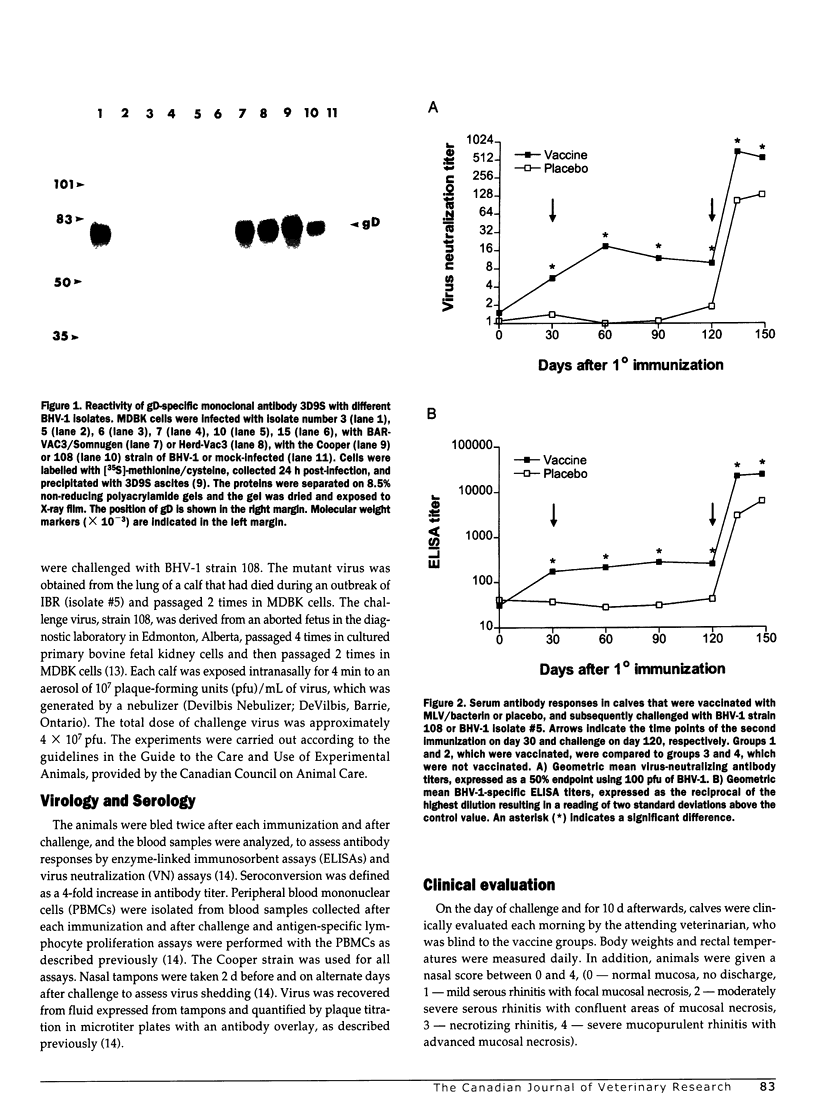
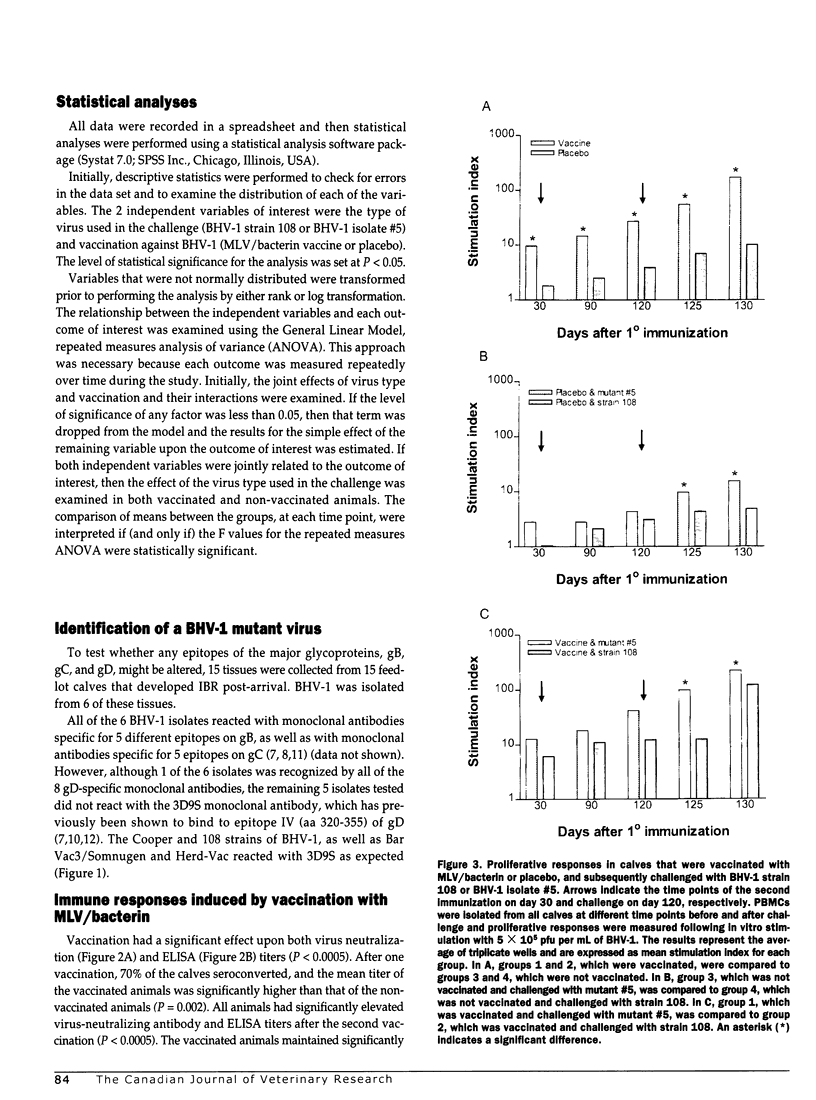
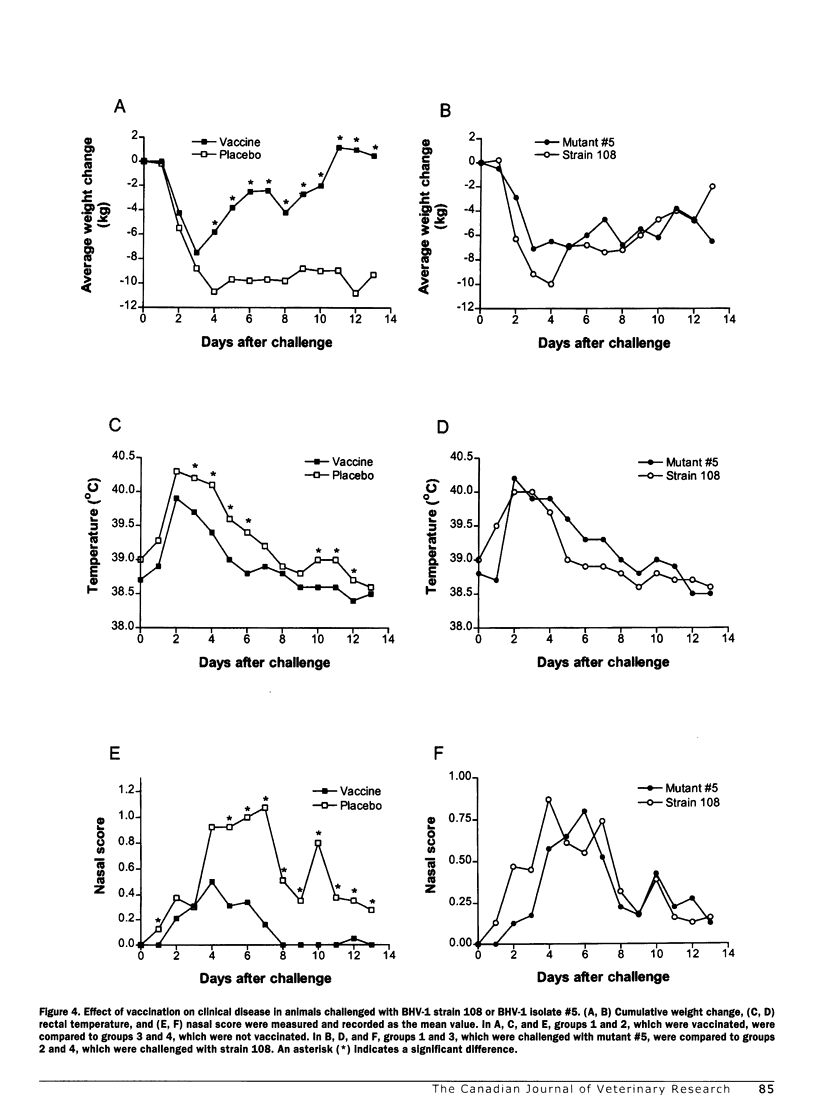
Abstract
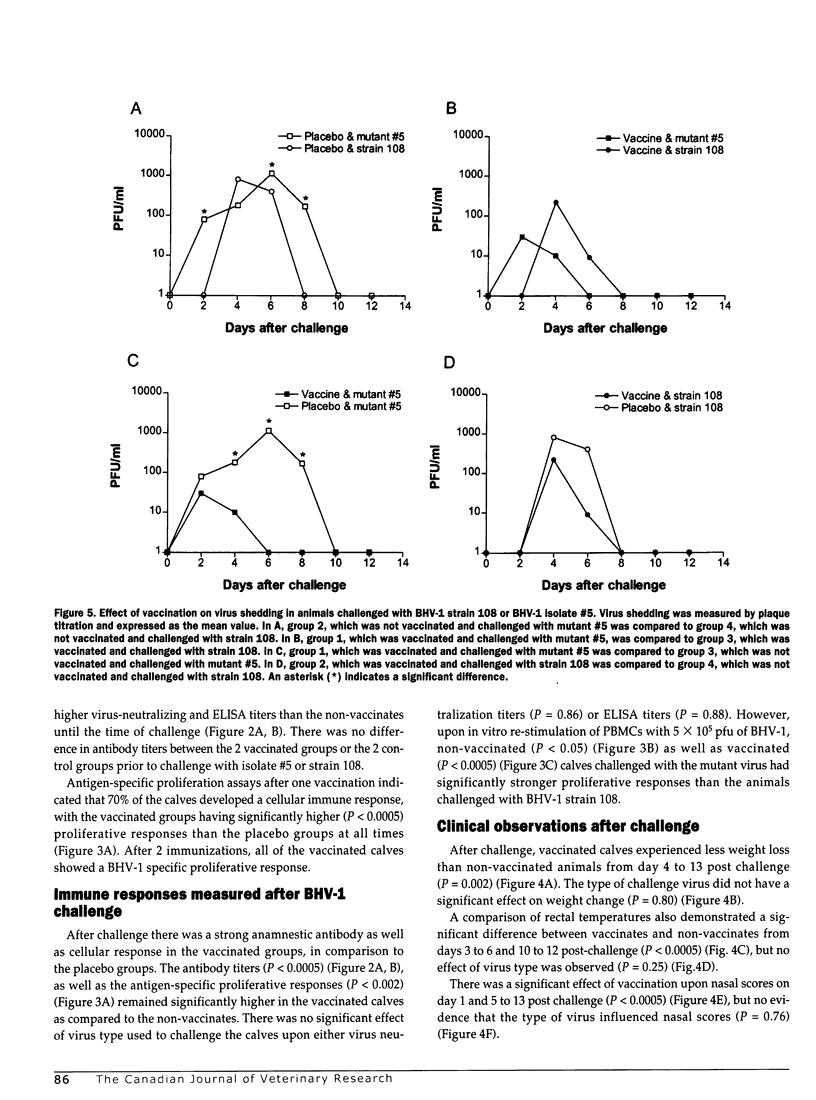
Outbreaks of infectious bovine rhinotracheitis (IBR) have recently been observed in vaccinated feedlot calves in Alberta a few months post-arrival. To investigate the cause of these outbreaks, lung and tracheal tissues were collected from calves that died of IBR during a post-arrival outbreak of disease. Bovine herpesvirus-1 (BHV-1), the causative agent of IBR, was isolated from 6 out of 15 tissues. Of these 6 isolates, 5 failed to react with a monoclonal antibody specific for one of the epitopes on glycoprotein D, one of the most important antigens of BHV-1. The ability of one of these mutant BHV-1 isolates to cause disease in calves vaccinated with a modified-live IBR vaccine was assessed in an experimental challenge study. After one vaccination, the majority of the calves developed humoral and cellular immune responses. Secondary vaccination resulted in a substantially enhanced level of immunity in all animals. Three months after the second vaccination, calves were either challenged with one of the mutant isolates or with a conventional challenge strain of BHV-1. Regardless of the type of virus