Abstract
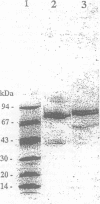
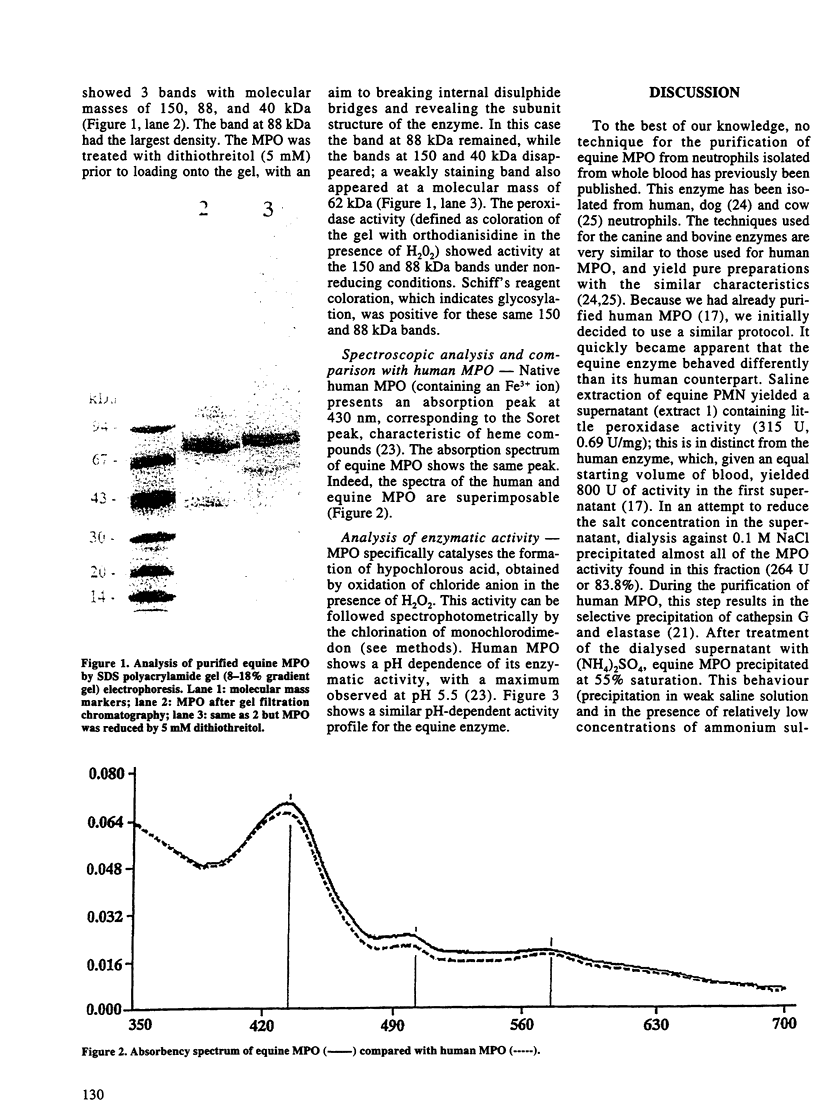
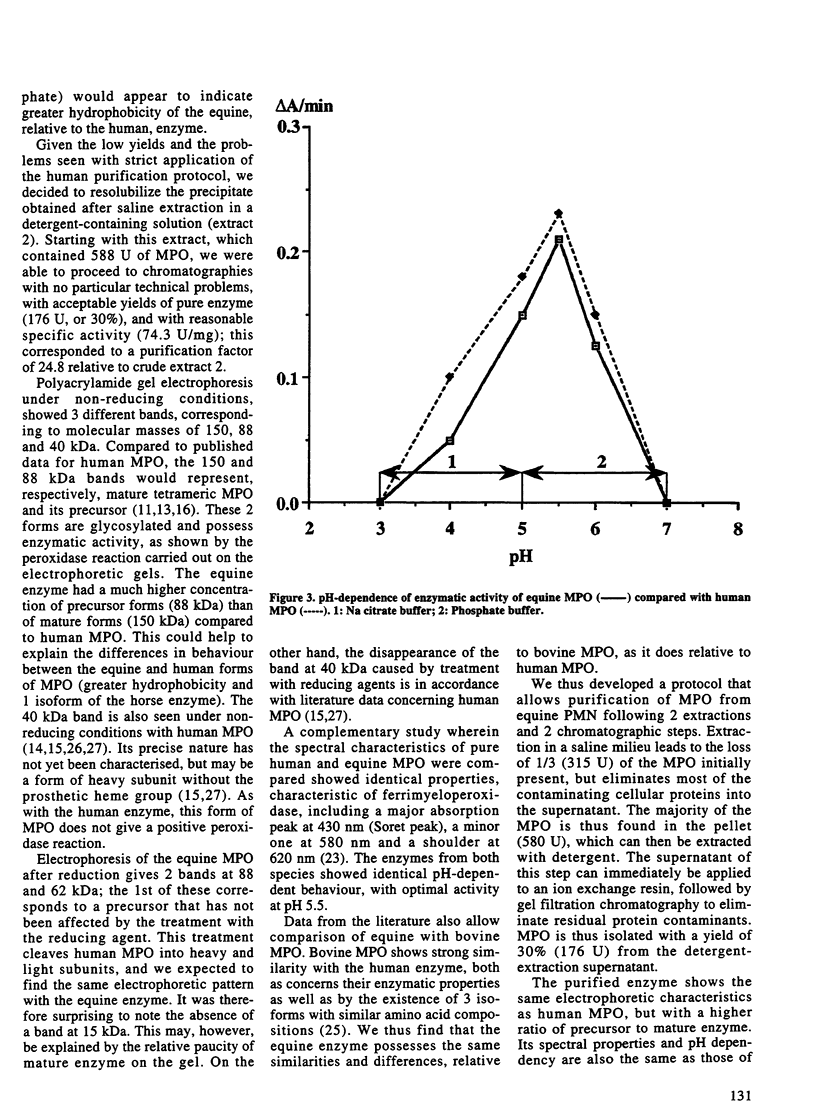
Increases of plasma concentrations of neutrophil myeloperoxidase (MPO) can be used as markers of polymorphonuclear leucocytes (PMN) activation in pathological situations (sepsis, acute lung injury, acute inflammation). To develop an assay for measurement of plasma MPO in horses during the above-mentioned infectious and inflammatory conditions, MPO was purified from equine PMN isolated from blood anticoagulated with citrate. PMN were extracted in a saline milieu (0.2 M Na acetate, 1 M NaCl, pH 4.7) to eliminate most of cellular proteins. Pellets were then extracted in the same buffer containing cationic detergent (1% cetyltrimethyl ammonium bromide). The supernatant was further purified by ion exchange chromatography (Hiload S Sepharose HP column 0.5 x 26 cm, equilibrated with 25 mM Na acetate, 0.2 M NaCl, pH 4.7) with a NaCl gradient (until 1 M). Most of the peroxidase activity of MPO (spectrophotometrically measured by the oxidation of orthodianisidine by hydrogen peroxide) was eluted at 0.65 M NaCl. MPO was further purified by gel filtration chromatography (Sephacryl S 200 column 2.6 x 42 cm with 25 mM Na acetate, 0.2 M NaCl, pH 4.7). MPO (specific activity: 74.3 U/mg) was obtained with a yield of 30% from the detergent extraction supernatant. Electrophoresis (non-reducing conditions) showed 3 bands identified, by comparison with human MPO, (i) the mature tetrameric enzyme (150 kDa) with 2 light and 2 heavy subunits, (ii) the precursor form (88 kDa) and (iii) a form of the heavy subunit without the prosthetic heme group (40 kDa). The mature enzyme and its precursor were glycosylated and possessed peroxidase activity. Equine MPO showed strong similarities with human and bovine MPO, with an absorption peak at 430 nm (Soret peak) characteristic of ferrimyeloperoxidase. Enzymatic activity was pH dependent (optimal value at pH 5.5).
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen M. R., Atkin C. L., Eyre H. J. Intact form of myeloperoxidase from normal human neutrophils. Arch Biochem Biophys. 1982 Mar;214(1):273–283. doi: 10.1016/0003-9861(82)90031-5. [DOI] [PubMed] [Google Scholar]
- Andrews P. C., Krinsky N. I. The reductive cleavage of myeloperoxidase in half, producing enzymically active hemi-myeloperoxidase. J Biol Chem. 1981 May 10;256(9):4211–4218. [PubMed] [Google Scholar]
