Abstract
Islet transplantation for type 1 diabetic patients shows promising results with the use of nondiabetogenic immunosuppressive therapy. However, in addition to compromising the immune system of transplant recipients, long-term studies demonstrate that islet viability is impaired. Here, we demonstrate that, in the absence of immunosuppressive agents, monotherapy with clinical-grade human α1-antitrypsin (hAAT), the major serum serine-protease inhibitor, prolongs islet graft survival and normoglycemia in transplanted allogeneic diabetic mice, lasting until the development of anti-hAAT antibodies. Compared to untreated or albumin-control-treated graft recipients, which rejected islets at day 10, AAT-treated mice displayed diminished cellular infiltrates and intact intragraft insulin production throughout treatment. Using peritoneal infiltration models, we demonstrate that AAT decreases allogeneic fibroblast-elicited natural-killer-cell influx by 89%, CD3-positive cell influx by 44%, and thioglycolate-elicited neutrophil emigration by 66%. ATT also extended islet viability in mice after streptozotocin-induced beta cell toxicity. In vitro, several islet responses to IL-1β/IFNγ stimulation were examined. In the presence of AAT, islets displayed enhanced viability and inducible insulin secretion. Islets also released 36% less nitric oxide and 82% less macrophage inflammatory protein 1 α and expressed 63% fewer surface MHC class II molecules. TNFα release from IL-1β/IFNγ-stimulated islet cells was reduced by 99%, accompanied by an 8-fold increase in the accumulation of membrane TNFα on CD45-positive islet cells. In light of the established safety record and the nondiabetogenic potential of AAT, these data suggest that AAT may be beneficial as adjunctive therapy in patients undergoing islet transplantation.
Keywords: interleukin 1, nitric oxide, TNFα
Islet damage and an increased incidence of diabetes are associated with the use of immunosuppressive drugs in organ transplantation and present a major obstacle for clinically applicable human islet transplantation (1). Therefore, the advent of nondiabetogenic steroid-free, immunosuppressive treatment protocols, such as rapamycin-based protocols, have greatly facilitated human islet transplantation (reviewed in ref. 2). However, although clinically effective, rapamycin-based protocols have been associated with an increased risk of hyperlipidemia and hypertension, limiting the applicability of islet transplantation to severe cases of diabetes type 1. Long-term follow-up studies reveal that the viability of engrafted islets is also compromised (3, 4).
α1-Antitrypsin (AAT), the major serum serine-protease inhibitor, inhibits the enzymatic activity of neutrophil elastase, cathepsin G, proteinase 3, thrombin, trypsin, and chymotrypsin (reviewed in ref. 5). AAT may facilitate the survival of islet transplants in engrafted patients, because the inhibitor prevents inflammatory cytokine production, blocks immune cell infiltration and function, inhibits complement activation, and delays the development of diabetes in nonobese diabetic mice (5-12).
In the present study, we demonstrate that monotherapy with clinical grade human AAT (hAAT) prolongs graft survival in a mouse model of islet allograft rejection. To examine the mechanisms responsible for this protection, we measured the effects of hAAT on peritoneal-cell infiltration and addressed the protective effects of hAAT on islets by studying IL-1β/IFNγ-induced islet responses in vitro and streptozotocin (STZ)-induced islet toxicity in vivo.
Materials and Methods
Mice. C57BL/6 and DBA/2 females were purchased from The Jackson Laboratory. Experiments were approved by the University of Colorado Institutional Animal Care and Use Committee.
Islet Isolation, Transplantation, and in Vitro Responses. Five- to 6-week-old C57BL/6 mice were rendered hyperglycemic by STZ (225 mg/kg of body weight i.p., Sigma) and were transplanted 5 days later. Islets were isolated from DBA/2 mice on the day of transplantation, as described in ref. 13. Briefly, mice were anesthetized, and pancreata were inflated with collagenase (1 mg/ml, type XI, Sigma), excised, and incubated for 40 min at 37°C. Digested pancreata were vortexed and filtered through a 500-μm sieve and the pellet washed in HBSS containing 0.5% BSA (Sigma). The pellet was resuspended in RPMI medium 1640 supplemented with 10% FCS, 50 units/ml penicillin, and 50 μg/ml streptomycin (Cellgro, Mediatech, Herndon, VA). Islets were collected on a 100-μm cell strainer (BD, Falcon) and hand picked. For transplantation, 450 islets were washed and mounted on a standard 0.2-ml tip. Recipient mice were anesthetized, an abdominal-wall incision was made over the left kidney, and the islets were released into the renal subcapsular space through a puncture in the capsule, which was immediately sealed with 1-mm3 sterile absorbable gelatin sponge (Surgifoam, Ethicon, Somerville, NJ). Blood glucose levels were determined three times a week from tail blood by using a glucometer (Roche).
For in vitro studies, the islets were incubated at 37°C for 24 h before experiments. An insulin-induction assay was performed as described in ref. 14, by using a mouse-insulin ELISA kit (Mercodia, Metuchen, NJ). Immunohistochemistry was performed as described in ref. 15, by using anti-mouse-insulin antibody (Sigma) and staining reagents (VECTASTAIN ABC, Vector Laboratories). NO in islet supernatants was measured by using Griess reagent (Promega). Islet viability was assessed by using an XTT-based toxicology assay (Sigma).
Detection of Anti-hAAT Antibodies. Serum anti-human-AAT antibody level was determined as described in ref. 16, with the following modifications. Microtiter plates were coated with hAAT (2 μg/ml, Aralast, Baxter, Westlake Village, CA) or human albumin (2 μg/ml). Goat-anti-mouse IgG-peroxide conjugate (R & D Systems) was used as secondary antibody to detect bound anti-hAAT antibodies.
