Abstract
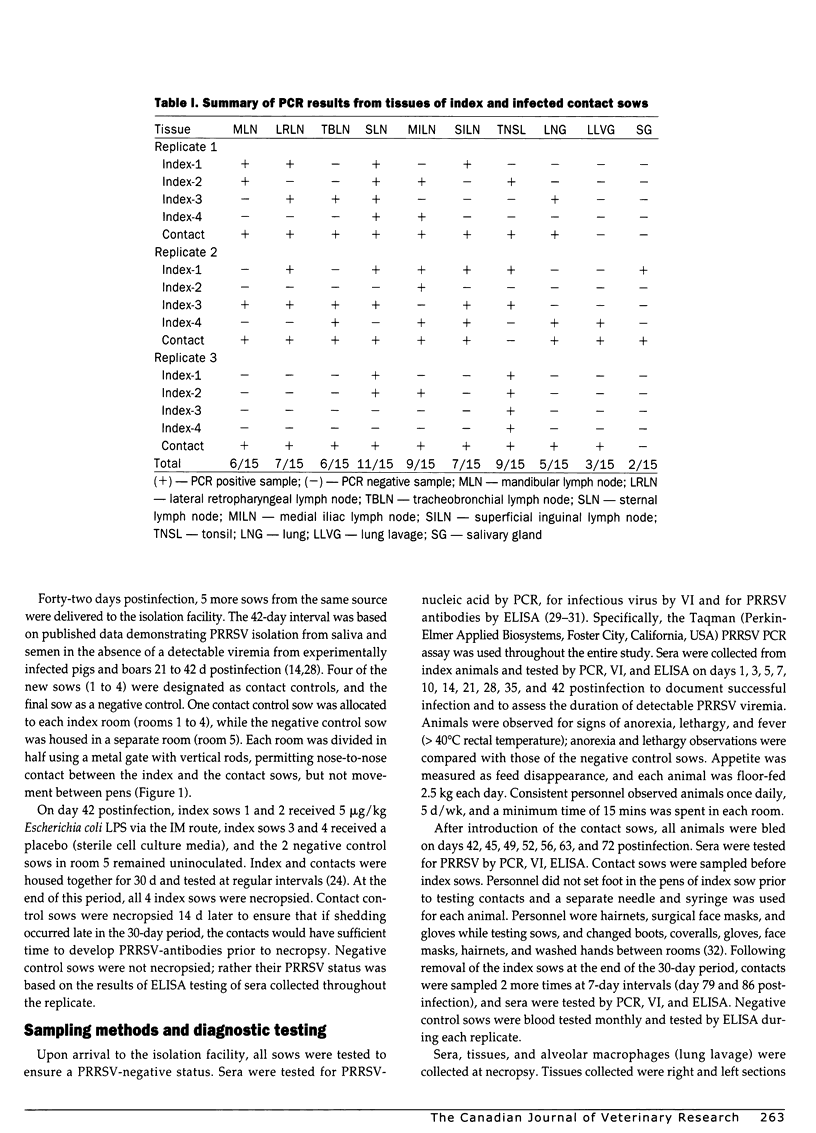
The objective of this study was to determine if porcine reproductive and respiratory syndrome virus (PRRSV) could persist in non-pregnant sows and if persistently infected sows could transmit virus to naive contact controls. Twelve PRRSV-naive, non-pregnant sows (index sows) were infected with a field isolate of PRRSV and housed in individual isolation rooms for 42 to 56 days postinfection. Following this period, 1 naive contact sow was placed in each room divided by a gate allowing nose-to-nose contact with a single index sow. Index sows were not viremic at the time of contact sow entry. Virus nucleic acid was detected by polymerase chain reaction, and infectious virus was detected by virus isolation in sera from 3 of the 12 contact sows at 49, 56, and 86 days postinfection. All 3 infected contacts developed PRRSV antibodies. Virus nucleic acid was detected in tissues of all of the 12 index sows at 72 or 86 days postinfection. Nucleic acid sequencing indicated that representative samples from index and infected contacts were homologous (> 99%) to the PRRSV used to infect index sows at the onset of the study. This study demonstrates that PRRSV can persist in sows and that persistently infected sows can transmit virus to naive contact animals.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albina E., Leforban Y., Baron T., Plana Duran J. P., Vannier P. An enzyme linked immunosorbent assay (ELISA) for the detection of antibodies to the porcine reproductive and respiratory syndrome (PRRS) virus. Ann Rech Vet. 1992;23(2):167–176. [PubMed] [Google Scholar]
- Albina E., Madec F., Cariolet R., Torrison J. Immune response and persistence of the porcine reproductive and respiratory syndrome virus in infected pigs and farm units. Vet Rec. 1994 May 28;134(22):567–573. doi: 10.1136/vr.134.22.567. [DOI] [PubMed] [Google Scholar]
- Allende R., Laegreid W. W., Kutish G. F., Galeota J. A., Wills R. W., Osorio F. A. Porcine reproductive and respiratory syndrome virus: description of persistence in individual pigs upon experimental infection. J Virol. 2000 Nov;74(22):10834–10837. doi: 10.1128/jvi.74.22.10834-10837.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bautista E. M., Goyal S. M., Yoon I. J., Joo H. S., Collins J. E. Comparison of porcine alveolar macrophages and CL 2621 for the detection of porcine reproductive and respiratory syndrome (PRRS) virus and anti-PRRS antibody. J Vet Diagn Invest. 1993 Apr;5(2):163–165. doi: 10.1177/104063879300500204. [DOI] [PubMed] [Google Scholar]
- Bierk M. D., Dee S. A., Rossow K. D., Collins J. E., Guedes M. I., Pijoan C., Molitor T. W. Diagnostic investigation of chronic porcine reproductive and respiratory syndrome virus in a breeding herd of pigs. Vet Rec. 2001 Jun 2;148(22):687–690. doi: 10.1136/vr.148.22.687. [DOI] [PubMed] [Google Scholar]
- Cavanagh D. Nidovirales: a new order comprising Coronaviridae and Arteriviridae. Arch Virol. 1997;142(3):629–633. [PubMed] [Google Scholar]
- Christopher-Hennings J., Nelson E. A., Hines R. J., Nelson J. K., Swenson S. L., Zimmerman J. J., Chase C. L., Yaeger M. J., Benfield D. A. Persistence of porcine reproductive and respiratory syndrome virus in serum and semen of adult boars. J Vet Diagn Invest. 1995 Oct;7(4):456–464. doi: 10.1177/104063879500700406. [DOI] [PubMed] [Google Scholar]
- Christopher-Hennings J., Nelson E. A., Nelson J. K., Rossow K. D., Shivers J. L., Yaeger M. J., Chase C. C., Garduno R. A., Collins J. E., Benfield D. A. Identification of porcine reproductive and respiratory syndrome virus in semen and tissues from vasectomized and nonvasectomized boars. Vet Pathol. 1998 Jul;35(4):260–267. doi: 10.1177/030098589803500404. [DOI] [PubMed] [Google Scholar]
