Google Scholar]
- 14.Gitan, R. S., Luo, H., Rodgers, J., Broderius, M. & Eide, D. (1998) J. Biol. Chem. 273, 28617-28624. [DOI] [PubMed] [Google Scholar]
- 15.Connolly, E. L., Fett, J. P. & Guerinot, M. L. (2002) Plant Cell 14, 1347-1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ramesh, S. A., Choimes, S. & Schachtman, D. P. (2004) Plant Mol. Biol. 54, 373-385. [DOI] [PubMed] [Google Scholar]
- 17.Chiu, W. L., Niwa, Y., Zeng, W., Hirano, T., Kobayashi, H. & Sheen, J. (1996) Curr. Biol. 6, 325-330. [DOI] [PubMed] [Google Scholar]
- 18.Jefferson, R. A., Kavanagh, T. A. & Bevan, M. W. (1987) EMBO J. 6, 3901-3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bevan, M. (1984) Nucleic Acids Res. 12, 8711-8721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clough, S. J. & Bend, A. F. (1998) Plant J. 16, 735-743. [DOI] [PubMed] [Google Scholar]
- 21.Ueda, T., Uemura, T., Sato, M. H. & Nakano, A. (2004) Plant J. 40, 783-789. [DOI] [PubMed] [Google Scholar]
- 22.Curtis, M. D. & Grossniklaus, U. (2003) Plant Physiol. 133, 462-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cutler, S. R., Ehrhardt, D. W., Griffitts, J. S. & Somerville, C. R. (2000) Proc. Natl. Acad. Sci. USA 97, 3718-3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fujiwara, T., Hirai, M. Y., Chino, M., Komeda, Y. & Naito, S. (1992) Plant Physiol. 99, 263-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ohkama, N., Takei, K., Sakakibara, H., Hayashi, H., Yoneyama, T. & Fujiwara, T. (2002) Plant Cell Physiol. 43, 1493-1501. [DOI] [PubMed] [Google Scholar]
- 26.Odell, J. T., Nagy, F. & Chua, N. H. (1985) Nature 313, 810-812. [DOI] [PubMed] [Google Scholar]
- 27.Dufner-Beattie, J., Langmade, S. J., Wang, F., Eide, D. & Andrews, G. K. (2003) J. Biol. Chem. 278, 50142-50150. [DOI] [PubMed] [Google Scholar]
- 28.Wang, F., Dufner-Beattie, J., Kim, B. E., Petris, M. J., Andrews, G. & Eide, D. J. (2004) J. Biol. Chem. 279, 24631-24639. [DOI] [PubMed] [Google Scholar]
- 29.Geldner, N. (2004) Planta 219, 547-560. [DOI] [PubMed] [Google Scholar]
- 30.Murphy, A. S., Bandyopadhyay, A., Holstein, S. E. & Peer, W.A. (2005) Annu. Rev. Plant Biol. 56, 221-251. [DOI] [PubMed] [Google Scholar]
- 31.Samaj, J., Baluska, F., Voigt, B., Schlicht, M., Volkmann, D. & Menzel, D. (2004) Plant Physiol. 135, 1150-1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vida, T. A. & Emr, S. D. (1995) J. Cell Biol. 128, 779-792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ueda, T., Yamaguchi, M., Uchimiya, H. & Nakano, A. (2001) EMBO J. 20, 4730-4741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meckel, T., Hurst, A. C., Thiel, G. & Homann, U. (2004) Plant J. 39, 182-193. [DOI] [PubMed] [Google Scholar]
- 35.Tse, Y. C., Mo, B., Hillmer, S., Zhao, M., Lo, S. W., Robinson, D. G. & Jiang, L. (2004) Plant Cell 16, 672-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Geldner, N., Friml, J., Stierhof, Y. D., Jurgens, G. & Palme, K. (2001) Nature 413, 425-428. [DOI] [PubMed] [Google Scholar]
- 37.Geldner, N., Anders, N., Wolters, H., Keicher, J., Kornberger, W., Muller, P., Delbarre, A., Ueda, T., Nakano, A. & Jurgens, G. (2003) Cell 112, 219-230. [DOI] [PubMed] [Google Scholar]
- 38.Grebe, M., Xu, J., Mobius, W., Ueda, T., Nakano, A., Geuze, H. J., Rook, M. B. & Scheres, B. (2003) Curr. Biol. 13, 1378-1387. [DOI] [PubMed] [Google Scholar]
- 39.Yu, Q., Hlavacka, A., Matoh, T., Volkmann, D., Menzel, D., Goldbach, H. E. & Baluska, F. (2002) Plant Physiol. 130, 415-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Drose, S., Bindseil, K. U., Bowman, E. J., Siebers, A., Zeeck, A. & Altendorf, K. (1993) Biochemistry 32, 3902-3906. [DOI] [PubMed] [Google Scholar]
- 41.Matsuoka, K., Higuchi, T., Maeshima, M. & Nakamura, K. (1997) Plant Cell 9, 533-546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tamura, K., Shimada, T., Ono, E., Tanaka, Y., Nagatani, A., Higashi, S., Watanabe, M., Nishimura, M. & Hara-Nishimura, I. (2003) Plant J. 35, 545-555. [DOI] [PubMed] [Google Scholar]
- 43.Dettmer, J., Schubert, D., Calvo-Weimar, O., Stierhof, Y. D., Schmidt, R. & Schumacher, K. (2005) Plant J. 41, 117-124. [DOI] [PubMed] [Google Scholar]
- 44.Robinson, D. G., Albrecht, S. & Moriysu, Y. (2004) Protoplasma 224, 255-260. [DOI] [PubMed] [Google Scholar]