Peritoneal Cellular Infiltrates. Thioglycolate (ThG) (1 ml, 3% wt/vol, Sigma) or allogeneic cells (1 × 107 freshly trypsinized NIH 3T3 cells per peritoneal inoculation) were injected i.p. into mice that were pretreated with 0.1 ml of saline or human albumin, AAT, or oxidized AAT. Lavage was performed at indicated time points. Mice were anesthetized by isoflurane inhalation and injected i.p. with PBS containing 5% FCS and 5 units/ml heparin. Peritoneal fluid was recovered, and red blood cells were lysed (RBC lysing buffer, BD Pharmingen). After counting, cells were centrifuged, resuspended in FACS buffer (PBS containing 2% BSA, 0.1% sodium azide, and 0.1% EDTA, pH 7.4), and incubated with anti-FcγRII/III antibodies (2.4G2, BD Pharmingen). Two separate groups of cells were stained: (i) panleukocyte mAb (anti-CD45-APC, 30-F11, BD Pharmingen) with mAbs for CD3-positive and natural killer (NK) cells (17A2-FITC, BD Pharmingen and DX5-PE, Miltenyi Biotec, Auburn, CA) and (ii) panleukocyte mAb with mAbs for neutrophils and macrophages (Gr1-FITC, BD Pharmingen and F4/80-PE, eBioscience, San Diego). Isotype control Abs were used according to the manufacturer's recommendations. Cells were washed and fixed in 2% EM-grade formaldehyde (Ted Pella). Analysis was performed on a FACSCalibur cytometer using cellquest software (BD Biosciences).
AAT Oxidation by Myeloperoxidase (MPO)-H2O2 System. As described in ref. 17, hAAT (4 mg/ml) was incubated at 37°C for 45 min with MPO (1 unit/ml, Sigma), H2O2 (80 μM, Sigma), and NaCl (2.5 mM) in PBS. The reaction was terminated by boiling for 1 h followed by filter-centrifugation (molecular weight 30,000 cutoff, Micron Bioseparations, Bedford, MA). Inhibition of elastase was evaluated 30 min after coincubation of oxidized AAT with porcine elastase (Sigma), as described in ref. 17.
Cytokine Assays. IFNγ was determined by using specific ELISA (R & D Systems). TNFα and macrophage inflammatory protein 1α (MIP-1α) were detected by electrochemiluminescence assay, as described in ref. 18. The amount of chemiluminescence was determined by using an Origen analyzer (BioVeris, Gaithersburg, MD).
Detection of Membrane TNFα and MHC Class II. Membrane TNFα on islet cells was detected by modification of a method used for the detection of membrane TNFα on human peripheral blood mononuclear cells (19). A single-cell suspension of islets was incubated with anti-mTNFα-PE mAb (MP6-XT22-PE, eBioscience) or anti-MHC class II (M5/114.15.2, BD Pharmingen).
Statistical Analysis. Comparisons between groups were analyzed by two-sided t test or by ANOVA for experiments with more than two subgroups. Results are presented as mean (±SEM).
Results
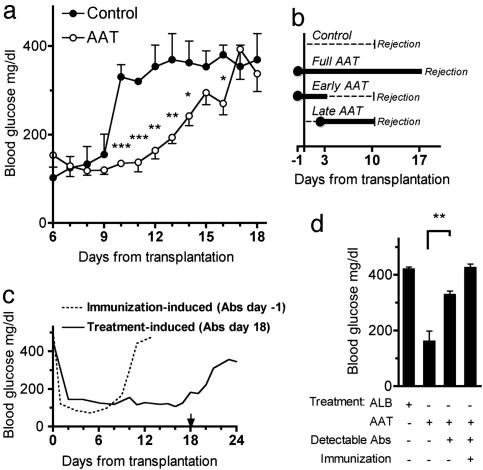
AAT Prolongs Islet Allograft Survival. Islets isolated from DBA/2 mice (H-2d) were transplanted under the left renal capsule of STZ-induced hyperglycemic C57BL/6 mice (H-2b). Blood glucose was followed throughout the study (Fig. 1a). Untreated recipient mice exhibited a rise in glucose levels after a short period of normoglycemia, reflecting the occurrence of acute graft failure. hAAT or albumin control was injected i.p. 1 day before transplantation and every 3 days thereafter. Recipient mice that received the full AAT treatment protocol exhibited prolonged graft function (Fig. 1 a and b). As depicted in Fig. 1b, partial treatment protocols, i.e., hAAT on days -1, 1, and 3 (early AAT) or on day 2 and thereafter (late AAT), did not prolong allograft survival.
Fig. 1.
Prolonged survival of islet allografts by treatment with AAT. Islets from DBA/2 mice (H-2d) were transplanted under the renal capsule of STZ-induced hyperglycemic C57BL/6 mice (H-2b). (a) Glucose levels from days 6-18. Control consists of graft recipients that were untreated (n = 3) or treated every 3 days (from day-1) with human albumin (6 mg, n = 3). Prolonged islet graft survival is observed in mice treated every 3 days (from day-1) with hAAT (2 mg, n = 10). *, P < 0.05; **, P < 0.01; ***, P < 0.001 between glucose levels on the same day. (b) Treatment protocols. Control and full AAT treatments are represented in a. Shown in b are AAT only on days -1, 1, and 3 (2 mg, n = 3; Early AAT) and AAT from day 2 and every 2 days thereafter (Late AAT) (2 mg, n = 3). The day that glucose levels exceed 300 mg/dl is indicated as Rejection. (c) Effect of mouse anti-hAAT antibodies. The dashed line indicates posttransplantation glucose levels of a mouse (1 representative of 3) under the full AAT treatment protocol (see a and b) that was immunized by prior administrations of hAAT. The solid line indicates glucose levels of a nonimmunized mouse (1 representative of 10) treated under the full AAT treatment protocol. The arrow indicates the day of detection of treatment-induced anti-hAAT antibodies. (d) Comparison of day 15 posttransplantation glucose levels in mice that were under full treatment protocol with albumin (ALB) (n = 3) or AAT (nonimmunized, n = 10; immunized, n = 3). Of the AAT-alone-treated group, antibodies were detected in 3 of 3 immunized mice and in 6 of 10 nonimmunized mice. **, P = 0.005 between mice that produced antibodies and mice that did not.
