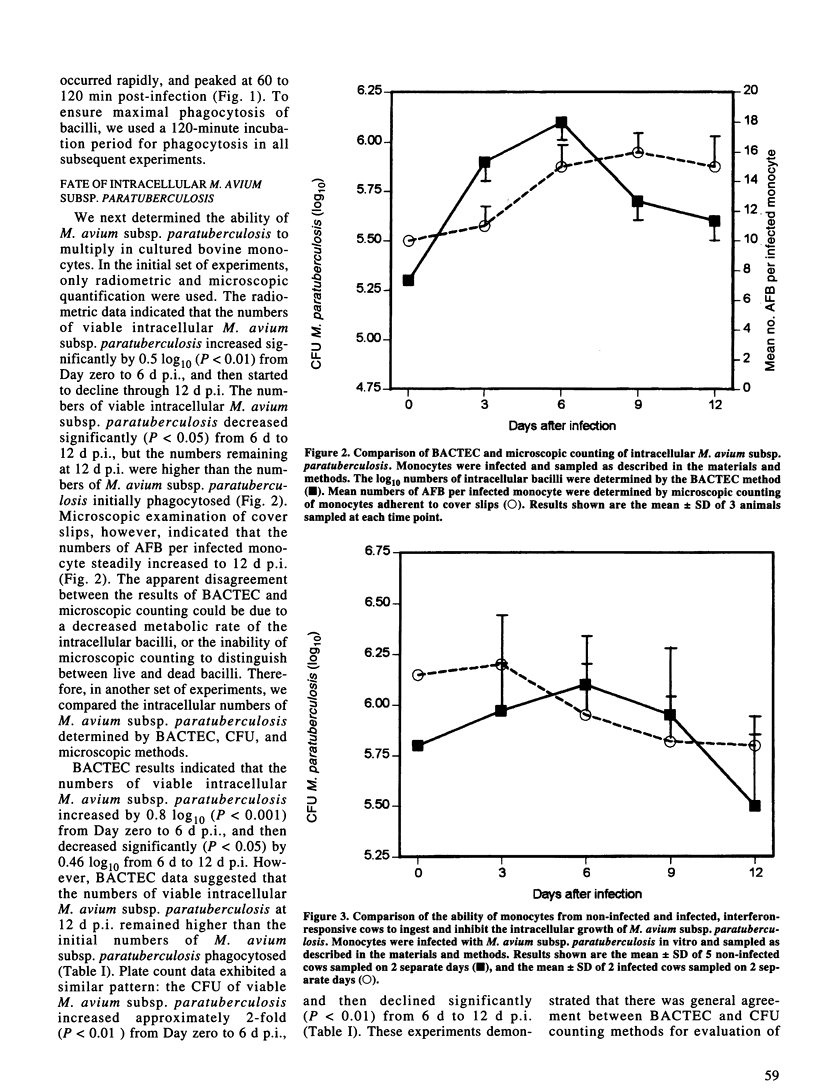
Abstract
The ability of Mycobacterium avium subsp. paratuberculosis to survive in bovine monocytes was studied using radiometric (BACTEC) culture, standard plate counting and microscopic counting of acid-fast stained monocyte monolayers. Results of microscopic counts sharply contrasted with results of viable counts determined both by plate counting and radiometric counting. We observed an early phase (the first 6 d after in vitro infection) of intracellular bacillary growth, followed by a later phase of mycobacteriostasis or killing (up to 12 d after in vitro infection) in monocytes from non-infected cows. The data suggest that multiplication and death of M. avium subsp. paratuberculosis occur simultaneously in bovine monocytes infected in vitro. Using the BACTEC method, we compared the ability of bovine monocytes from normal cows and cows infected with M. avium subsp. paratuberculosis and showing evidence of a strong Thl-like cellular immune response to ingest and inhibit the intracellular growth of M. avium subsp. paratuberculosis. There was a trend toward greater phagocytosis and faster killing of Mycobacterium avium subsp. paratuberculosis by monocytes from the infected, immune responder cows. However, the observed numbers of viable M. avium subsp. paratuberculosis at each time after monocyte infection were not significantly different between normal and infected cows.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. L., Collins M. T., Czuprynski C. J. Polymerase chain reaction analysis of TNF-alpha and IL-6 mRNA levels in whole blood from cattle naturally or experimentally infected with Mycobacterium paratuberculosis. Can J Vet Res. 1996 Oct;60(4):257–262. [PMC free article] [PubMed] [Google Scholar]
- Bendixen P. H., Bloch B., Jorgensen J. B. Lack of intracellular degradation of Mycobacterium paratuberculosis by bovine macrophages infected in vitro and in vivo: light microscopic and electron microscopic observations. Am J Vet Res. 1981 Jan;42(1):109–113. [PubMed] [Google Scholar]
- Chan J., Fujiwara T., Brennan P., McNeil M., Turco S. J., Sibille J. C., Snapper M., Aisen P., Bloom B. R. Microbial glycolipids: possible virulence factors that scavenge oxygen radicals. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2453–2457. doi: 10.1073/pnas.86.7.2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiodini R. J., Van Kruiningen H. J., Merkal R. S. Ruminant paratuberculosis (Johne's disease): the current status and future prospects. Cornell Vet. 1984 Jul;74(3):218–262. [PubMed] [Google Scholar]
