Abstract
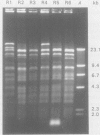
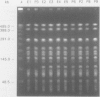
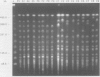
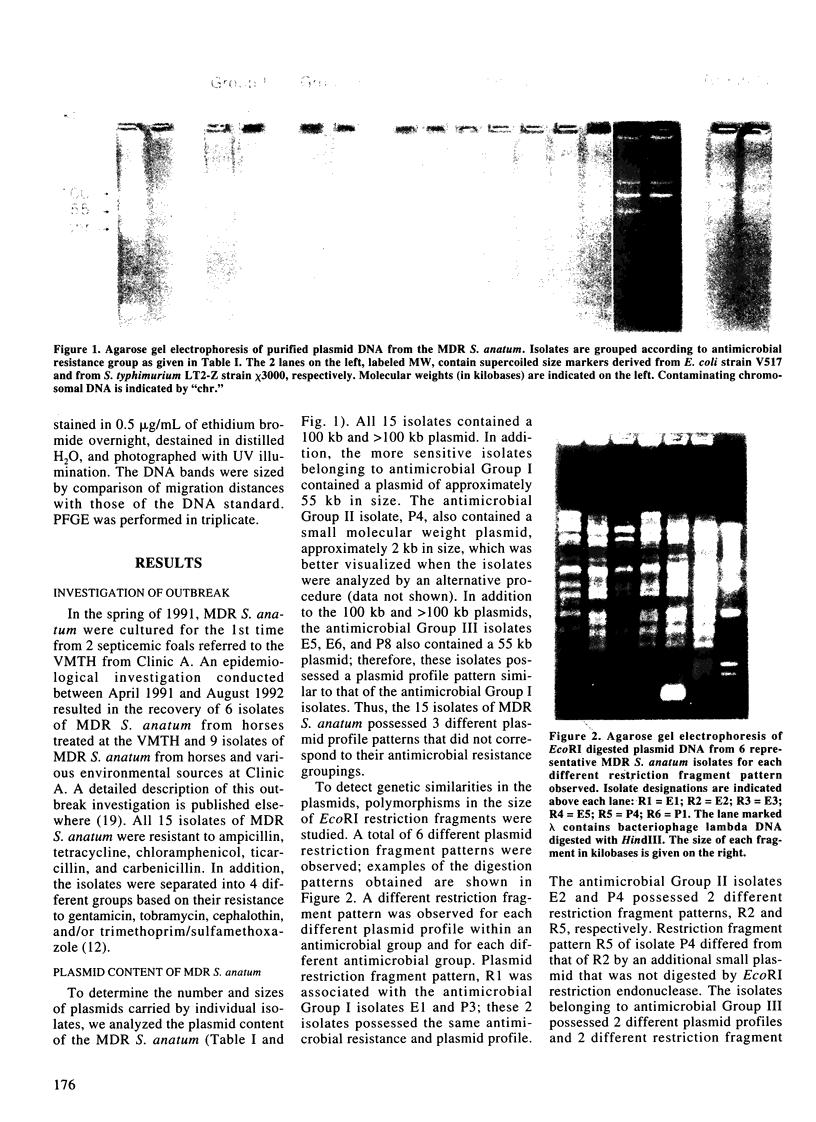
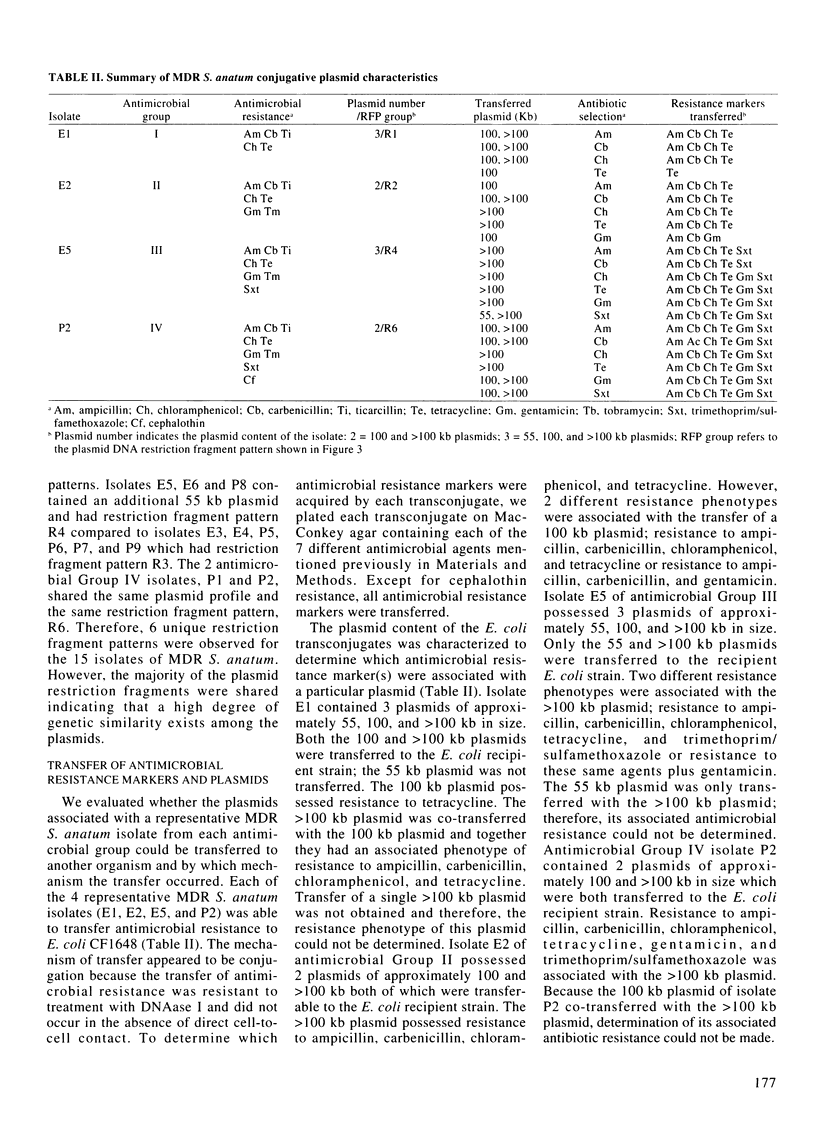
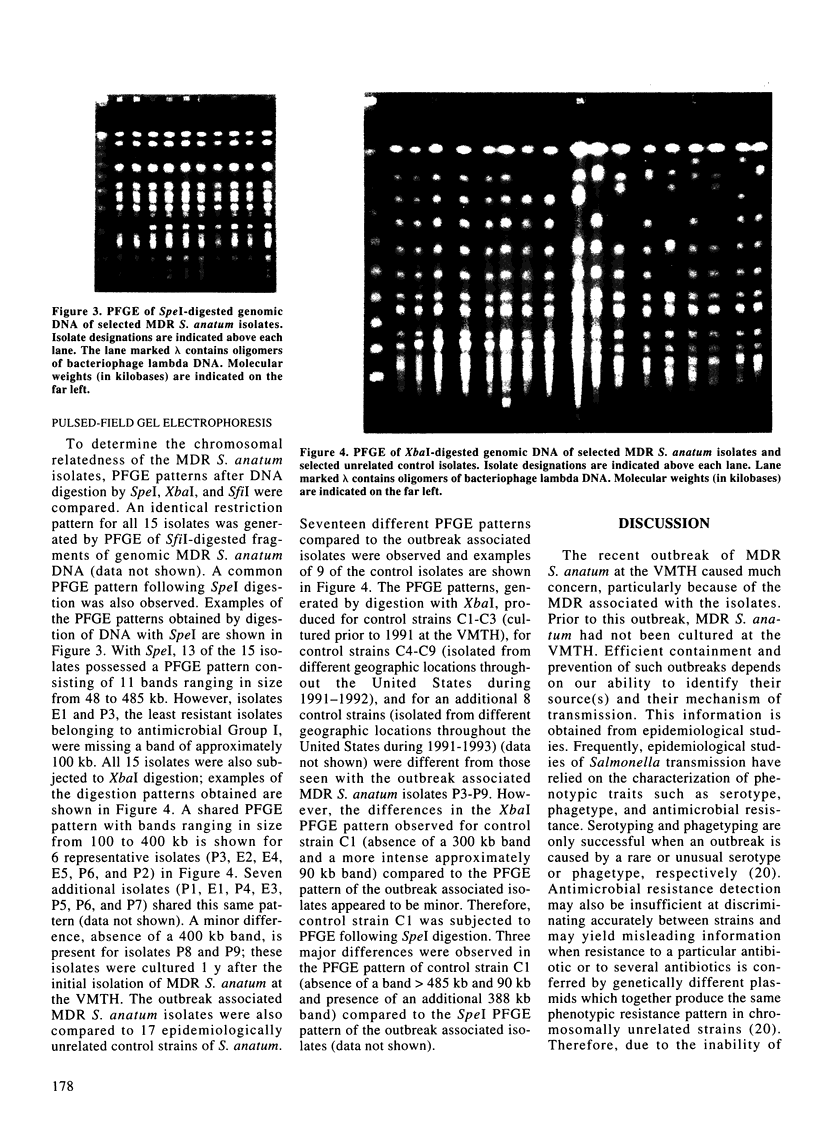
Phenotypic and molecular techniques, including antimicrobial susceptibility testing, plasmid analysis, and pulsed-field gel electrophoresis (PFGE) were used to characterize 15 isolates of multidrug-resistant (MDR) Salmonella anatum cultured during a 16 mo period from horses and a veterinary clinic environment. The isolates were resistant to multiple antimicrobial agents and could be placed into 4 groups based on their antimicrobial resistance patterns. The isolates contained multiple plasmids ranging in size from 2 to > 100 kb that could be grouped into 3 different plasmid profile patterns; these patterns did not correlate with the antimicrobial resistance groupings. Furthermore, antimicrobial resistance was conjugatively transferable. Digestion of genomic DNA from the 15 isolates with 3 different restriction endonucleases, SfiI, SpeI, and XbaI followed by PFGE revealed a highly conserved restriction endonuclease digestion pattern. In contrast, diverse banding patterns were observed with S. anatum obtained from other sources. These observations suggest that the MDR S. anatum isolates represent a common outbreak strain even though they possess different, albeit similar, antibiograms and plasmid profiles. The study showed that PFGE is a useful epidemiological tool for discriminating between unrelated and outbreak-related strains of S. anatum. In conclusion, epidemiological studies of outbreaks caused by MDR isolates of S. anatum should consist of both genotypic and phenotypic methods of analysis.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allardet-Servent A., Bouziges N., Carles-Nurit M. J., Bourg G., Gouby A., Ramuz M. Use of low-frequency-cleavage restriction endonucleases for DNA analysis in epidemiological investigations of nosocomial bacterial infections. J Clin Microbiol. 1989 Sep;27(9):2057–2061. doi: 10.1128/jcm.27.9.2057-2061.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baquar N., Burnens A., Stanley J. Comparative evaluation of molecular typing of strains from a national epidemic due to Salmonella brandenburg by rRNA gene and IS200 probes and pulsed-field gel electrophoresis. J Clin Microbiol. 