hAAT-treated mice developed anti-hAAT antibodies (Fig. 1 c and d). To ascertain that the antibodies were responsible for the reduction in the protective effect of hAAT, a group of mice was preexposed (”immunized”) to hAAT. The mice were injected i.p. with 10 mg of hAAT at intervals of 1 week for a total of four times and were transplanted 2 months later with allogenic islets. The mice were treated with the full AAT protocol, despite exhibiting high titers of specific antibodies before engraftment. These recipients, not unexpectedly, exhibited rapid graft rejection (Fig. 1c). In addition, day 15 from transplantation was chosen to depict an association between antibody formation and loss of hAAT-protective activity; at this time point, hAAT-treated mice were divided into those that exhibited anti-hAAT antibodies and those that did not. As shown in Fig. 1d, all antibody-positive mice were hyperglycemic, and all antibody-negative mice were normoglycemic.
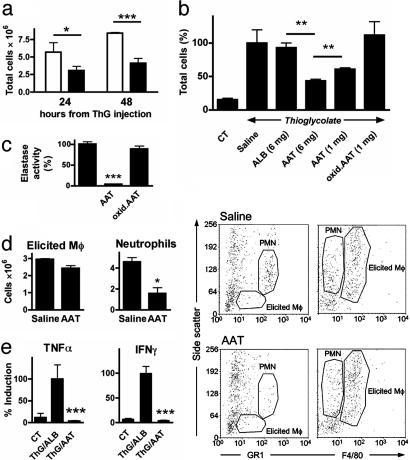
AAT Inhibits Cellular Infiltration. Two models of cell emigration were performed: ThG-elicited peritoneal infiltration, and cellular infiltration elicited by i.p. injection of major-MHC-incompatible fibroblasts. For both setups, C57BL/6 mice were pretreated with saline, human albumin, hAAT, or oxidized hAAT.
ThG Model. Peritoneal lavage was performed 24 and 48 h after ThG injection, and cells were counted and identified by FACS analysis. As shown in Fig. 2a, at 24 and 48 h, the total cell count was lower in mice pretreated with AAT. At 48 h, the total cell count in peritoneal lavage of AAT-treated mice was 50% of that in control mice (Fig. 2 a and b). The total cell count in mice that received albumin control was similar to that of saline-treated mice. Oxidized AAT that had lost its in vitro anti-elastase activity (Fig. 2c) failed to inhibit cellular infiltration (Fig. 2b). The decrease in the total cell count is primarily attributed to a decrease in the number of neutrophils, identified by their GR-1high/intermediate side-scatter (SSC) profile (20) (Fig. 2d Left) quantified FACS analysis and Fig. 2d Right, FACS analysis. No major differences were observed with the infiltration of macrophages, identified by their F4/80int, GR-1int, intermediate SSC profile (20), which is distinct from the F4/80very high, GR-1low, high-SSC profile of resident macrophages. Spleens were removed 48 h after ThG injection, and splenocytes were cultured. In the absence of inducers, the splenocytes spontaneously produced TNFα (Fig. 2e). Pretreatment of mice with AAT decreased TNFα release from cultured splenocytes (mean 96.4%). A similar suppression was found in IFNγ (mean 95.0%), implying a sustained effect of AAT on isolated, washed splenocytes 48 h after in vivo exposure of the mouse to ThG.
Fig. 2.
Effect of AAT on ThG-elicited peritoneal cellular infiltrates. Mice were administered saline, albumin (ALB), AAT, or oxidized AAT (oxid.ATT), followed by either saline or ThG (3% wt/vol, n = 3 per group). Peritoneal lavage was performed on separate groups after 24 and 48 h. (a) Total cell population of lavaged cells of saline-(open bars) or AAT-treated (5 mg, black bars) ThG-injected mice. *, P < 0.05; ***, P < 0.001. (b) Percent cell population from saline-treated mice at 48 h. **, P < 0.01. CT, control. (c) Oxidation of AAT. AAT was subjected to oxidative radicals, and loss of activity was assessed in an elastase assay. Activity of elastase in the absence of native AAT was set at 100%, and the percentage of activity in the presence of native and oxidized AAT was calculated (n = 3). ***, P < 0.001. (d) Identification of elicited macrophages (Mô) and neutrophils. Peritoneal infiltrates from 48-h lavages of ALB-(6 mg) and AAT-treated (6 mg) ThG-injected mice were identified by FACS analysis. Macrophages and neutrophils were identified on the basis of F4/80 and Gr1 vs. side-scatter flow cytometry profiles. (Left) Quantified FACS results (n = 3). (Right) FACS analysis, representative graphs (n = 3). PMN, polymorphonuclear leukocyte. (e) Production of TNFα by cultured splenocytes from ThG-injected mice. Splenocytes were harvested and washed 48 h after i.p. injection of 1 ml ThG or saline (CT) in mice that were pretreated with albumin (ThG/ALB, 5 mg) or AAT (ThG/AAT, 5 mg). After 48 h of culture in the absence of inducers, levels of secreted TNFα and IFNγ were determined. Mean ± SEM from ThG/ALB group (n = 3 per group).