used for challenge, vaccinated calves experienced significantly (P < 0.05) less weight loss and temperature rises, had lower nasal scores, and shed less virus than non-vaccinated animals. The only statistically significant (P < 0.05) difference between the 2 challenge viruses was the amount of virus shed, which was higher in non-vaccinated calves challenged with the mutant virus than in those challenged with the conventional virus. These data show that calves vaccinated with a modified-live IBR vaccine are protected from challenge with either the mutant or the conventional virus.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babiuk L. A., Wardley R. C., Rouse B. T. Defense mechanisms against bovine herpesvirus: relationship of virus-host cell events to susceptibility to antibody-complement cell lysis. Infect Immun. 1975 Nov;12(5):958–963. doi: 10.1128/iai.12.5.958-963.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egyed L., Brocchi E., Rusvai M., Bartha A. Study of bovine herpesvirus type 1 strains with monoclonal antibodies. Acta Vet Hung. 1992;40(3):225–230. [PubMed] [Google Scholar]
- Fitzpatrick D. R., Redmond M. J., Attah-Poku S. K., van Drunen Littel-van den Hurk S., Babiuk L. A., Zamb T. J. Mapping of 10 epitopes on bovine herpesvirus type 1 glycoproteins gI and gIII. Virology. 1990 May;176(1):145–157. doi: 10.1016/0042-6822(90)90239-n. [DOI] [PubMed] [Google Scholar]