- Clemens D. L., Horwitz M. A. Characterization of the Mycobacterium tuberculosis phagosome and evidence that phagosomal maturation is inhibited. J Exp Med. 1995 Jan 1;181(1):257–270. doi: 10.1084/jem.181.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins M. T., Sockett D. C., Goodger W. J., Conrad T. A., Thomas C. B., Carr D. J. Herd prevalence and geographic distribution of, and risk factors for, bovine paratuberculosis in Wisconsin. J Am Vet Med Assoc. 1994 Feb 15;204(4):636–641. [PubMed] [Google Scholar]
- Lambrecht R. S., Carriere J. F., Collins M. T. A model for analyzing growth kinetics of a slowly growing Mycobacterium sp. Appl Environ Microbiol. 1988 Apr;54(4):910–916. doi: 10.1128/aem.54.4.910-916.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonough K. A., Kress Y., Bloom B. R. The interaction of Mycobacterium tuberculosis with macrophages: a study of phagolysosome fusion. Infect Agents Dis. 1993 Aug;2(4):232–235. [PubMed] [Google Scholar]
- Reddy M. V., Srinivasan S., Andersen B., Gangadharam P. R. Rapid assessment of mycobacterial growth inside macrophages and mice, using the radiometric (BACTEC) method. Tuber Lung Dis. 1994 Apr;75(2):127–131. doi: 10.1016/0962-8479(94)90041-8. [DOI] [PubMed] [Google Scholar]
- Saxegaard F., Baess I. Relationship between Mycobacterium avium, Mycobacterium paratuberculosis and "wood pigeon mycobacteria". Determinations by DNA-DNA hybridization. APMIS. 1988 Jan;96(1):37–42. [PubMed] [Google Scholar]
- Sturgill-Koszycki S., Schlesinger P. H., Chakraborty P., Haddix P. L., Collins H. L., Fok A. K., Allen R. D., Gluck S. L., Heuser J., Russell D. G. Lack of acidification in Mycobacterium phagosomes produced by exclusion of the vesicular proton-ATPase. Science. 1994 Feb 4;263(5147):678–681. doi: 10.1126/science.8303277. [DOI] [PubMed] [Google Scholar]
- Thorel M. F., Krichevsky M., Lévy-Frébault V. V. Numerical taxonomy of mycobactin-dependent mycobacteria, emended description of Mycobacterium avium, and description of Mycobacterium avium subsp. avium subsp. nov., Mycobacterium avium subsp. paratuberculosis subsp. nov., and Mycobacterium avium subsp. silvaticum subsp. nov. Int J Syst Bacteriol. 1990 Jul;40(3):254–260. doi: 10.1099/00207713-40-3-254. [DOI] [PubMed] [Google Scholar]
- Xu S., Cooper A., Sturgill-Koszycki S., van Heyningen T., Chatterjee D., Orme I., Allen P., Russell D. G. Intracellular trafficking in Mycobacterium tuberculosis and Mycobacterium avium-infected macrophages. J Immunol. 1994 Sep 15;153(6):2568–2578. [PubMed] [Google Scholar]
- Zurbrick B. G., Czuprynski C. J. Ingestion and intracellular growth of Mycobacterium paratuberculosis within bovine blood monocytes and monocyte-derived macrophages. Infect Immun. 1987 Jul;55(7):1588–1593. doi: 10.1128/iai.55.7.1588-1593.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]