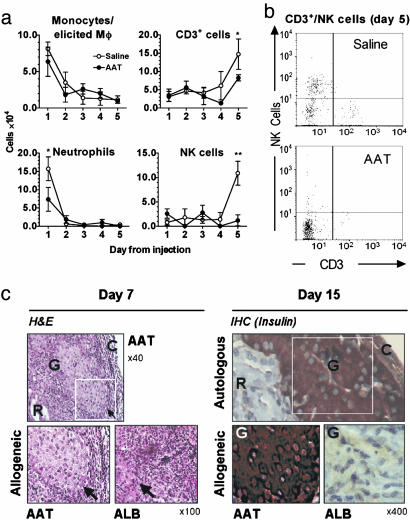
Major-MHC-Incompatible Fibroblast Model. Freshly harvested NIH 3T3 cells, derived from BALB/c mice (H-2d), were injected i.p. into C57BL/6 mice (H-2b). Lavage was performed on days 1-5. As shown in Fig. 3a, the introduction of allogeneic cells evoked a cellular infiltrate that consisted of early appearing neutrophils and activated macrophages and late appearing CD3+ and NK cells (Fig. 3 a and b). hAAT-treated mice exhibited a reduction in neutrophils by 53%, CD3+ by 44%, and NK cells by 89%.
Fig. 3.
Effect of AAT on MHC-incompatible fibroblast-elicited peritoneal cellular infiltrates. Mice (C57BL/6; H-2b) were administered saline or AAT (1 mg) followed by NIH 3T3 cells (1 × 107 cells; H-2d). Peritoneal lavage was performed daily on days 1-5, and cell subpopulations were identified by FACS analysis (n = 3 per treatment). (a) Cell numbers. The number of cells in each subpopulation was calculated from the percentages obtained by FACS analysis and total number of cells in the infiltrate. *, P < 0.05; **, P < 0.01 between cell numbers on the same day. (b) Representative FACS analysis. (c) Effect of AAT on intensity and function of infiltrate elicited by islet allograft. (Left) Hematoxylin and eosin (H&E) staining of day-7 islet allografts. A section of AAT-treated islet graft (in white frame) is compared with an equivalent section from an ALB-treated diabetic recipient mouse (full AAT treatment protocol, see Fig. 1a). The arrow indicates the border between the islet and surrounding infiltrate. (Right) Immunohistochemistry for insulin in islet grafts on day 15. A section of autologous islet graft (in white frame) represents intact transplanted islets and is compared with equivalent sections of allografts of AAT- and ALB-treated recipient mice. R, renal parenchyma; G, graft; C, renal capsule.
To evaluate infiltration into grafted islets, grafts from AAT- and ALB-treated recipient mice were examined. As depicted in Fig. 3c Left, a cellular infiltrate is demonstrable regardless of AAT treatment and includes neutrophils and lymphocytes. However, infiltrates evoked by grafts of AAT-treated recipient mice were small and accumulated around intact islets, whereas infiltrates evoked by grafts of ALB-treated recipient mice were large and caused disruption of islet borders. To evaluate islet graft function, insulin content was evaluated in day-15 grafts from AAT- and ALB-treated recipient mice. As depicted in Fig. 3c Right, insulin production was preserved in islets of hAAT-treated recipients.
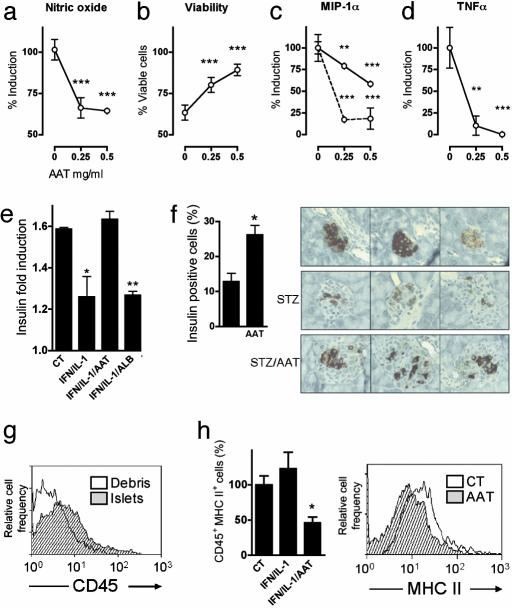
AAT Modifies Islet Response to Proinflammatory Mediators in Vitro.Islet responses to IL-1β/IFNγ stimulation were examined in vitro. Stimulated islets produce NO and exhibit NO-dependent loss of viability (14). As shown in Fig. 4 a and b, in the presence of AAT, less NO is produced and greater islet viability is observed. The production of MIP-1α was decreased in the presence of AAT, particularly when stimulated by low concentrations of IL-1β/IFNγ (Fig. 4c). Notably, TNFα levels in islet supernatants were markedly diminished by AAT (Fig. 4d). Insulin induction (Fig. 4e) was inhibited by IL-1β/IFNγ but was intact in the presence of AAT.
Fig. 4.
Effect of AAT on islet responses. (a-d) Islets from C57BL/6 mice were cultured (100 islets per well, in duplicate). AAT was incubated at the indicated concentrations for 1 h before the addition of IFNγ (5 ng/ml) and IL-1β (10 ng/ml). Seventy-two hours later, supernatants were collected, and islet viability was assessed. Islet responses in the absence of AAT were set at 100%. The data are combined from three individual experiments. **, P < 0.01; ***, P < 0.001 between AAT-treated and untreated islets. The dashed line represents islets incubated at 1/30th the concentration of IFNγ/IL-1β. (e) Insulin-induction assay. Islets were incubated (20 islets per well, in triplicate) in the presence of AAT (0.5 mg/ml) or ALB (0.5 mg/ml) 1 h before the addition of IFNγ (5 ng/ml) and IL-1β (10 ng/ml). Twenty-four hours later, islets were transferred to a 3 mM or 20 mM glucose solution for 30 min, and insulin levels were measured. The vertical axis represents the ratio between insulin levels at both glucose concentrations. *, P < 0.05 between AAT-treated and ALB-treated islets. (f) In vivo STZ-induced beta cell toxicity. C57BL/6 mice were injected with AAT (5 mg) or saline, 1 day before, on the same day as, and 1 day after injection of STZ (225 mg/kg of body weight) or saline (n = 3 per group). Forty-eight hours later, pancreata were removed, and insulin-containing cells were identified by immunohistochemistry. (Left) Mean ± SEM percent change of insulin-containing cells, as determined from images of two islets per pancreas. (Right) Each image depicts a representative islet from one pancreas (n = 6 per treatment group). *, P < 0.05. (g) Cellular content of islets. Freshly isolated islets (100 islets, in triplicate) and residual nonislet pancreatic debris were dissociated into single-cell suspensions and stained for FACS analysis with anti-CD45-APC or isotype control antibody. (h) MHC class II expression. Islets from C57BL/6 mice were cultured (100 islets per well, in duplicate) in the presence of AAT (0.5 mg/ml) 1 h before the addition of IFNγ (5 ng/ml) and IL-1β (10 ng/ml). Twenty-four hours later, islets were dissociated into single-cell suspensions and double-stained for FACS analysis with anti-CD45-APC and anti-MHCII-PE or isotype control antibodies. (Left) Mean ± SEM percent change from control (CT) unstimulated islets. *, P < 0.05 between AAT-treated and untreated islets. (Right) Representative FACS analysis; events are gated for CD45.