- Halbur P. G., Miller L. D., Paul P. S., Meng X. J., Huffman E. L., Andrews J. J. Immunohistochemical identification of porcine reproductive and respiratory syndrome virus (PRRSV) antigen in the heart and lymphoid system of three-week-old colostrum-deprived pigs. Vet Pathol. 1995 Mar;32(2):200–204. doi: 10.1177/030098589503200218. [DOI] [PubMed] [Google Scholar]
- Hopper S. A., White M. E., Twiddy N. An outbreak of blue-eared pig disease (porcine reproductive and respiratory syndrome) in four pig herds in Great Britain. Vet Rec. 1992 Aug 15;131(7):140–144. doi: 10.1136/vr.131.7.140. [DOI] [PubMed] [Google Scholar]
- Jolie R., Bäckström L., Olson L., Chase C. A 15-week experimental exposure of pigs to airborne dust with added endotoxin in a continuous flow exposure chamber. Can J Vet Res. 1999 Apr;63(2):129–137. [PMC free article] [PubMed] [Google Scholar]
- Klir J. J., Shahbazian L. M., Matteri R. L., Fritsche K. L., Becker B. A. Effects of thermal environment on response to acute peripheral lipopolysaccharide challenge exposure in neonatal pigs. Am J Vet Res. 1997 Apr;58(4):364–369. [PubMed] [Google Scholar]
- Murtaugh M. P., Elam M. R., Kakach L. T. Comparison of the structural protein coding sequences of the VR-2332 and Lelystad virus strains of the PRRS virus. Arch Virol. 1995;140(8):1451–1460. doi: 10.1007/BF01322671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmberg L., Larsson B. M., Malmberg P., Larsson K. Induction of IL-8 production in human alveolar macrophages and human bronchial epithelial cells in vitro by swine dust. Thorax. 1998 Apr;53(4):260–264. doi: 10.1136/thx.53.4.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossow K. D., Bautista E. M., Goyal S. M., Molitor T. W., Murtaugh M. P., Morrison R. B., Benfield D. A., Collins J. E. Experimental porcine reproductive and respiratory syndrome virus infection in one-, four-, and 10-week-old pigs. J Vet Diagn Invest. 1994 Jan;6(1):3–12. doi: 10.1177/104063879400600102. [DOI] [PubMed] [Google Scholar]
- Rossow K. D., Morrison R. B., Goyal S. M., Singh G. S., Collins J. E. Lymph node lesions in neonatal pigs congenitally exposed to porcine reproductive and respiratory syndrome virus. J Vet Diagn Invest. 1994 Jul;6(3):368–371. doi: 10.1177/104063879400600316. [DOI] [PubMed] [Google Scholar]
- Swenson S. L., Hill H. T., Zimmerman J. J., Evans L. E., Landgraf J. G., Wills R. W., Sanderson T. P., McGinley M. J., Brevik A. K., Ciszewski D. K. Excretion of porcine reproductive and respiratory syndrome virus in semen after experimentally induced infection in boars. J Am Vet Med Assoc. 1994 Jun 15;204(12):1943–1948. [PubMed] [Google Scholar]
- Wang Z., Malmberg P., Ek A., Larsson K., Palmberg L. Swine dust induces cytokine secretion from human epithelial cells and alveolar macrophages. Clin Exp Immunol. 1999 Jan;115(1):6–12. doi: 10.1046/j.1365-2249.1999.00776.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills R. W., Zimmerman J. J., Yoon K. J., Swenson S. L., Hoffman L. J., McGinley M. J., Hill H. T., Platt K. B. Porcine reproductive and respiratory syndrome virus: routes of excretion. Vet Microbiol. 1997 Jun 30;57(1):69–81. doi: 10.1016/S0378-1135(97)00079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills R. W., Zimmerman J. J., Yoon K. J., Swenson S. L., McGinley M. J., Hill H. T., Platt K. B., Christopher-Hennings J., Nelson E. A. Porcine reproductive and respiratory syndrome virus: a persistent infection. Vet Microbiol. 1997 Apr;55(1-4):231–240. doi: 10.1016/s0378-1135(96)01337-5. [DOI] [PubMed] [Google Scholar]