1994 Aug;32(8):1876–1880. doi: 10.1128/jcm.32.8.1876-1880.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrego J. J., Castro D., Jimenez-Notario M., Luque A., Martinez-Manzanares E., Rodriguez-Avial C., Picazo J. J. Comparison of epidemiological markers of Salmonella strains isolated from different sources in Spain. J Clin Microbiol. 1992 Dec;30(12):3058–3064. doi: 10.1128/jcm.30.12.3058-3064.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordillo M. E., Reeve G. R., Pappas J., Mathewson J. J., DuPont H. L., Murray B. E. Molecular characterization of strains of enteroinvasive Escherichia coli O143, including isolates from a large outbreak in Houston, Texas. J Clin Microbiol. 1992 Apr;30(4):889–893. doi: 10.1128/jcm.30.4.889-893.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulig P. A., Curtiss R., 3rd Plasmid-associated virulence of Salmonella typhimurium. Infect Immun. 1987 Dec;55(12):2891–2901. doi: 10.1128/iai.55.12.2891-2901.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haertl R., Bandlow G. Epidemiological fingerprinting of Enterobacter cloacae by small-fragment restriction endonuclease analysis and pulsed-field gel electrophoresis of genomic restriction fragments. J Clin Microbiol. 1993 Jan;31(1):128–133. doi: 10.1128/jcm.31.1.128-133.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann F. A., Callan R. J., McGuirk S. M., West S. E. Control of an outbreak of salmonellosis caused by drug-resistant Salmonella anatum in horses at a veterinary hospital and measures to prevent future infections. J Am Vet Med Assoc. 1996 Aug 1;209(3):629–631. [PubMed] [Google Scholar]
- Hartmann F. A., West S. E. Antimicrobial susceptibility profiles of multidrug-resistant Salmonella anatum isolated from horses. J Vet Diagn Invest. 1995 Jan;7(1):159–161. doi: 10.1177/104063879500700128. [DOI] [PubMed] [Google Scholar]