- Hughes G., Babiuk L. A., van Drunen Littel-van den Hurk S. Functional and topographical analyses of epitopes on bovine herpesvirus type 1 glycoprotein IV. Arch Virol. 1988;103(1-2):47–60. doi: 10.1007/BF01319808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall R. L., Rodriguez L. L., Letchworth G. J., 3rd Characterization of envelope proteins of infectious bovine rhinotracheitis virus (bovine herpesvirus 1) by biochemical and immunological methods. J Virol. 1986 Mar;57(3):745–753. doi: 10.1128/jvi.57.3.745-753.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tikoo S. K., Campos M., Babiuk L. A. Bovine herpesvirus 1 (BHV-1): biology, pathogenesis, and control. Adv Virus Res. 1995;45:191–223. doi: 10.1016/s0065-3527(08)60061-5. [DOI] [PubMed] [Google Scholar]
- Tikoo S. K., Zamb T. J., Babiuk L. A. Analysis of bovine herpesvirus 1 glycoprotein gIV truncations and deletions expressed by recombinant vaccinia viruses. J Virol. 1993 Apr;67(4):2103–2109. doi: 10.1128/jvi.67.4.2103-2109.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates W. D. A review of infectious bovine rhinotracheitis, shipping fever pneumonia and viral-bacterial synergism in respiratory disease of cattle. Can J Comp Med. 1982 Jul;46(3):225–263. [PMC free article] [PubMed] [Google Scholar]
