Abstract
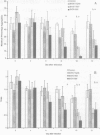
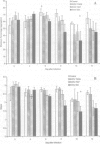
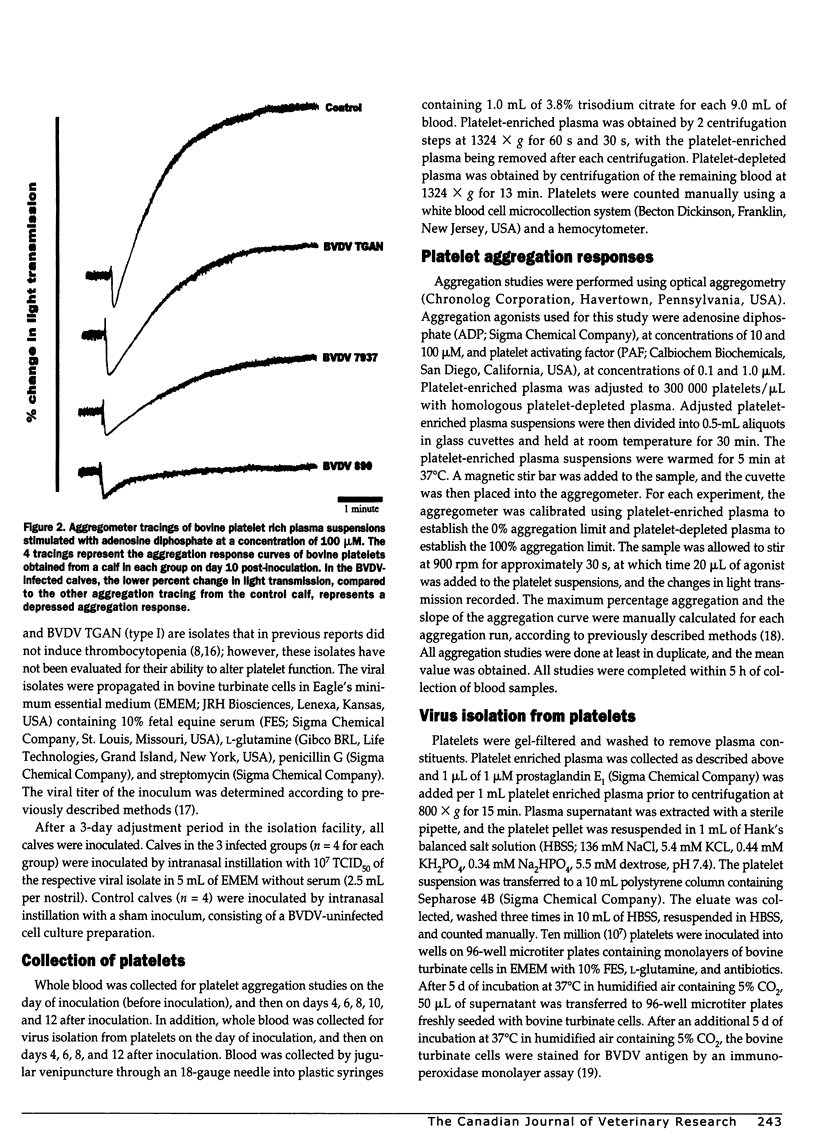
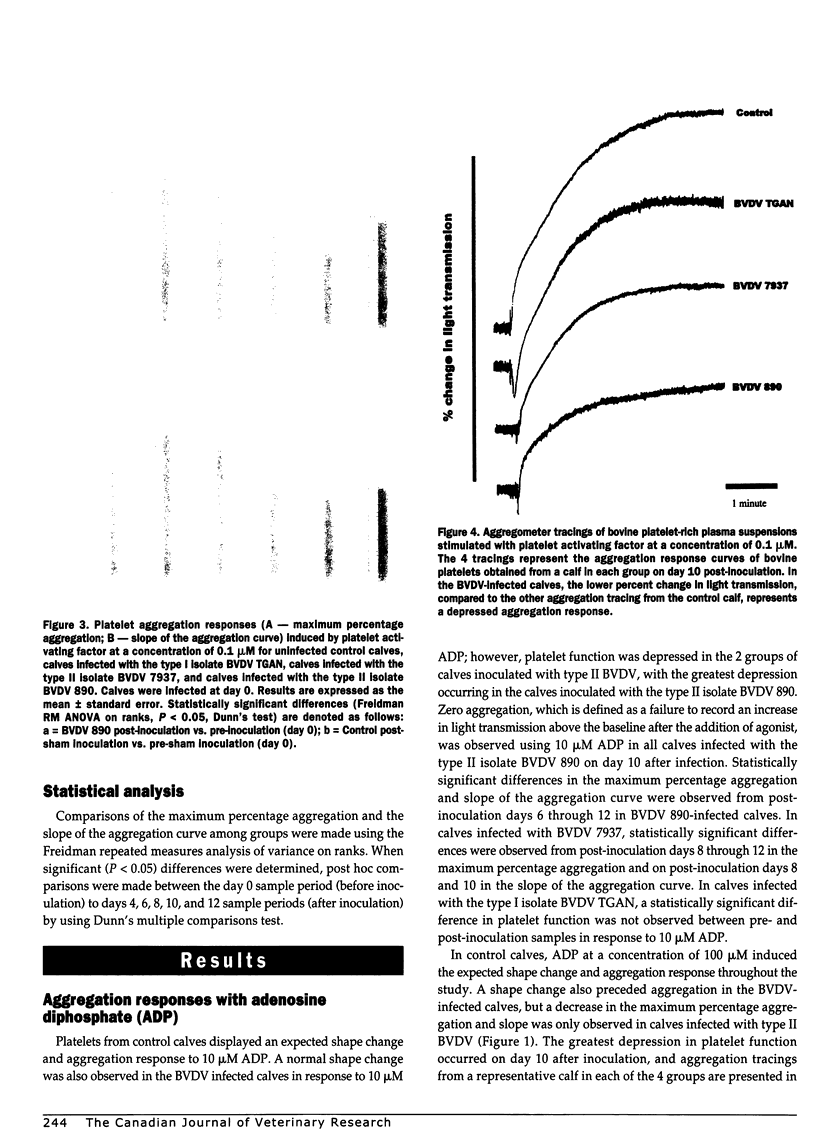
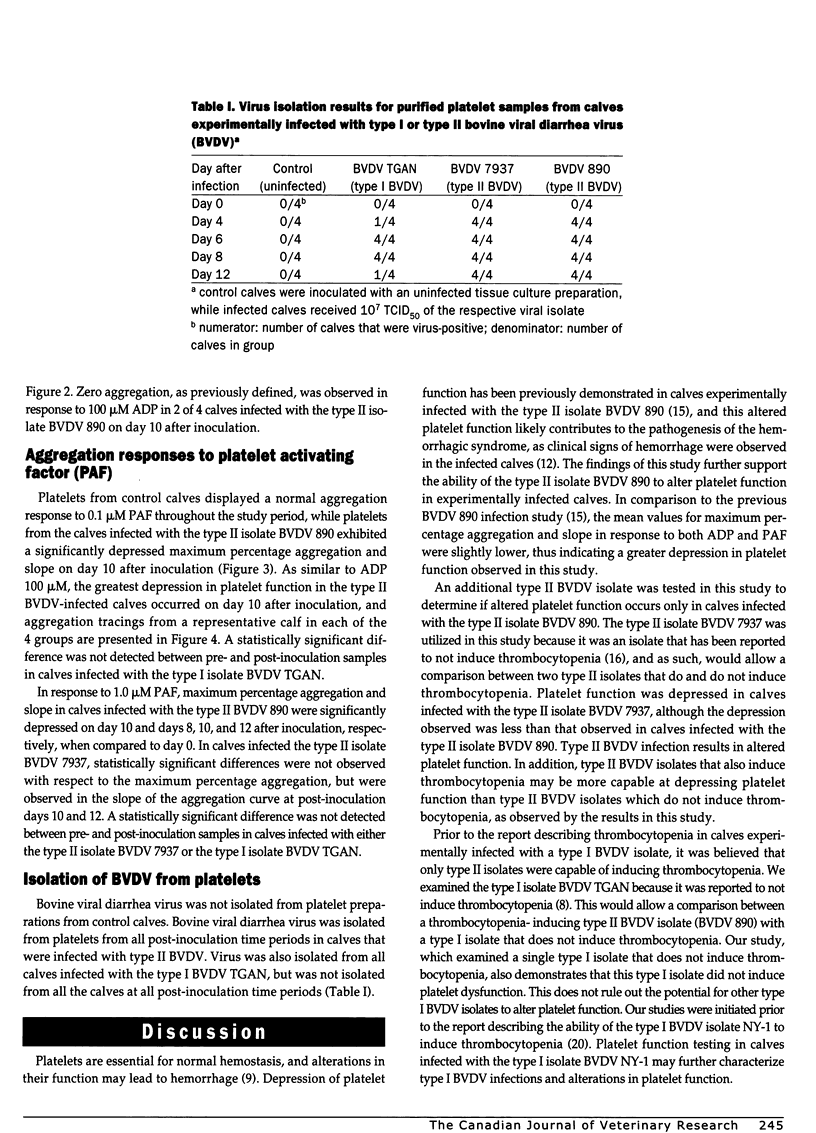
Altered platelet function has been reported in calves experimentally infected with type II bovine viral diarrhea virus (BVDV). The purpose of the present study was to further evaluate the ability of BVDV isolates to alter platelet function and to examine for the presence of a virus-platelet interaction during BVDV infection. Colostrum-deprived Holstein calves were obtained immediately after birth, housed in isolation, and assigned to 1 of 4 groups (1 control and 3 treatment groups). Control calves (n = 4) were sham inoculated, while calves in the infected groups (n = 4 for each group) were inoculated by intranasal instillation with 10(7) TCID50 of either BVDV 890 (type II), BVDV 7937 (type II), or BVDV TGAN (type I). Whole blood was collected prior to inoculation (day 0) and on days 4, 6, 8, 10, and 12 after inoculation for platelet function testing by optical aggregometry by using adenosine diphosphate and platelet activating factor. The maximum percentage aggregation and the slope of the aggregation curve decreased over time in BVDV-infected calves; however, statistically significant differences (Freidman repeated measures ANOVA on ranks, P < 0.05) were only observed in calves infected with the type II BVDV isolates. Bovine viral diarrhea virus was not isolated from control calves, but was isolated from all calves infected with both type II BVDV isolates from days 4 through 12 after inoculation. In calves infected with type I BVDV, virus was isolated from 1 of 4 calves on days 4 and 12 after inoculation and from all calves on days 6 and 8 after inoculation. Altered platelet function was observed in calves infected with both type II BVDV isolates, but was not observed in calves infected with type I BVDV. Altered platelet function may be important as a difference in virulence between type I and type II BVDV infection.