Effect of AAT on Beta Cells in Vivo. STZ-mediated toxicity was evaluated in vivo. AAT was administered 1 day before, on the same day as, and 1 day after STZ injection. Immunohistochemistry of pancreata with anti-insulin antibodies at 48 h after STZ injection reveals larger numbers of insulin-producing cells in islets of AAT-treated mice than in ALB-treated mice (Fig. 4f; mean 26.3% and 12.8% insulin-producing cells per islet, respectively). Freshly isolated islets were found to contain CD45+ cells, according to FACS analysis (Fig. 4g). These cells are also positive for the monocytic/granulocytic markers Gr1 and F4/80 (data not shown). The CD45+ cell population responded to AAT with decreased surface MHC class II expression (Fig. 4h).
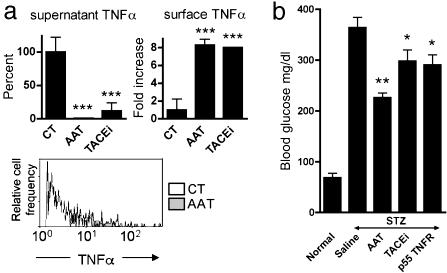
AAT Inhibits Release of Membrane TNFα from Islets. Soluble TNFα is released from activated cells by the action of TNFα-converting enzyme (TACE) (21). We examined the levels of membrane TNFα in cytokine-stimulated islet cells. The effect of AAT was compared with that of a TACE inhibitor (TACE inhibitor described in ref. 22). Both AAT and the TACE inhibitor decreased TNFα levels in supernatants of islets exposed to IL-1β/IFNγ (Fig. 5a Left). Under these conditions, membrane TNFα accumulated on the cell surface of CD45+ islet cells (Fig. 5a Right and Lower).
Fig. 5.
Effect of AAT on TNFα in islets. (a) Islets from C57BL/6 mice were cultured (100 islets per well, in triplicate) in the presence of AAT (0.5 mg/ml) or TACE inhibitor (10 mM) 1 h before stimulation by IFNγ (5 ng/ml) and IL-1β (10 ng/ml). (Left) Mean (±SEM) change in TNFα levels in supernatants after 72 h of incubation. (Right) Mean (±SEM) fold change in membrane TNFα on islet cells after 5 h of incubation. ***, P < 0.001 compared with control (CT) levels in the absence of AAT. (Lower) Representative FACS analysis of membrane TNFα on stimulated islet cells in the absence (open area) or presence (shaded area) of AAT. Events are gated for CD45. (b) In vivo STZ-induced beta cell toxicity. C57BL/6 mice were injected with saline (n = 3), AAT (5 mg, n = 3) or soluble p55 TNF receptor (p55 TNFR, 1 mg/kg of body weight, n = 3) or administered oral TACE inhibitor (TACEi, 60 mg/kg of body weight, n = 6) 1 day before injection of STZ (225 mg/kg of body weight, i.p.). Subsequently, AAT and soluble TNF receptor were injected daily, and TACE inhibitor was administered twice a day. At 48 h, mean (±SEM) glucose levels are compared with those of normal littermates (n = 3). *, P < 0.05; **, P < 0.01, compared with saline-treated, STZ-injected mice.
To assess the possibility that islet protection occurs through inhibition of release-of-membrane TNFα in vivo, we compared treatment of mice with TACE inhibitor, the soluble form of p55 TNF receptor (23) and AAT, before STZ injection. Although, as anticipated, mice developed hyperglycemia 4 days after STZ administration, the progression of beta cell toxicity was significantly affected by the different treatments. As shown in Fig. 5b, the effect of STZ at 48 h was decreased in the presence of AAT (46.5% lower fasting glucose levels than STZ/saline injected mice). The effect of STZ was also diminished, although to a lesser extent, by administration of TACE inhibitor or a soluble form of p55 TNF receptor (22.3% and 24.7%, respectively, lower fasting glucose levels than STZ/saline-injected mice).
Discussion
In this study, monotherapy with clinical grade AAT prolonged the survival of insulin-secreting islet allografts in a mouse model of islet allograft rejection. Excised grafts showed intact islets and diminished intragraft cellular infiltration. In mice, hAAT activity is limited in vivo by the development of anti-hAAT antibodies (7). In this study, the association between antibody formation and loss of hAAT protective effect was particularly evident after 15 days of hAAT treatment and was verified by using mice that were induced to produce anti-hAAT prior to transplantation. The dosage used in this study is based on mouse gene-transfer studies, in which plasma levels of 0.8-1.0 mg/ml hAAT were attained (16) and provided protection from type I diabetes in nonobese diabetic mice (7). Similarly, 0.3-1.0 mg of i.p. AAT protected mice from TNFα-mediated lethal response (24), and 0.8 mg of AAT protected against d-galactosamine/LPS-induced hepatic injury (25). Normal human plasma contains 0.8-2.4 mg/ml AAT (26).
