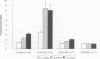Abstract
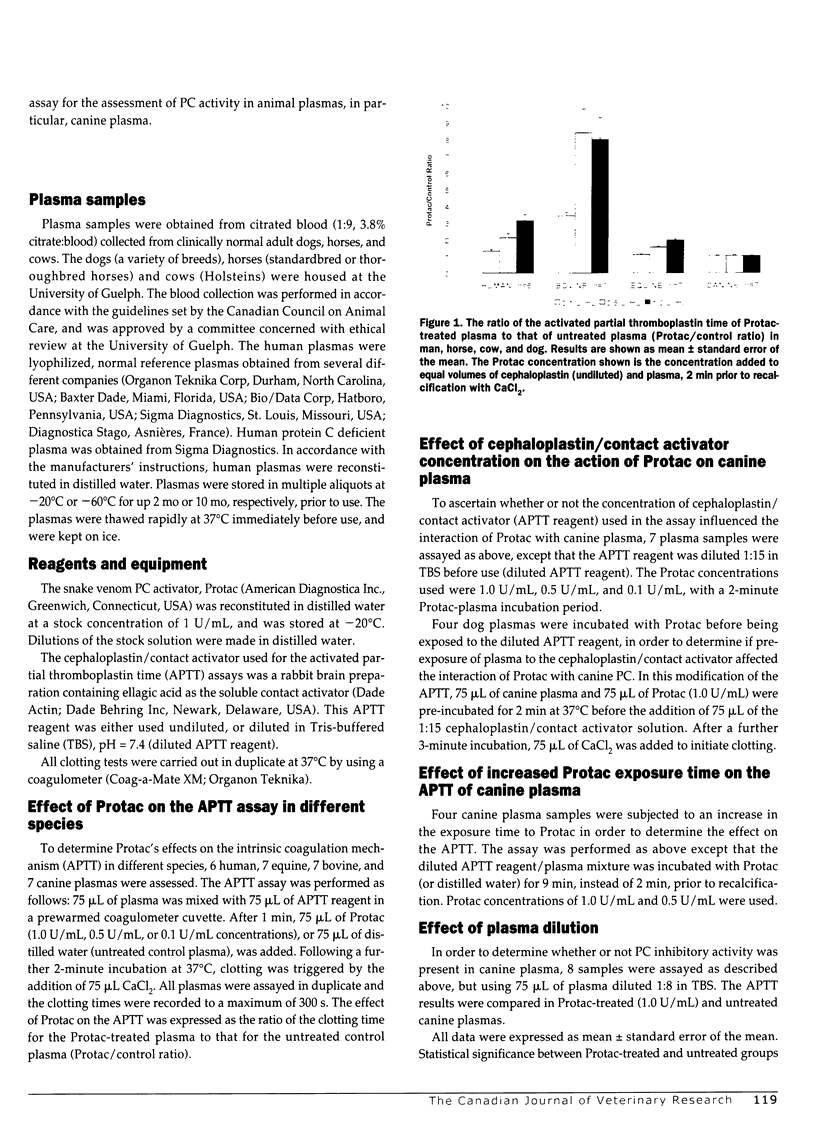
The commercial snake venom extract, Protac, is a specific activator of the anticoagulant zymogen, protein C (PC) in human plasma. This specific action has led to its use in developing coagulation-based and amidolytic-based assays for the diagnosis of quantitative and/or qualitative PC deficiency states in human beings. The purpose of the present study was to compare the effects of Protac on the activated partial thromboplastin times (APTT) of human, bovine, equine, and canine plasmas in order to determine the potential value of this venom extract as an activator in functional PC assays in these domestic animal species. As expected, Protac significantly prolonged the APTT of normal human plasma, but had no effect on plasma known to be devoid of PC. Clotting times were prolonged by 34%-214% with concentrations of venom activator ranging from 0.1-1.0 U/mL. Under identical conditions, Protac prolonged the APTT of equine plasma by 11%-98% over control times. Even more dramatic was the inhibitory effect of Protac on the clotting of bovine plasma, extending the APTT more than 3-fold at a venom concentration of 0.1 U/mL. At higher venom concentrations, most bovine plasmas remained unclotted after 300 s (control time 34.1 s). Under similar conditions, the canine APTT was unaffected by Protac, even when the venom concentration was increased to 3 U/mL. In order to determine the reason for the lack in response of canine plasma, the concentration of the APTT reagent was altered (decreased), exposure time of the plasma to the Protac was increased from 2 min to 9 min, and the plasma was diluted to assess for the potential existence of plasma PC inhibitors. Protac caused an unexpected shortening of the APTT when the contact activator reagent was diluted. Increasing the exposure time had no effect. Although a slight prolongation of the canine APTT was detected when the plasma was diluted, the presence of strong plasma PC inhibition was considered an unlikely cause of the lack of significant anticoagulant action. The failure of Protac to exert a strong inhibitory effect on the canine APTT, as well as to generate amidolytic activity, suggests that this venom extract does not stimulate the production of activated PC activity in canine plasma. This may result from molecular differences in the canine PC molecule that prevent the formation of the stoichiometric complex of venom extract, APTT reagent, and canine protein, a complex thought to be essential for the PC-activating function of Protac. Protac may be suitable as an activator of PC in bovine and equine plasmas; however, it appears ineffective in generating anticoagulant activity in canine plasma.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aiach M., Gandrille S. Molecular basis for protein C hereditary deficiency. Haemostasis. 1996 Oct;26 (Suppl 4):9–19. doi: 10.1159/000217280. [DOI] [PubMed] [Google Scholar]
