Abstract
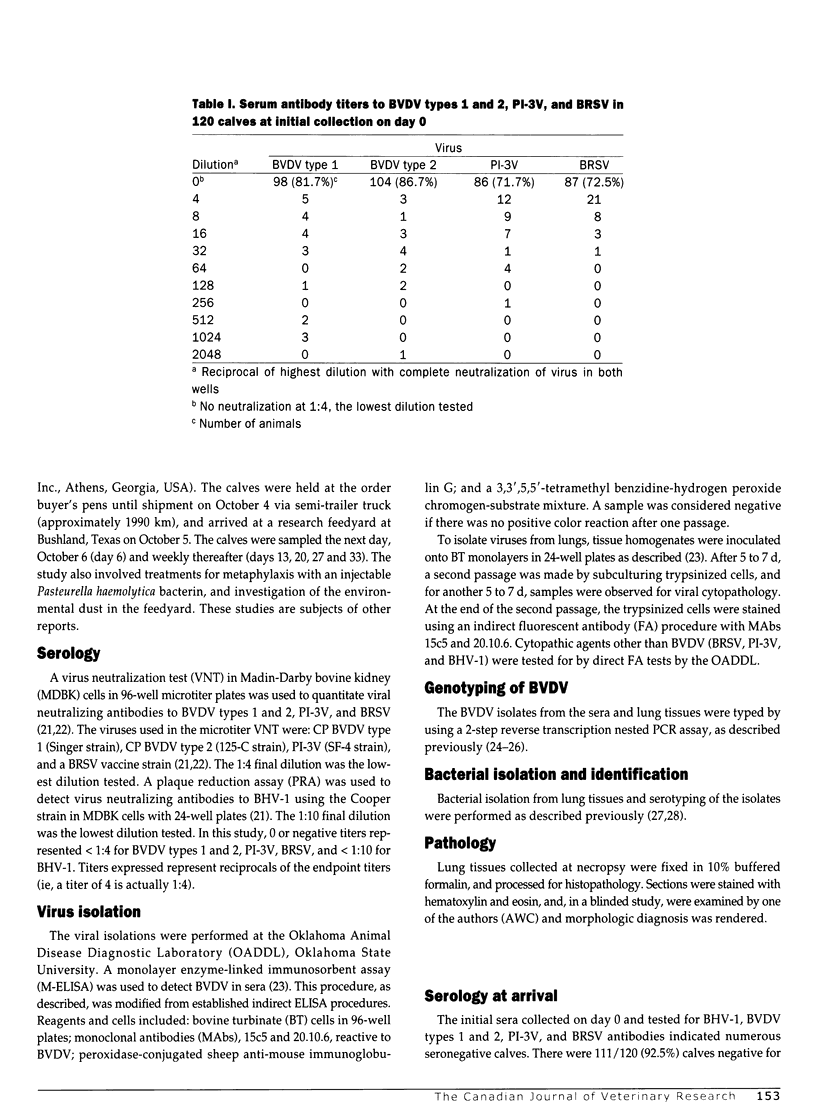
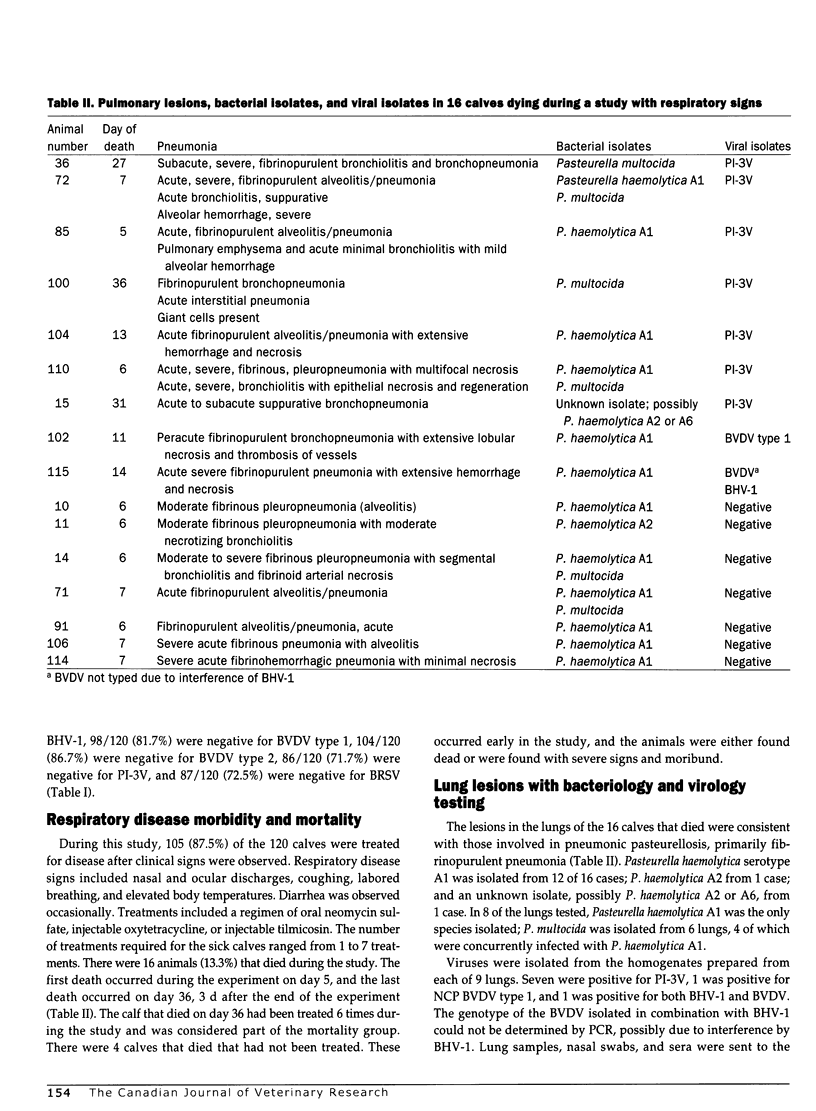
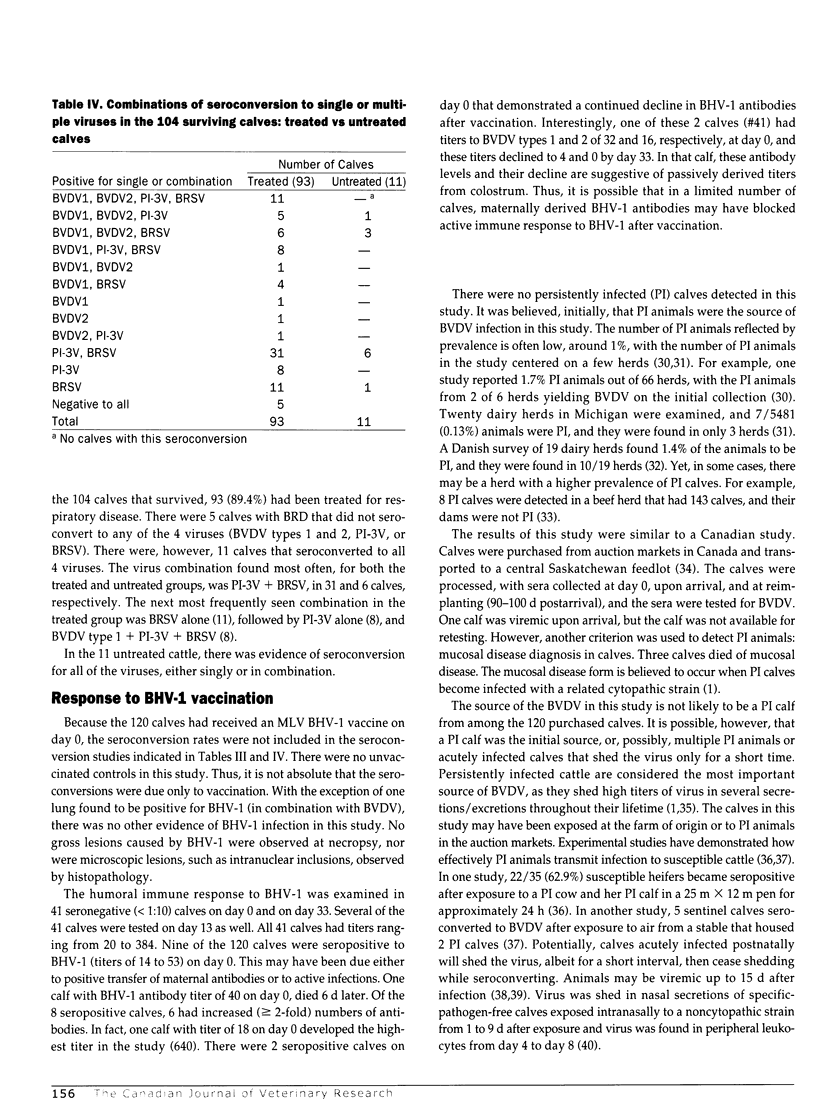
The prevalence of bovine viral diarrhea virus (BVDV) infections was determined in a group of stocker calves suffering from acute respiratory disease. The calves were assembled after purchase from Tennessee auctions and transported to western Texas. Of the 120 calves, 105 (87.5%) were treated for respiratory disease. Sixteen calves died during the study (13.3%). The calves received a modified live virus BHV-1 vaccine on day 0 of the study. During the study, approximately 5 wk in duration, sera from the cattle, collected at weekly intervals, were tested for BVDV by cell culture. Sera were also tested for neutralizing antibodies to BVDV types 1 and 2, bovine herpesvirus-1 (BHV-1), parainfluenza-3 virus (PI-3V), and bovine respiratory syncytial virus (BRSV). The lungs from the 16 calves that died during the study were collected and examined by histopathology, and lung homogenates were inoculated onto cell cultures for virus isolation. There were no calves persistently infected with BVDV detected in the study, as no animals were viremic on day 0, nor were any animals viremic at the 2 subsequent serum collections. There were, however, 4 animals with BVDV type 1 noncytopathic (NCP) strains in the sera from subsequent collections. Viruses were isolated from 9 lungs: 7 with PI-3V, 1 with NCP BVDV type 1, and 1 with both BVHV-1 and BVDV. The predominant bacterial species isolated from these lungs was Pasteurella haemolytica serotype 1. There was serologic evidence of infection with BVDV types 1 and 2, PI-3V, and BRSV, as noted by seroconversion (> or = 4-fold rise in antibody titer) in day 0 to day 34 samples collected from the 104 survivors: 40/104 (38.5%) to BVDV type 1; 29/104 (27.9%) to BVDV type 2; 71/104 (68.3%) to PI-3V; and 81/104 (77.9%) to BRSV. In several cases, the BVDV type 2 antibody titers may have been due to crossreacting BVDV type 1 antibodies; however, in 7 calves the BVDV type 2 antibodies were higher, indicating BVDV type 2 infection. At the outset of the study, the 120 calves were at risk (susceptible to viral infections) on day 0 because they