Abstract
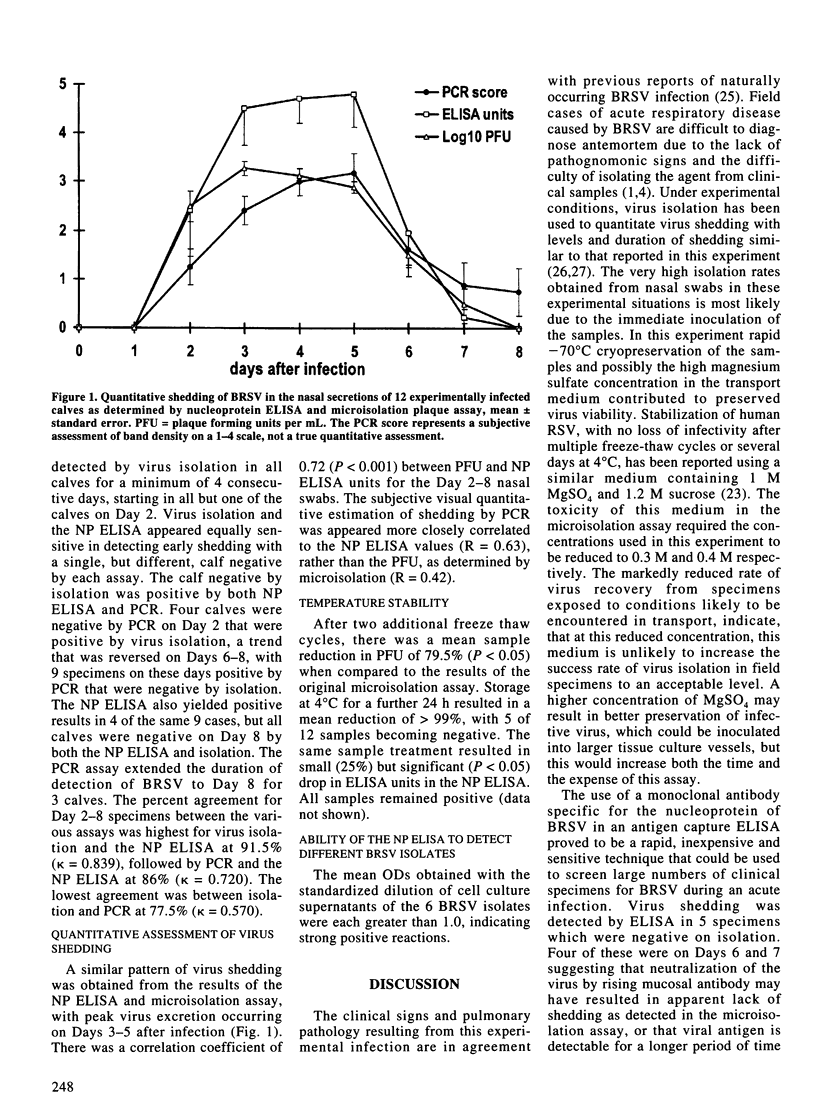
Virus shedding was monitored in nasal secretions of 12 calves experimentally infected with bovine respiratory syncytial virus (BRSV) using an antigen capture enzyme-linked immunosorbent assay (ELISA) detecting the nucleoprotein (NP) antigen of BRSV, by a polymerase chain reaction (PCR) amplifying the fusion protein of BRSV, and by a microisolation assay combined with immunoperoxidase staining for the F protein of BRSV. Under the conditions of this study, similar limits of detection and quantitative results were obtained from all three assays. BRSV was detected in nasal secretions of all calves for a minimum of 4 d. Virus shedding began on Day 2 after infection, peaked on Days 3-5, and was cleared in most calves by Day 8. The PCR, and to a lesser extent the ELISA, may detect virus shedding for a longer period after infection than virus isolation, possibly due to neutralization of the virus by rising mucosal antibody. Simulated environmental conditions likely to be experienced during transport of clinical field specimens markedly reduced the sensitivity of virus isolation but had a minimal effect on the results of the NP ELISA. Actual field transport conditions (overnight on ice) had minimal apparent effect on the results of the PCR assay. The less stringent specimen handling requirements, combined with low limits of detection, of both the nucleoprotein ELISA and PCR, indicate either of these assays are more suitable for diagnostic applications than virus isolation.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker J. C., Frey M. L. Bovine respiratory syncytial virus. Vet Clin North Am Food Anim Pract. 1985 Jul;1(2):259–275. [PubMed] [Google Scholar]
- Baker J. C., Werdin R. E., Ames T. R., Markham R. J., Larson V. L. Study on the etiologic role of bovine respiratory syncytial virus in pneumonia of dairy calves. J Am Vet Med Assoc. 1986 Jul 1;189(1):66–70. [PubMed] [Google Scholar]
- Baker J. C., Wilson E. G., McKay G. L., Stanek R. J., Underwood W. J., Velicer L. F., Mufson M. A. Identification of subgroups of bovine respiratory syncytial virus. J Clin Microbiol. 1992 May;30(5):1120–1126. doi: 10.1128/jcm.30.5.1120-1126.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeler J. A., van Wyke Coelingh K. Neutralization epitopes of the F glycoprotein of respiratory syncytial virus: effect of mutation upon fusion function. J Virol. 1989 Jul;63(7):2941–2950. doi: 10.1128/jvi.63.7.2941-2950.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belknap E. B., Baker J. C., Patterson J. S., Walker R. D., Haines D. M., Clark E. G. The role of passive immunity in bovine respiratory syncytial virus-infected calves. J Infect Dis. 1991 Mar;163(3):470–476. doi: 10.1093/infdis/163.3.470. [DOI] [PubMed] [Google Scholar]
- Fernie B. F., Gerin J. L. The stabilization and purification of respiratory syncytial virus using MgSO4. Virology. 1980 Oct 15;106(1):141–144. doi: 10.1016/0042-6822(80)90229-9. [DOI] [PubMed] [Google Scholar]
- Haines D. M., Clark E. G., Chelack B. J. The detection of bovine respiratory syncytial virus in formalin fixed bovine lung with commercially available monoclonal antibodies and avidin biotin complex immunohistochemistry. Can J Vet Res. 1989 Jul;53(3):366–368. [PMC free article] [PubMed] [Google Scholar]
- Hamel A. L., Wasylyshen M. D., Nayar G. P. Rapid detection of bovine viral diarrhea virus by using RNA extracted directly from assorted specimens and a one-tube reverse transcription PCR assay. J Clin Microbiol. 1995 Feb;33(2):287–291. doi: 10.1128/jcm.33.2.287-291.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimman T. G., Straver P. J., Zimmer G. M. Pathogenesis of naturally acquired bovine respiratory syncytial virus infection in calves: morphologic and serologic findings. Am J Vet Res. 1989 May;50(5):684–693. [PubMed] [Google Scholar]