We demonstrate here that i.p. ThG induced local immune-cell migration that was inhibited by AAT. Specifically, ThG-induced infiltration contained 66% fewer neutrophils, the presence of which is detrimental to islet survival (27). The effect of AAT on neutrophil function is well documented; AAT blocks IL-8 secretion from human leukocytes (28) and reduces whole-lung expression of macrophage inflammatory protein 2, monocyte chemotactic protein 1 (MCP-1), and intercellular adhesion molecule 1 (11). AAT reduces neutrophil infiltration into kidneys during ischemia/reperfusion injury (10) and into lung tissue after intratracheal silica administration (11). The essential inhibitory effect of AAT on neutrophils probably involves direct inhibition of elastase, the prototypic neutrophil protease and an avid substrate for AAT anti-protease activity. Elastase can promote islet graft destruction not only by facilitation of neutrophil migration (29) but also by induction of platelet-activating factor (6) and processing of tyrosine-tRNA synthase into fragments with IL-8-like properties (30).
Macrophages infiltrate islets before the onset of insulitis in nonobese diabetic mice (31), and the depletion of macrophages protects transplanted islets from acute rejection in rats (32). It was shown that islets produce MCP-1 (33) and that islet rejection is associated with an increased expression of intragraft macrophage chemokines and their receptors (34). Indeed, knockout mice for these receptors exhibit prolongation of islet allograft survival (34, 35). Once activated inside transplanted tissues, macrophages can secrete TNFα and IL-1β, which cause cell damage before antigen recognition (36). Therefore, it was interesting to find that macrophages reside within freshly isolated islets (as also described by Toyama et al. in ref. 37). i.p. injection of allogeneic NIH 3T3 cells evoked infiltration of macrophages and neutrophils on days 1 and 2 after injection and of CD3+ and NK cells on days 4 and 5 after injection. The intensity of the latter type of infiltrate was decreased by administration of AAT before injection of allogeneic cells but not by administration of AAT 3 days after cell injection (data not shown), indicating that the decrease in CD3+ and NK cells is secondary to AAT-induced suppression of the preceding infiltrate. Concurrently, late treatment with AAT was ineffective in prolonging islet graft survival, whereas full AAT treatment diminished infiltration of neutrophils and lymphocytes and was associated with improved islet viability and intragraft insulin content.
AAT decreased in vitro NO production by IL-1β/IFNγ-stimulated islets, resulting in enhancement of islet viability and intact glucose-mediated insulin induction. In vivo, AAT protected islets from STZ-induced toxicity, as demonstrated by a lower degree of hyperglycemia after 48 h. In that experiment, we observed that the relative number of viable beta cells inside islets was proportional to the decrease in serum glucose levels, as assessed by insulin content. Considering the ability of AAT to diminish NO production by islet cells, these data suggest that AAT interferes with NO-dependent events that mediate STZ toxicity (38).
MHC class II antigens on the surface of islet cells are critical for directing immune responses against islets (39). Here, we show that AAT reduces MHC class II expression in islets to levels below those found in IL-1β/IFNγ-stimulated islets and in unstimulated islets. Because the procedure of islet-isolation provokes the activation of inflammatory pathways (40), the ability of AAT to decrease islet expression levels of MHC class II below isolation-induced levels is highly relevant.
In the presence of AAT, IL-1β/IFNγ-stimulated islets produced strikingly less TNFα (mean decreases of 89.9% and 99.2% at 0.5 mg/ml and 1.0 mg/ml AAT, respectively). Because AAT blocks TNFα release from stimulated human peripheral blood mononuclear cells without affecting TNFα mRNA levels (41), we believe that AAT might also inhibit the release of TNFα from islet cells. Membrane TNFα is released from cell surfaces by the action of the metalloproteinase TACE (21). Accordingly, inhibitors of TACE reduce TNFα release and increase the amounts of membrane TNFα (19). Here, we demonstrate that, in the presence of AAT, TNFα accumulates on the surface of IL-1β/IFNγ-stimulated CD45+ islet cells. TACE-inhibition may be relevant for the process of graft rejection, because an inhibitor of TACE decreased injury in a rat model of posttransplant lung injury (42). Although there are no reports that describe prolongation of islet graft survival by TACE inhibition alone, locally secreted TNFα was found to be detrimental to islet graft function (43), and islet allograft survival was prolonged after ex vivo transduction with adenovirus-encoded soluble type 1 TNF receptor decoy (44).
In an animal model of renal ischemia/reperfusion injury, administration of human AAT (0.5 mg) protected kidney function and was associated with reduced neutrophil infiltration, intragraft TNFα levels, and frequency of apoptosis (10). It is recognized that ischemia/reperfusion injury during human renal transplantation correlates with poor clinical outcome (45). Specifically, in gene expression studies performed on biopsies of renal grafts at 15-min postperfusion (”zero-hour”), Avihingsanon et al. (45) demonstrate that intragraft proinflammatory and early immune-activating gene-expression patterns correlate with poor transplant outcome; abundance of TNFα and ICAM-1 transcripts and graft ischemic time individually predicted acute rejection, and suboptimal expression of antiapoptotic genes predicted delayed graft function. The ability of AAT to diminish inflammatory parameters and cytopathic tissue injury in the ischemia/reperfusion model may provide the basis for the diminished alloimmune response and prolonged graft survival in the islet-transplantation model observed here.
Our data suggest, therefore, that the mechanism by which AAT prolongs islet graft survival involves multiple aspects of immune and inflammatory responses and, importantly, islet cell viability. (i) AAT reduces the degree of inflammation below that which damages islets, (ii) AAT promotes the viability of islets in the presence of inflammatory agents, (iii) AAT decreases islet immunogeneity in the form of low MHC class II expression, and (iv) AAT reduces infiltration of immune cells elicited by a variety of inducers, including those that are independent of MHC recognition. This impressive array of activities, which includes the crucial preservation of islet viability, is distinctive to AAT and has not been reported to be the outcome of other clinical or experimental immunosuppressive regimens.