were seronegative to the viruses: 98/120 (81.7%), < 1:4 to BVDV type 1; 104/120 (86.7%) < 1:4 to BVDV type 2; 86/120 (71.7%) < 1:4 to PI-3V; 87/120 (72.5%) < 1:4 to BRSV; and 111/120 (92.5%) < 1:10 to BHV-1. The results of this study indicate that BVDV types 1 and 2 are involved in acute respiratory disease of calves with pneumonic pasteurellosis. The BVDV may be detected by virus isolation from sera and/or lung tissues and by serology. The BVDV infections occurred in conjunction with infections by other viruses associated with respiratory disease, namely, PI-3V and BRSV. These other viruses may occur singly or in combination with each other. Also, the study indicates that purchased calves may be highly susceptible, after weaning, to infections by BHV-1, BVDV types 1 and 2, PI-3V, and BRSV early in the marketing channel.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen J. W., Viel L., Bateman K. G., Nagy E., Røsendal S., Shewen P. E. Serological titers to bovine herpesvirus 1, bovine viral diarrhea virus, parainfluenza 3 virus, bovine respiratory syncytial virus and Pasteurella haemolytica in feedlot calves with respiratory disease: associations with bacteriological and pulmonary cytological variables. Can J Vet Res. 1992 Oct;56(4):281–288. [PMC free article] [PubMed] [Google Scholar]
- Baker J. C. The clinical manifestations of bovine viral diarrhea infection. Vet Clin North Am Food Anim Pract. 1995 Nov;11(3):425–445. doi: 10.1016/s0749-0720(15)30460-6. [DOI] [PubMed] [Google Scholar]
- Bolin S. R., McClurkin A. W., Coria M. F. Frequency of persistent bovine viral diarrhea virus infection in selected cattle herds. Am J Vet Res. 1985 Nov;46(11):2385–2387. [PubMed] [Google Scholar]
- Bolin S. R., Ridpath J. F. Assessment of protection from systemic infection or disease afforded by low to intermediate titers of passively acquired neutralizing antibody against bovine viral diarrhea virus in calves. Am J Vet Res. 1995 Jun;56(6):755–759. [PubMed] [Google Scholar]
- Brownlie J., Clarke M. C., Howard C. J., Pocock D. H. Pathogenesis and epidemiology of bovine virus diarrhoea virus infection of cattle. Ann Rech Vet. 1987;18(2):157–166. [PubMed] [Google Scholar]