- Bakkenist A. R., Wever R., Vulsma T., Plat H., van Gelder B. F. Isolation procedure and some properties of myeloperoxidase from human leucocytes. Biochim Biophys Acta. 1978 May 11;524(1):45–54. doi: 10.1016/0005-2744(78)90101-8. [DOI] [PubMed] [Google Scholar]
- Cooray R., Petersson C. G., Holmberg O. Isolation and purification of bovine myeloperoxidase from neutrophil granules. Vet Immunol Immunopathol. 1993 Oct;38(3-4):261–272. doi: 10.1016/0165-2427(93)90086-j. [DOI] [PubMed] [Google Scholar]
- Faymonville M. E., Pincemail J., Duchateau J., Paulus J. M., Adam A., Deby-Dupont G., Deby C., Albert A., Larbuisson R., Limet R. Myeloperoxidase and elastase as markers of leukocyte activation during cardiopulmonary bypass in humans. J Thorac Cardiovasc Surg. 1991 Aug;102(2):309–317. [PubMed] [Google Scholar]
- Fujishima S., Aikawa N. Neutrophil-mediated tissue injury and its modulation. Intensive Care Med. 1995 Mar;21(3):277–285. doi: 10.1007/BF01701489. [DOI] [PubMed] [Google Scholar]
- Green E. M., Adams H. R. New perspectives in circulatory shock: pathophysiologic mediators of the mammalian response to endotoxemia and sepsis. J Am Vet Med Assoc. 1992 Jun 15;200(12):1834–1841. [PubMed] [Google Scholar]
- Hager L. P., Morris D. R., Brown F. S., Eberwein H. Chloroperoxidase. II. Utilization of halogen anions. J Biol Chem. 1966 Apr 25;241(8):1769–1777. [PubMed] [Google Scholar]
- Harrison J. E., Pabalan S., Schultz J. The subunit structure of crystalline canine myeloperoxidase. Biochim Biophys Acta. 1977 Aug 23;493(2):247–259. doi: 10.1016/0005-2795(77)90181-7. [DOI] [PubMed] [Google Scholar]
- Jacquet A., Deby C., Mathy M., Moguilevsky N., Deby-Dupont G., Thirion A., Goormaghtigh E., Garcia-Quintana L., Bollen A., Pincemail J. Spectral and enzymatic properties of human recombinant myeloperoxidase: comparison with the mature enzyme. Arch Biochem Biophys. 1991 Nov 15;291(1):132–138. doi: 10.1016/0003-9861(91)90115-y. [DOI] [PubMed] [Google Scholar]
- Klebanoff S. J., Hamon C. B. Role of myeloperoxidase-mediated antimicrobial systems in intact leukocytes. J Reticuloendothel Soc. 1972 Aug;12(2):170–196. [PubMed] [Google Scholar]
- Matheson N. R., Wong P. S., Travis J. Isolation and properties of human neutrophil myeloperoxidase. Biochemistry. 1981 Jan 20;20(2):325–330. doi: 10.1021/bi00505a015. [DOI] [PubMed] [Google Scholar]