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Archambault D., Béliveau C., Couture Y., Carman S. Clinical response and immunomodulation following experimental challenge of calves with type 2 noncytopathogenic bovine viral diarrhea virus. Vet Res. 2000 Mar-Apr;31(2):215–227. doi: 10.1051/vetres:2000117. [DOI] [PubMed] [Google Scholar]
- Baker J. C. The clinical manifestations of bovine viral diarrhea infection. Vet Clin North Am Food Anim Pract. 1995 Nov;11(3):425–445. doi: 10.1016/s0749-0720(15)30460-6. [DOI] [PubMed] [Google Scholar]
- Bezek D. M., Gröhn Y. T., Dubovi E. J. Effect of acute infection with noncytopathic or cytopathic bovine viral diarrhea virus isolates on bovine platelets. Am J Vet Res. 1994 Aug;55(8):1115–1119. [PubMed] [Google Scholar]
- Bolin S. R., Ridpath J. F. Differences in virulence between two noncytopathic bovine viral diarrhea viruses in calves. Am J Vet Res. 1992 Nov;53(11):2157–2163. [PubMed] [Google Scholar]
- Carman S., van Dreumel T., Ridpath J., Hazlett M., Alves D., Dubovi E., Tremblay R., Bolin S., Godkin A., Anderson N. Severe acute bovine viral diarrhea in Ontario, 1993-1995. J Vet Diagn Invest. 1998 Jan;10(1):27–35. doi: 10.1177/104063879801000106. [DOI] [PubMed] [Google Scholar]
- Carty D. J., Gear A. R. Fractionation of platelets according to size: functional and biochemical characteristics. Am J Hematol. 1986 Jan;21(1):1–14. doi: 10.1002/ajh.2830210102. [DOI] [PubMed] [Google Scholar]