- Bruschke C. J., Weerdmeester K., Van Oirschot J. T., Van Rijn P. A. Distribution of bovine virus diarrhoea virus in tissues and white blood cells of cattle during acute infection. Vet Microbiol. 1998 Nov;64(1):23–32. doi: 10.1016/s0378-1135(98)00249-1. [DOI] [PubMed] [Google Scholar]
- Caldow G. L., Edwards S., Peters A. R., Nixon P., Ibata G., Sayers R. Associations between viral infections and respiratory disease in artificially reared calves. Vet Rec. 1993 Jul 24;133(4):85–89. doi: 10.1136/vr.133.4.85. [DOI] [PubMed] [Google Scholar]
- Carman S., van Dreumel T., Ridpath J., Hazlett M., Alves D., Dubovi E., Tremblay R., Bolin S., Godkin A., Anderson N. Severe acute bovine viral diarrhea in Ontario, 1993-1995. J Vet Diagn Invest. 1998 Jan;10(1):27–35. doi: 10.1177/104063879801000106. [DOI] [PubMed] [Google Scholar]
- Duffell S. J., Harkness J. W. Bovine virus diarrhoea-mucosal disease infection in cattle. Vet Rec. 1985 Sep 7;117(10):240–245. doi: 10.1136/vr.117.10.240. [DOI] [PubMed] [Google Scholar]
- Fulton R. W., Confer A. W., Burge L. J., Perino L. J., d'Offay J. M., Payton M. E., Mock R. E. Antibody responses by cattle after vaccination with commercial viral vaccines containing bovine herpesvirus-1, bovine viral diarrhea virus, parainfluenza-3 virus, and bovine respiratory syncytial virus immunogens and subsequent revaccination at day 140. Vaccine. 1995;13(8):725–733. doi: 10.1016/0264-410x(94)00072-u. [DOI] [PubMed] [Google Scholar]
- Fulton R. W., Saliki J. T., Burge L. J., d'Offay J. M., Bolin S. R., Maes R. K., Baker J. C., Frey M. L. Neutralizing antibodies to type 1 and 2 bovine viral diarrhea viruses: detection by inhibition of viral cytopathology and infectivity by immunoperoxidase assay. Clin Diagn Lab Immunol. 1997 May;4(3):380–383. doi: 10.1128/cdli.4.3.380-383.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulton R. W., Saliki J. T., Confer A. W., Burge L. J., d'Offay J. M., Helman R. G., Bolin S. R., Ridpath J. F., Payton M. E. Bovine viral diarrhea virus cytopathic and noncytopathic biotypes and type 1 and 2 genotypes in diagnostic laboratory accessions: clinical and necropsy samples from cattle. J Vet Diagn Invest. 2000 Jan;12(1):33–38. doi: 10.1177/104063870001200106. [DOI] [PubMed] [Google Scholar]