The notable safety record of large-dose AAT administration in AAT-deficient patients (46) suggests that the use of AAT to block graft rejection may be well tolerated. The combination of allograft preservation, reduced graft toxicity, and a feasible safety profile in a single agent is unique and strongly suggests that AAT should be further studied as adjunctive therapy for islet transplantation in diabetic patients.
Acknowledgments
This work was supported, in part, by National Institutes of Health Grants AI 15614 and HL-68743 and Colorado Cancer Center Grant CA-04 6934.
Author contributions: E.C.L., L.S., and C.A.D. designed research; E.C.L., L.S., O.J.B., and C.A.D. performed research; E.C.L., L.S., and O.J.B. contributed new reagents/analytic tools; E.C.L., L.S., O.J.B., and C.A.D. analyzed data; and E.C.L., L.S., O.J.B., and C.A.D. wrote the paper.
Abbreviations: hAAT, human α1-antitrypsin; NK, natural killer; STZ, streptozotocin; TACE, TNFα-converting enzyme; ThG, thioglycolate.
References
- 1.Sato, T., Inagaki, A., Uchida, K., Ueki, T., Goto, N., Matsuoka, S., Katayama, A., Haba, T., Tominaga, Y., Okajima, Y., et al. (2003) Transplantation 76, 1320-1326. [DOI] [PubMed] [Google Scholar]
- 2.Ricordi, C. & Strom, T. B. (2004) Nat. Rev. Immunol. 4, 259-268. [DOI] [PubMed] [Google Scholar]
- 3.Couzin, J. (2004) Science 306, 34-37. [DOI] [PubMed] [Google Scholar]
- 4.Kobayashi, N., Okitsu, T., Lakey, J. R. & Tanaka, N. (2004) J. Artif. Organs 7, 1-8. [DOI] [PubMed] [Google Scholar]
- 5.Breit, S. N., Wakefield, D., Robinson, J. P., Luckhurst, E., Clark, P. & Penny, R. (1985) Clin. Immunol. Immunopathol. 35, 363-380. [DOI] [PubMed] [Google Scholar]
- 6.Camussi, G., Tetta, C., Bussolino, F. & Baglioni, C. (1988) J. Exp. Med. 168, 1293-1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Song, S., Goudy, K., Campbell-Thompson, M., Wasserfall, C., Scott-Jorgensen, M., Wang, J., Tang, Q., Crawford, J. M., Ellis, T. M., et al. (2004) Gene Ther. 11, 181-186. [DOI] [PubMed] [Google Scholar]
- 8.Fischer, D. C., Siebertz, B., van de Leur, E., Schiwy-Bochat, K. H., Graeve, L., Heinrich, P. C. & Haubeck, H. D. (1999) Arthritis Rheum. 42, 1936-1945. [DOI] [PubMed] [Google Scholar]
- 9.Knoell, D. L., Ralston, D. R., Coulter, K. R. & Wewers, M. D. (1998) Am. J. Respir. Crit. Care Med. 157, 246-255. [DOI] [PubMed] [Google Scholar]
- 10.Daemen, M. A., Heemskerk, V. H., van't Veer, C., Denecker, G., Wolfs, T. G., Vandenabeele, P. & Buurman, W. A. (2000) Circulation 102, 1420-1426. [DOI] [PubMed] [Google Scholar]
- 11.Churg, A., Dai, J., Zay, K., Karsan, A., Hendricks, R., Yee, C., Martin, R., MacKenzie, R., Xie, C., Zhang, L., et al. (2001) Lab. Invest. 81, 1119-1131. [DOI] [PubMed] [Google Scholar]
- 12.Jie, Z., Cai, Y., Yang, W., Jin, M., Zhu, W. & Zhu, C. (2003) Chin. Med. J. (Engl. Ed.) 116, 1678-1682. [PubMed] [Google Scholar]
- 13.Salvalaggio, P. R., Deng, S., Ariyan, C. E., Millet, I., Zawalich, W. S., Basadonna, G. P. & Rothstein, D. M. (2002) Transplantation 74, 877-879. [DOI] [PubMed] [Google Scholar]
- 14.Thomas, H. E., Darwiche, R., Corbett, J. A. & Kay, T. W. (2002) Diabetes 51, 311-316. [DOI] [PubMed] [Google Scholar]
- 15.Noguchi, H., Matsushita, M., Okitsu, T., Moriwaki, A., Tomizawa, K., Kang, S., Li, S. T., Kobayashi, N., Matsumoto, S., Tanaka, K., et al. (2004) Nat. Med. 10, 305-309. [DOI] [PubMed] [Google Scholar]
- 16.Song, S., Morgan, M., Ellis, T., Poirier, A., Chesnut, K., Wang, J., Brantly, M., Muzyczka, N., Byrne, B. J., Atkinson, M. & Flotte, T. R. (1998) Proc. Natl. Acad. Sci. USA 95, 14384-14388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wasil, M., Halliwell, B., Hutchison, D. C. & Baum, H. (1987) Biochem. J. 243, 219-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fantuzzi, G. & Dinarello, C. A. (1998) Eur. Cytokine Network 9, 85-92. [PubMed] [Google Scholar]
- 19.Bueno, C., Rodriguez-Caballero, A., Garcia-Montero, A., Pandiella, A., Almeida, J. & Orfao, A. (2002) J. Immunol. Methods 264, 77-87. [DOI] [PubMed] [Google Scholar]
- 20.Gordon, S., Lawson, L., Rabinowitz, S., Crocker, P. R., Morris, L. & Perry, V. H. (1992) Curr. Top. Microbiol. Immunol. 181, 1-37. [DOI] [PubMed] [Google Scholar]