- van Donkersgoed J., Klassen P. Serological study of a modified-live virus IBR vaccine given to feedlot calves after arrival. Can Vet J. 1995 Jun;36(6):394–394. [PMC free article] [PubMed] [Google Scholar]
- van Drunen Littel-van den Hurk, Braun R. P., Lewis P. J., Karvonen B. C., Baca-Estrada M. E., Snider M., McCartney D., Watts T., Babiuk L. A. Intradermal immunization with a bovine herpesvirus-1 DNA vaccine induces protective immunity in cattle. J Gen Virol. 1998 Apr;79(Pt 4):831–839. doi: 10.1099/0022-1317-79-4-831. [DOI] [PubMed] [Google Scholar]
- van Drunen Littel-van den Hurk S., Babiuk L. A. Polypeptide specificity of the antibody response after primary and recurrent infection with bovine herpesvirus 1. J Clin Microbiol. 1986 Feb;23(2):274–282. doi: 10.1128/jcm.23.2.274-282.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Drunen Littel-van den Hurk S., van den Hurk J. V., Babiuk L. A. Topographical analysis of bovine herpesvirus type-1 glycoproteins: use of monoclonal antibodies to identify and characterize functional epitopes. Virology. 1985 Jul 15;144(1):216–227. doi: 10.1016/0042-6822(85)90319-8. [DOI] [PubMed] [Google Scholar]