- Corapi W. V., Elliott R. D., French T. W., Arthur D. G., Bezek D. M., Dubovi E. J. Thrombocytopenia and hemorrhages in veal calves infected with bovine viral diarrhea virus. J Am Vet Med Assoc. 1990 Feb 15;196(4):590–596. [PubMed] [Google Scholar]
- Corapi W. V., French T. W., Dubovi E. J. Severe thrombocytopenia in young calves experimentally infected with noncytopathic bovine viral diarrhea virus. J Virol. 1989 Sep;63(9):3934–3943. doi: 10.1128/jvi.63.9.3934-3943.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis J. A., West K. H., Cortese V. S., Myers S. L., Carman S., Martin K. M., Haines D. M. Lesions and distribution of viral antigen following an experimental infection of young seronegative calves with virulent bovine virus diarrhea virus-type II. Can J Vet Res. 1998 Jul;62(3):161–169. [PMC free article] [PubMed] [Google Scholar]
- Gentry P. A., Cheryk L. A., Shanks R. D., Healey R. An inherited platelet function defect in a Simmental crossbred herd. Can J Vet Res. 1997 Apr;61(2):128–133. [PMC free article] [PubMed] [Google Scholar]
- Marshall D. J., Moxley R. A., Kelling C. L. Distribution of virus and viral antigen in specific pathogen-free calves following inoculation with noncytopathic bovine viral diarrhea virus. Vet Pathol. 1996 May;33(3):311–318. doi: 10.1177/030098589603300308. [DOI] [PubMed] [Google Scholar]
- Marshall D. J., Moxley R. A., Kelling C. L. Severe disease following experimental exposure of calves to noncytopathic bovine viral diarrhoea virus isolate New York-1. Aust Vet J. 1998 Jun;76(6):428–430. doi: 10.1111/j.1751-0813.1998.tb12397.x. [DOI] [PubMed] [Google Scholar]
- Pellerin C., van den Hurk J., Lecomte J., Tijssen P. Identification of a new group of bovine viral diarrhea virus strains associated with severe outbreaks and high mortalities. Virology. 1994 Sep;203(2):260–268. doi: 10.1006/viro.1994.1483. [DOI] [PubMed] [Google Scholar]