- Fulton R. W., d'Offay J. M., Saliki J. T., Burge L. J., Helman R. G., Confer A. W., Bolin S. R., Ridpath J. F. Nested reverse transcriptase-polymerase chain reaction (RT-PCR) for typing ruminant pestiviruses: bovine viral diarrhea viruses and border disease virus. Can J Vet Res. 1999 Oct;63(4):276–281. [PMC free article] [PubMed] [Google Scholar]
- Ganaba R., Bélanger D., Dea S., Bigras-Poulin M. A seroepidemiological study of the importance in cow-calf pairs of respiratory and enteric viruses in beef operations from northwestern Quebec. Can J Vet Res. 1995 Jan;59(1):26–33. [PMC free article] [PubMed] [Google Scholar]
- Houe H., Baker J. C., Maes R. K., Wuryastuti H., Wasito R., Ruegg P. L., Lloyd J. W. Prevalence of cattle persistently infected with bovine viral diarrhea virus in 20 dairy herds in two counties in central Michigan and comparison of prevalence of antibody-positive cattle among herds with different infection and vaccination status. J Vet Diagn Invest. 1995 Jul;7(3):321–326. doi: 10.1177/104063879500700304. [DOI] [PubMed] [Google Scholar]
- Houe H. Epidemiology of bovine viral diarrhea virus. Vet Clin North Am Food Anim Pract. 1995 Nov;11(3):521–547. doi: 10.1016/s0749-0720(15)30465-5. [DOI] [PubMed] [Google Scholar]
- Kelling C. L., Stine L. C., Rump K. K., Parker R. E., Kennedy J. E., Stone R. T., Ross G. S. Investigation of bovine viral diarrhea virus infections in a range beef cattle herd. J Am Vet Med Assoc. 1990 Sep 1;197(5):589–593. [PubMed] [Google Scholar]
- Lehmkuhl H. D., Gough P. M. Investigation of causative agents of bovine respiratory tract disease in a beef cow-calf herd with an early weaning program. Am J Vet Res. 1977 Nov;38(11):1717–1720. [PubMed] [Google Scholar]
- Mars M. H., Bruschke C. J., van Oirschot J. T. Airborne transmission of BHV1, BRSV, and BVDV among cattle is possible under experimental conditions. Vet Microbiol. 1999 Apr 19;66(3):197–207. doi: 10.1016/s0378-1135(99)00009-7. [DOI] [PubMed] [Google Scholar]
- Martin S. W., Bateman K. G., Shewen P. E., Rosendal S., Bohac J. G., Thorburn M. A group level analysis of the associations between antibodies to seven putative pathogens and respiratory disease and weight gain in Ontario feedlot calves. Can J Vet Res. 1990 Jun;54(3):337–342. [PMC free article] [PubMed] [Google Scholar]