- Barton M. H., Morris D. D., Crowe N., Collatos C., Prasse K. W. Hemostatic indices in healthy foals from birth to one month of age. J Vet Diagn Invest. 1995 Jul;7(3):380–385. doi: 10.1177/104063879500700314. [DOI] [PubMed] [Google Scholar]
- Comp P. C., Nixon R. R., Esmon C. T. Determination of functional levels of protein C, an antithrombotic protein, using thrombin-thrombomodulin complex. Blood. 1984 Jan;63(1):15–21. [PubMed] [Google Scholar]
- Dahlbäck B., Carlsson M., Svensson P. J. Familial thrombophilia due to a previously unrecognized mechanism characterized by poor anticoagulant response to activated protein C: prediction of a cofactor to activated protein C. Proc Natl Acad Sci U S A. 1993 Feb 1;90(3):1004–1008. doi: 10.1073/pnas.90.3.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edens L. M., Morris D. D., Prasse K. W., Anver M. R. Hypercoagulable state associated with a deficiency of protein C in a thoroughbred colt. J Vet Intern Med. 1993 May-Jun;7(3):190–193. doi: 10.1111/j.1939-1676.1993.tb03185.x. [DOI] [PubMed] [Google Scholar]
- Esmon C. T., Fukudome K. Cellular regulation of the protein C pathway. Semin Cell Biol. 1995 Oct;6(5):259–268. doi: 10.1006/scel.1995.0035. [DOI] [PubMed] [Google Scholar]
- Esmon C. T., Owen W. G. Identification of an endothelial cell cofactor for thrombin-catalyzed activation of protein C. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2249–2252. doi: 10.1073/pnas.78.4.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exner T., Vaasjoki R. Characterisation and some properties of the protein C activator from Agkistrodon Contortrix Contortrix venom. Thromb Haemost. 1988 Feb 25;59(1):40–44. [PubMed] [Google Scholar]
- Francis R. B., Jr, Seyfert U. Rapid amidolytic assay of protein C in whole plasma using an activator from the venom of Agkistrodon contortrix. Am J Clin Pathol. 1987 May;87(5):619–625. doi: 10.1093/ajcp/87.5.619. [DOI] [PubMed] [Google Scholar]
- Gable P. S., Le D. T., McGehee W., Rapaport S. I. A Protac-based screening test for activated protein C-resistant factor Va and other defects of the protein C anticoagulant pathway. Blood Coagul Fibrinolysis. 1997 Sep;8(6):327–335. doi: 10.1097/00001721-199709000-00002. [DOI] [PubMed] [Google Scholar]
- Heeb M. J., España F., Griffin J. H. Inhibition and complexation of activated protein C by two major inhibitors in plasma. Blood. 1989 Feb;73(2):446–454. [PubMed] [Google Scholar]
- Kraus M., Noah M., Fickenscher K. The PCAT--a simple screening assay for assessing the functionality of the protein C anticoagulant pathway. Thromb Res. 1995 Jul 15;79(2):217–222. doi: 10.1016/0049-3848(95)91525-p. [DOI] [PubMed] [Google Scholar]