- Peng J., Friese P., Heilmann E., George J. N., Burstein S. A., Dale G. L. Aged platelets have an impaired response to thrombin as quantitated by P-selectin expression. Blood. 1994 Jan 1;83(1):161–166. [PubMed] [Google Scholar]
- Rebhun W. C., French T. W., Perdrizet J. A., Dubovi E. J., Dill S. G., Karcher L. F. Thrombocytopenia associated with acute bovine virus diarrhea infection in cattle. J Vet Intern Med. 1989 Jan-Mar;3(1):42–46. doi: 10.1111/j.1939-1676.1989.tb00327.x. [DOI] [PubMed] [Google Scholar]
- Ridpath J. F., Bolin S. R., Dubovi E. J. Segregation of bovine viral diarrhea virus into genotypes. Virology. 1994 Nov 15;205(1):66–74. doi: 10.1006/viro.1994.1620. [DOI] [PubMed] [Google Scholar]
- Spagnuolo-Weaver M., Allan G. M., Kennedy S., Foster J. C., Adair B. M. Distribution of cytopathic and noncytopathic bovine viral diarrhea virus antigens in tissues of calves following acute experimental infection. J Vet Diagn Invest. 1997 Jul;9(3):287–297. doi: 10.1177/104063879700900310. [DOI] [PubMed] [Google Scholar]
- Stoffregen B., Bolin S. R., Ridpath J. F., Pohlenz J. Morphologic lesions in type 2 BVDV infections experimentally induced by strain BVDV2-1373 recovered from a field case. Vet Microbiol. 2000 Nov 15;77(1-2):157–162. doi: 10.1016/s0378-1135(00)00272-8. [DOI] [PubMed] [Google Scholar]
- Walz P. H., Bell T. G., Steficek B. A., Kaiser L., Maes R. K., Baker J. C. Experimental model of type II bovine viral diarrhea virus-induced thrombocytopenia in neonatal calves. J Vet Diagn Invest. 1999 Nov;11(6):505–514. doi: 10.1177/104063879901100604. [DOI] [PubMed] [Google Scholar]
- Walz P. H., Steficek B. A., Baker J. C., Kaiser L., Bell T. G. Effect of experimentally induced type II bovine viral diarrhea virus infection on platelet function in calves. Am J Vet Res. 1999 Nov;60(11):1396–1401. [PubMed] [Google Scholar]