- Martin S. W., Bohac J. G. The association between serological titers in infectious bovine rhinotracheitis virus, bovine virus diarrhea virus, parainfluenza-3 virus, respiratory syncytial virus and treatment for respiratory disease in Ontario feedlot calves. Can J Vet Res. 1986 Jul;50(3):351–358. [PMC free article] [PubMed] [Google Scholar]
- Martin S. W., Meek A. H., Davis D. G., Johnson J. A., Curtis R. A. Factors associated with mortality and treatment costs in feedlot calves: the Bruce County Beef Project, years 1978, 1979, 1980. Can J Comp Med. 1982 Oct;46(4):341–349. [PMC free article] [PubMed] [Google Scholar]
- McClurkin A. W., Littledike E. T., Cutlip R. C., Frank G. H., Coria M. F., Bolin S. R. Production of cattle immunotolerant to bovine viral diarrhea virus. Can J Comp Med. 1984 Apr;48(2):156–161. [PMC free article] [PubMed] [Google Scholar]
- McGowan M. R., Kirkland P. D., Richards S. G., Littlejohns I. R. Increased reproductive losses in cattle infected with bovine pestivirus around the time of insemination. Vet Rec. 1993 Jul 10;133(2):39–43. doi: 10.1136/vr.133.2.39. [DOI] [PubMed] [Google Scholar]
- Pellerin C., van den Hurk J., Lecomte J., Tijssen P. Identification of a new group of bovine viral diarrhea virus strains associated with severe outbreaks and high mortalities. Virology. 1994 Sep;203(2):260–268. doi: 10.1006/viro.1994.1483. [DOI] [PubMed] [Google Scholar]
- Potgieter L. N., McCracken M. D., Hopkins F. M., Guy J. S. Comparison of the pneumopathogenicity of two strains of bovine viral diarrhea virus. Am J Vet Res. 1985 Jan;46(1):151–153. [PubMed] [Google Scholar]
- Potgieter L. N., McCracken M. D., Hopkins F. M., Walker R. D. Effect of bovine viral diarrhea virus infection on the distribution of infectious bovine rhinotracheitis virus in calves. Am J Vet Res. 1984 Apr;45(4):687–690. [PubMed] [Google Scholar]
- Potgieter L. N., McCracken M. D., Hopkins F. M., Walker R. D., Guy J. S. Experimental production of bovine respiratory tract disease with bovine viral diarrhea virus. Am J Vet Res. 1984 Aug;45(8):1582–1585. [PubMed] [Google Scholar]