- Holmberg S. D., Wachsmuth I. K., Hickman-Brenner F. W., Cohen M. L. Comparison of plasmid profile analysis, phage typing, and antimicrobial susceptibility testing in characterizing Salmonella typhimurium isolates from outbreaks. J Clin Microbiol. 1984 Feb;19(2):100–104. doi: 10.1128/jcm.19.2.100-104.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kado C. I., Liu S. T. Rapid procedure for detection and isolation of large and small plasmids. J Bacteriol. 1981 Mar;145(3):1365–1373. doi: 10.1128/jb.145.3.1365-1373.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapperud G., Gustavsen S., Hellesnes I., Hansen A. H., Lassen J., Hirn J., Jahkola M., Montenegro M. A., Helmuth R. Outbreak of Salmonella typhimurium infection traced to contaminated chocolate and caused by a strain lacking the 60-megadalton virulence plasmid. J Clin Microbiol. 1990 Dec;28(12):2597–2601. doi: 10.1128/jcm.28.12.2597-2601.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macrina F. L., Kopecko D. J., Jones K. R., Ayers D. J., McCowen S. M. A multiple plasmid-containing Escherichia coli strain: convenient source of size reference plasmid molecules. Plasmid. 1978 Jun;1(3):417–420. doi: 10.1016/0147-619x(78)90056-2. [DOI] [PubMed] [Google Scholar]
- Maslow J. N., Mulligan M. E., Arbeit R. D. Molecular epidemiology: application of contemporary techniques to the typing of microorganisms. Clin Infect Dis. 1993 Aug;17(2):153–164. doi: 10.1093/clinids/17.2.153. [DOI] [PubMed] [Google Scholar]
- Morse E. V., Duncan M. A. Salmonellosis--an environmental health problem. J Am Vet Med Assoc. 1974 Dec 1;165(11):1015–1019. [PubMed] [Google Scholar]
- Olsen J. E. An improved method for rapid isolation of plasmid DNA from wild-type gram-negative bacteria for plasmid restriction profile analysis. Lett Appl Microbiol. 1990 May;10(5):209–212. doi: 10.1111/j.1472-765x.1990.tb01335.x. [DOI] [PubMed] [Google Scholar]
- Owen R. Post stress diarrhoea in the horse. Vet Rec. 1975 Mar 22;96(12):267–270. doi: 10.1136/vr.96.12.267. [DOI] [PubMed] [Google Scholar]
- Riley L. W., DiFerdinando G. T., Jr, DeMelfi T. M., Cohen M. L. Evaluation of isolated cases of salmonellosis by plasmid profile analysis: introduction and transmission of a bacterial clone by precooked roast beef. J Infect Dis. 1983 Jul;148(1):12–17. doi: 10.1093/infdis/148.1.12. [DOI] [PubMed] [Google Scholar]