- 21.Black, R. A. (2002) Int. J. Biochem. Cell Biol. 34, 1-5. [DOI] [PubMed] [Google Scholar]
- 22.Beck, G., Bottomley, G., Bradshaw, D., Brewster, M., Broadhurst, M., Devos, R., Hill, C., Johnson, W., Kim, H. J., Kirtland, S., et al. (2002) J. Pharmacol. Exp. Ther. 302, 390-396. [DOI] [PubMed] [Google Scholar]
- 23.Santos, A. A., Shapiro, L., Lynch, E. A., Brown, E. F., Chambers, E., Jacobs, D. O., Dinarello, C. A., Mannick, J. & Wilmore, D. W. (1993) Surg. Forum 44, 119-121. [Google Scholar]
- 24.Libert, C., Van Molle, W., Brouckaert, P. & Fiers, W. (1996) J. Immunol. 157, 5126-5129. [PubMed] [Google Scholar]
- 25.Niehorster, M., Tiegs, G., Schade, U. F. & Wendel, A. (1990) Biochem. Pharmacol. 40, 1601-1603. [DOI] [PubMed] [Google Scholar]
- 26.Macen, J. L., Upton, C., Nation, N. & McFadden, G. (1993) Virology 195, 348-363. [DOI] [PubMed] [Google Scholar]
- 27.Allison, J., Georgiou, H. M., Strasser, A. & Vaux, D. L. (1997) Proc. Natl. Acad. Sci. USA 94, 3943-3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nita, I., Hollander, C., Westin, U. & Janciauskiene, S. M. (2005) Respir. Res. 6, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cai, T. Q. & Wright, S. D. (1996) J. Exp. Med. 184, 1213-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wakasugi, K. & Schimmel, P. (1999) Science 284, 147-151. [DOI] [PubMed] [Google Scholar]
- 31.Lee, K. U., Amano, K. & Yoon, J. W. (1988) Diabetes 37, 989-991. [DOI] [PubMed] [Google Scholar]
- 32.Bottino, R., Fernandez, L. A., Ricordi, C., Lehmann, R., Tsan, M. F., Oliver, R. & Inverardi, L. (1998) Diabetes 47, 316-323. [DOI] [PubMed] [Google Scholar]
- 33.Johansson, U., Olsson, A., Gabrielsson, S., Nilsson, B. & Korsgren, O. (2003) Biochem. Biophys. Res. Commun. 308, 474-479. [DOI] [PubMed] [Google Scholar]
- 34.Abdi, R., Means, T. K., Ito, T., Smith, R. N., Najafian, N., Jurewicz, M., Tchipachvili, V., Charo, I., Auchincloss, H., Jr., Sayegh, M. H. & Luster, A. D. (2004) J. Immunol. 172, 767-775. [DOI] [PubMed] [Google Scholar]
- 35.Baker, M. S., Chen, X., Rotramel, A. R., Nelson, J. J., Lu, B., Gerard, C., Kanwar, Y. & Kaufman, D. B. (2003) Surgery 134, 126-133. [DOI] [PubMed] [Google Scholar]
- 36.Teshima, T., Ordemann, R., Reddy, P., Gagin, S., Liu, C., Cooke, K. R. & Ferrara, J. L. (2002) Nat. Med. 8, 575-581. [DOI] [PubMed] [Google Scholar]
- 37.Toyama, H., Takada, M., Tanaka, T., Suzuki, Y. & Kuroda, Y. (2003) Transplant Proc. 35, 1503-1505. [DOI] [PubMed] [Google Scholar]
- 38.Wada, R. & Yagihashi, S. (2004) Virchows Arch. 444, 375-382. [DOI] [PubMed] [Google Scholar]
- 39.McInerney, M. F., Rath, S. & Janeway, C. A., Jr. (1991) Diabetes 40, 648-651. [DOI] [PubMed] [Google Scholar]
- 40.Abdelli, S., Ansite, J., Roduit, R., Borsello, T., Matsumoto, I., Sawada, T., Allaman-Pillet, N., Henry, H., Beckmann, J. S., Hering, B. J. & Bonny, C. (2004) Diabetes 53, 2815-2823. [DOI] [PubMed] [Google Scholar]
- 41.Scuderi, P., Dorr, R. T., Liddil, J. D., Finley, P. R., Meltzer, P., Raitano, A. B. & Rybski, J. (1989) Eur. J. Immunol. 19, 939-942. [DOI] [PubMed] [Google Scholar]
- 42.Goto, T., Ishizaka, A., Kobayashi, F., Kohno, M., Sawafuji, M., Tasaka, S., Ikeda, E., Okada, Y., Maruyama, I. & Kobayashi, K. (2004) Am. J. Respir. Crit. Care Med. 27, 27. [DOI] [PubMed] [Google Scholar]
- 43.de Vos, P., Smedema, I., van Goor, H., Moes, H., van Zanten, J., Netters, S., de Leij, L. F., de Haan, A. & de Haan, B. J. (2003) Diabetologia 46, 666-673. [DOI] [PubMed] [Google Scholar]
- 44.Machen, J., Bertera, S., Chang, Y., Bottino, R., Balamurugan, A. N., Robbins, P. D., Trucco, M. & Giannoukakis, N. (2004) Gene Ther. 11, 1506-1514. [DOI] [PubMed] [Google Scholar]
- 45.Avihingsanon, Y., Ma, N., Pavlakis, M., Chon, W. J., Uknis, M. E., Monaco, A. P., Ferran, C., Stillman, I., Schachter, A. D., Mottley, C., et al. (2005) J. Am. Soc. Nephrol. 16, 1542-1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wewers, M. D., Casolaro, M. A., Sellers, S. E., Swayze, S. C., McPhaul, K. M., Wittes, J. T. & Crystal, R. G. (1987) N. Engl. J. Med. 316, 1055-1062. [DOI] [PubMed] [Google Scholar]