- Purdy C. W., Scanlan C. M., Loan R. W., Foster G. S. Identification of Pasteurella haemolytica A1 isolates from market-stressed feeder calves by use of enzyme and antimicrobial susceptibility profiles. Am J Vet Res. 1993 Jan;54(1):92–98. [PubMed] [Google Scholar]
- Purdy C. W., Straus D. C., Sutherland R. J., Ayres J. R. Efficacy of a subcutaneously administered, ultraviolet light-killed Pasteurella haemolytica A1-containing vaccine against transthoracic challenge exposure in goats. Am J Vet Res. 1996 Aug;57(8):1168–1174. [PubMed] [Google Scholar]
- Ridpath J. F., Bolin S. R., Dubovi E. J. Segregation of bovine viral diarrhea virus into genotypes. Virology. 1994 Nov 15;205(1):66–74. doi: 10.1006/viro.1994.1620. [DOI] [PubMed] [Google Scholar]
- Saliki J. T., Fulton R. W., Hull S. R., Dubovi E. J. Microtiter virus isolation and enzyme immunoassays for detection of bovine viral diarrhea virus in cattle serum. J Clin Microbiol. 1997 Apr;35(4):803–807. doi: 10.1128/jcm.35.4.803-807.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storz J., Purdy C. W., Lin X., Burrell M., Truax R. E., Briggs R. E., Frank G. H., Loan R. W. Isolation of respiratory bovine coronavirus, other cytocidal viruses, and Pasteurella spp from cattle involved in two natural outbreaks of shipping fever. J Am Vet Med Assoc. 2000 May 15;216(10):1599–1604. doi: 10.2460/javma.2000.216.1599. [DOI] [PubMed] [Google Scholar]
- Stott E. J., Thomas L. H., Collins A. P., Crouch S., Jebbett J., Smith G. S., Luther P. D., Caswell R. A survey of virus infections of the respiratory tract of cattle and their association with disease. J Hyg (Lond) 1980 Oct;85(2):257–270. doi: 10.1017/s0022172400063294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan D. G., Akkina R. K. A nested polymerase chain reaction assay to differentiate pestiviruses. Virus Res. 1995 Oct;38(2-3):231–239. doi: 10.1016/0168-1702(95)00065-x. [DOI] [PubMed] [Google Scholar]
- Taylor L. F., Van Donkersgoed J., Dubovi E. J., Harland R. J., van den Hurk J. V., Ribble C. S., Janzen E. D. The prevalence of bovine viral diarrhea virus infection in a population of feedlot calves in western Canada. Can J Vet Res. 1995 Apr;59(2):87–93. [PMC free article] [PubMed] [Google Scholar]