- Mathy-Hartert M., Deby-Dupont G., Melin P., Lamy M., Deby C. Bactericidal activity against Pseudomonas aeruginosa is acquired by cultured human monocyte-derived macrophages after uptake of myeloperoxidase. Experientia. 1996 Feb 15;52(2):167–174. doi: 10.1007/BF01923364. [DOI] [PubMed] [Google Scholar]
- Miyasaki K. T., Wilson M. E., Cohen E., Jones P. C., Genco R. J. Evidence for and partial characterization of three major and three minor chromatographic forms of human neutrophil myeloperoxidase. Arch Biochem Biophys. 1986 May 1;246(2):751–764. doi: 10.1016/0003-9861(86)90332-2. [DOI] [PubMed] [Google Scholar]
- Moore J. N., Morris D. D. Endotoxemia and septicemia in horses: experimental and clinical correlates. J Am Vet Med Assoc. 1992 Jun 15;200(12):1903–1914. [PubMed] [Google Scholar]
- Nauseef W. M., Root R. K., Malech H. L. Biochemical and immunologic analysis of hereditary myeloperoxidase deficiency. J Clin Invest. 1983 May;71(5):1297–1307. doi: 10.1172/JCI110880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen R. L., Little C. Studies on the subunits of human myeloperoxidase. Biochem J. 1984 Sep 15;222(3):701–709. doi: 10.1042/bj2220701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pember S. O., Fuhrer-Krüsi S. M., Barnes K. C., Kinkade J. M., Jr Isolation of three native forms of myeloperoxidase from human polymorphonuclear leukocytes. FEBS Lett. 1982 Apr 5;140(1):103–108. doi: 10.1016/0014-5793(82)80530-9. [DOI] [PubMed] [Google Scholar]
- Pember S. O., Shapira R., Kinkade J. M., Jr Multiple forms of myeloperoxidase from human neutrophilic granulocytes: evidence for differences in compartmentalization, enzymatic activity, and subunit structure. Arch Biochem Biophys. 1983 Mar;221(2):391–403. doi: 10.1016/0003-9861(83)90158-3. [DOI] [PubMed] [Google Scholar]
- Seahorn T. L., Gaunt S. D., Berry C. Blood cell deformability in horses with intestinal colic. Am J Vet Res. 1994 Mar;55(3):321–324. [PubMed] [Google Scholar]
- Weiland J. E., Davis W. B., Holter J. F., Mohammed J. R., Dorinsky P. M., Gadek J. E. Lung neutrophils in the adult respiratory distress syndrome. Clinical and pathophysiologic significance. Am Rev Respir Dis. 1986 Feb;133(2):218–225. doi: 10.1164/arrd.1986.133.2.218. [DOI] [PubMed] [Google Scholar]
- Weiss S. J. Tissue destruction by neutrophils. N Engl J Med. 1989 Feb 9;320(6):365–376. doi: 10.1056/NEJM198902093200606. [DOI] [PubMed] [Google Scholar]