- Leeb T., Kopp T., Deppe A., Breen M., Matis U., Brunnberg L., Brenig B. Molecular characterization and chromosomal assignment of the canine protein C gene. Mamm Genome. 1999 Feb;10(2):134–139. doi: 10.1007/s003359900958. [DOI] [PubMed] [Google Scholar]
- Madden R. M., Ward M., Marlar R. A. Protein C activity levels in endotoxin-induced disseminated intravascular coagulation in a dog model. Thromb Res. 1989 Aug 1;55(3):297–307. doi: 10.1016/0049-3848(89)90062-5. [DOI] [PubMed] [Google Scholar]
- Marlar R. A. Protein C in thromboembolic disease. Semin Thromb Hemost. 1985 Oct;11(4):387–393. doi: 10.1055/s-2007-1004399. [DOI] [PubMed] [Google Scholar]
- Pabinger I. Clinical relevance of protein C. Blut. 1986 Aug;53(2):63–75. doi: 10.1007/BF00321089. [DOI] [PubMed] [Google Scholar]
- Prasse K. W., Allen D., Jr, Moore J. N., Duncan A. Evaluation of coagulation and fibrinolysis during the prodromal stages of carbohydrate-induced acute laminitis in horses. Am J Vet Res. 1990 Dec;51(12):1950–1955. [PubMed] [Google Scholar]
- Ritt M. G., Rogers K. S., Thomas J. S. Nephrotic syndrome resulting in thromboembolic disease and disseminated intravascular coagulation in a dog. J Am Anim Hosp Assoc. 1997 Sep-Oct;33(5):385–391. doi: 10.5326/15473317-33-5-385. [DOI] [PubMed] [Google Scholar]
- Stocker K., Fischer H., Meier J., Brogli M., Svendsen L. Characterization of the protein C activator Protac from the venom of the southern copperhead (Agkistrodon contortrix) snake. Toxicon. 1987;25(3):239–252. doi: 10.1016/0041-0101(87)90253-4. [DOI] [PubMed] [Google Scholar]
- Stocker K., Fischer H., Meier J., Brogli M., Svendsen L. Protein C activators in snake venoms. Behring Inst Mitt. 1986 Feb;(79):37–47. [PubMed] [Google Scholar]
- Stocker K., Fischer H., Meier J. Practical application of the protein C activator Protac from Agkistrodon contortrix venom. Folia Haematol Int Mag Klin Morphol Blutforsch. 1988;115(3):260–264. [PubMed] [Google Scholar]
- Stürzebecher J., Neumann U., Meier J. Inhibition of the protein C activator Protac, a serine proteinase from the venom of the southern copperhead snake Agkistrodon contortrix contortrix. Toxicon. 1991;29(2):151–155. doi: 10.1016/0041-0101(91)90099-d. [DOI] [PubMed] [Google Scholar]
- Vasse M., Borg J. Y., Monconduit M. Protein C: Rouen, a new hereditary protein C abnormality with low anticoagulant but normal amidolytic activities. Thromb Res. 1989 Nov 1;56(3):387–398. doi: 10.1016/0049-3848(89)90251-x. [DOI] [PubMed] [Google Scholar]
- de Moerloose P., Reber G., Bouvier C. A. Spuriously low levels of protein C with a Protac activation clotting assay. Thromb Haemost. 1988 Jun 16;59(3):543–543. [PubMed] [Google Scholar]