- Rivera M. J., Rivera N., Castillo J., Rubio M. C., Gómez-Lus R. Molecular and epidemiological study of Salmonella clinical isolates. J Clin Microbiol. 1991 May;29(5):927–932. doi: 10.1128/jcm.29.5.927-932.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwalbe R. S., Hoge C. W., Morris J. G., Jr, O'Hanlon P. N., Crawford R. A., Gilligan P. H. In vivo selection for transmissible drug resistance in Salmonella typhi during antimicrobial therapy. Antimicrob Agents Chemother. 1990 Jan;34(1):161–163. doi: 10.1128/aac.34.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley J., Baquar N., Threlfall E. J. Genotypes and phylogenetic relationships of Salmonella typhimurium are defined by molecular fingerprinting of IS200 and 16S rrn loci. J Gen Microbiol. 1993 Jun;139(Pt 6):1133–1140. doi: 10.1099/00221287-139-6-1133. [DOI] [PubMed] [Google Scholar]
- Stanley J., Burnens A., Powell N., Chowdry N., Jones C. The insertion sequence IS200 fingerprints chromosomal genotypes and epidemiological relationships in Salmonella heidelberg. J Gen Microbiol. 1992 Nov;138(11):2329–2336. doi: 10.1099/00221287-138-11-2329. [DOI] [PubMed] [Google Scholar]
- Tacket C. O. Molecular epidemiology of salmonella. Epidemiol Rev. 1989;11:99–108. doi: 10.1093/oxfordjournals.epirev.a036047. [DOI] [PubMed] [Google Scholar]
- Taylor D. N., Wachsmuth I. K., Shangkuan Y. H., Schmidt E. V., Barrett T. J., Schrader J. S., Scherach C. S., McGee H. B., Feldman R. A., Brenner D. J. Salmonellosis associated with marijuana: a multistate outbreak traced by plasmid fingerprinting. N Engl J Med. 1982 May 27;306(21):1249–1253. doi: 10.1056/NEJM198205273062101. [DOI] [PubMed] [Google Scholar]
- Thong K. L., Cheong Y. M., Puthucheary S., Koh C. L., Pang T. Epidemiologic analysis of sporadic Salmonella typhi isolates and those from outbreaks by pulsed-field gel electrophoresis. J Clin Microbiol. 1994 May;32(5):1135–1141. doi: 10.1128/jcm.32.5.1135-1141.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Threlfall E. J., Frost J. A. The identification, typing and fingerprinting of Salmonella: laboratory aspects and epidemiological applications. J Appl Bacteriol. 1990 Jan;68(1):5–16. doi: 10.1111/j.1365-2672.1990.tb02542.x. [DOI] [PubMed] [Google Scholar]
- Wachsmuth K. Molecular epidemiology of bacterial infections: examples of methodology and of investigations of outbreaks. Rev Infect Dis. 1986 Sep-Oct;8(5):682–692. doi: 10.1093/clinids/8.5.682. [DOI] [PubMed] [Google Scholar]