- Kimman T. G., Westenbrink F., Straver P. J. Priming for local and systemic antibody memory responses to bovine respiratory syncytial virus: effect of amount of virus, virus replication, route of administration and maternal antibodies. Vet Immunol Immunopathol. 1989 Sep;22(2):145–160. doi: 10.1016/0165-2427(89)90057-3. [DOI] [PubMed] [Google Scholar]
- Kimman T. G., Zimmer G. M., Straver P. J., de Leeuw P. W. Diagnosis of bovine respiratory syncytial virus infections improved by virus detection in lung lavage samples. Am J Vet Res. 1986 Jan;47(1):143–147. [PubMed] [Google Scholar]
- Lehmkuhl H. D., Gough P. M., Reed D. E. Characterization and identification of a bovine respiratory syncytial virus isolated from young calves. Am J Vet Res. 1979 Jan;40(1):124–126. [PubMed] [Google Scholar]
- Lerch R. A., Stott E. J., Wertz G. W. Characterization of bovine respiratory syncytial virus proteins and mRNAs and generation of cDNA clones to the viral mRNAs. J Virol. 1989 Feb;63(2):833–840. doi: 10.1128/jvi.63.2.833-840.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malvano R., Boniolo A., Dovis M., Zannino M. ELISA for antibody measurement: aspects related to data expression. J Immunol Methods. 1982;48(1):51–60. doi: 10.1016/0022-1759(82)90209-5. [DOI] [PubMed] [Google Scholar]
- McIntosh K., Hendry R. M., Fahnestock M. L., Pierik L. T. Enzyme-linked immunosorbent assay for detection of respiratory syncytial virus infection: application to clinical samples. J Clin Microbiol. 1982 Aug;16(2):329–333. doi: 10.1128/jcm.16.2.329-333.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohanty S. B., Ingling A. L., Lillie M. G. Experimentally induced respiratory syncytial viral infection in calves. Am J Vet Res. 1975 Apr;36(4 PT1):417–419. [PubMed] [Google Scholar]
- Oberst R. D., Hays M. P., Evermann J. F., Kelling C. L. Characteristic differences in reverse transcription-polymerase chain reaction products of ovine, bovine, and human respiratory syncytial viruses. J Vet Diagn Invest. 1993 Jul;5(3):322–328. doi: 10.1177/104063879300500303. [DOI] [PubMed] [Google Scholar]
- Oberst R. D., Hays M. P., Hennessy K. J., Stine L. C., Evermann J. F., Kelling C. L. Identifying bovine respiratory syncytial virus by reverse transcription-polymerase chain reaction and oligonucleotide hybridizations. J Clin Microbiol. 1993 May;31(5):1237–1240. doi: 10.1128/jcm.31.5.1237-1240.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osorio F. A., Anderson G. A., Sanders J., Grotelueschen D. Detection of bovine respiratory syncytial virus using a heterologous antigen-capture enzyme immunoassay. J Vet Diagn Invest. 1989 Jul;1(3):210–214. doi: 10.1177/104063878900100302. [DOI] [PubMed] [Google Scholar]
- Stott E. J., Bew M. H., Taylor G., Jebbett J., Collins A. P. The characterization and uses of monoclonal antibodies to respiratory syncytial virus. Dev Biol Stand. 1984;57:237–244. [PubMed] [Google Scholar]
- Taylor G., Stott E. J., Furze J., Ford J., Sopp P. Protective epitopes on the fusion protein of respiratory syncytial virus recognized by murine and bovine monoclonal antibodies. J Gen Virol. 1992 Sep;73(Pt 9):2217–2223. doi: 10.1099/0022-1317-73-9-2217. [DOI] [PubMed] [Google Scholar]
- Taylor G., Thomas L. H., Wyld S. G., Furze J., Sopp P., Howard C. J. Role of T-lymphocyte subsets in recovery from respiratory syncytial virus infection in calves. J Virol. 1995 Nov;69(11):6658–6664. doi: 10.1128/jvi.69.11.6658-6664.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilcek S., Elvander M., Ballagi-Pordány A., Belák S. Development of nested PCR assays for detection of bovine respiratory syncytial virus in clinical samples. J Clin Microbiol. 1994 Sep;32(9):2225–2231. doi: 10.1128/jcm.32.9.2225-2231.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westenbrink F., Kimman T. G. Immunoglobulin M-specific enzyme-linked immunosorbent assay for serodiagnosis of bovine respiratory syncytial virus infections. Am J Vet Res. 1987 Jul;48(7):1132–1137. [PubMed] [Google Scholar]


