Abstract
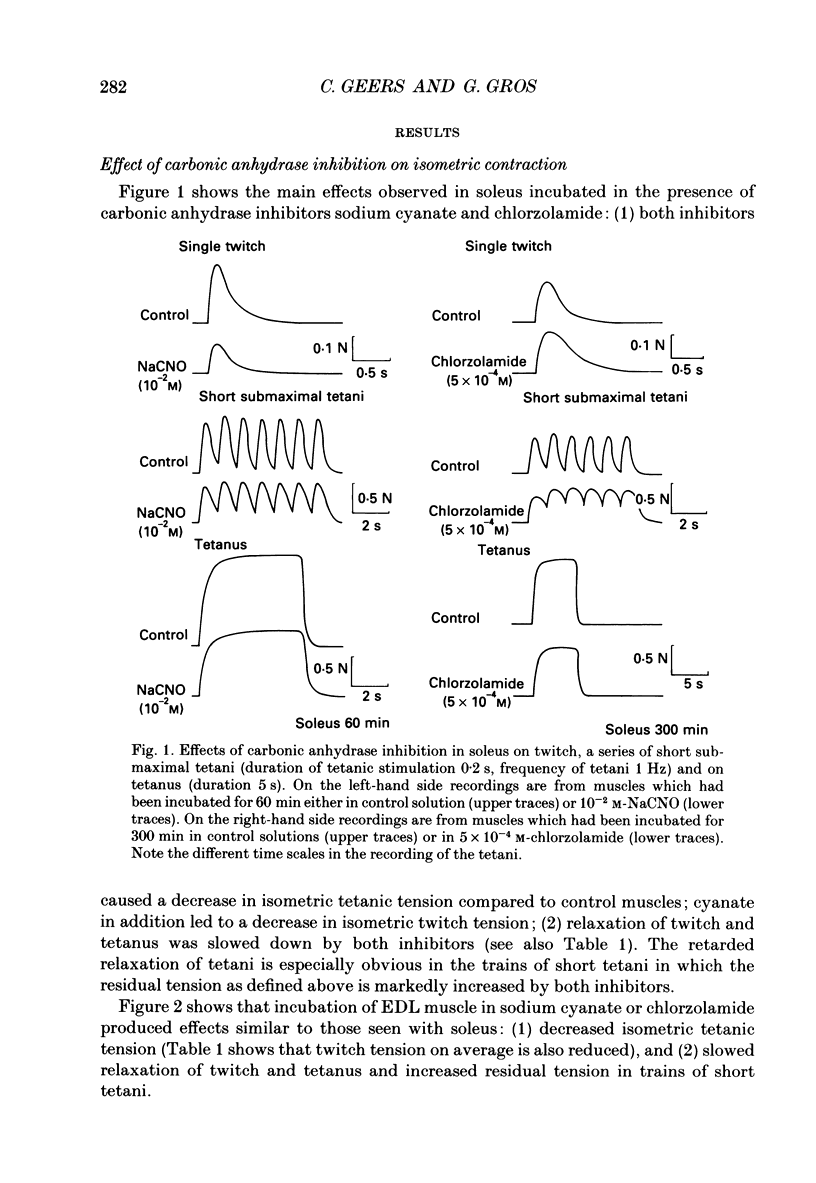
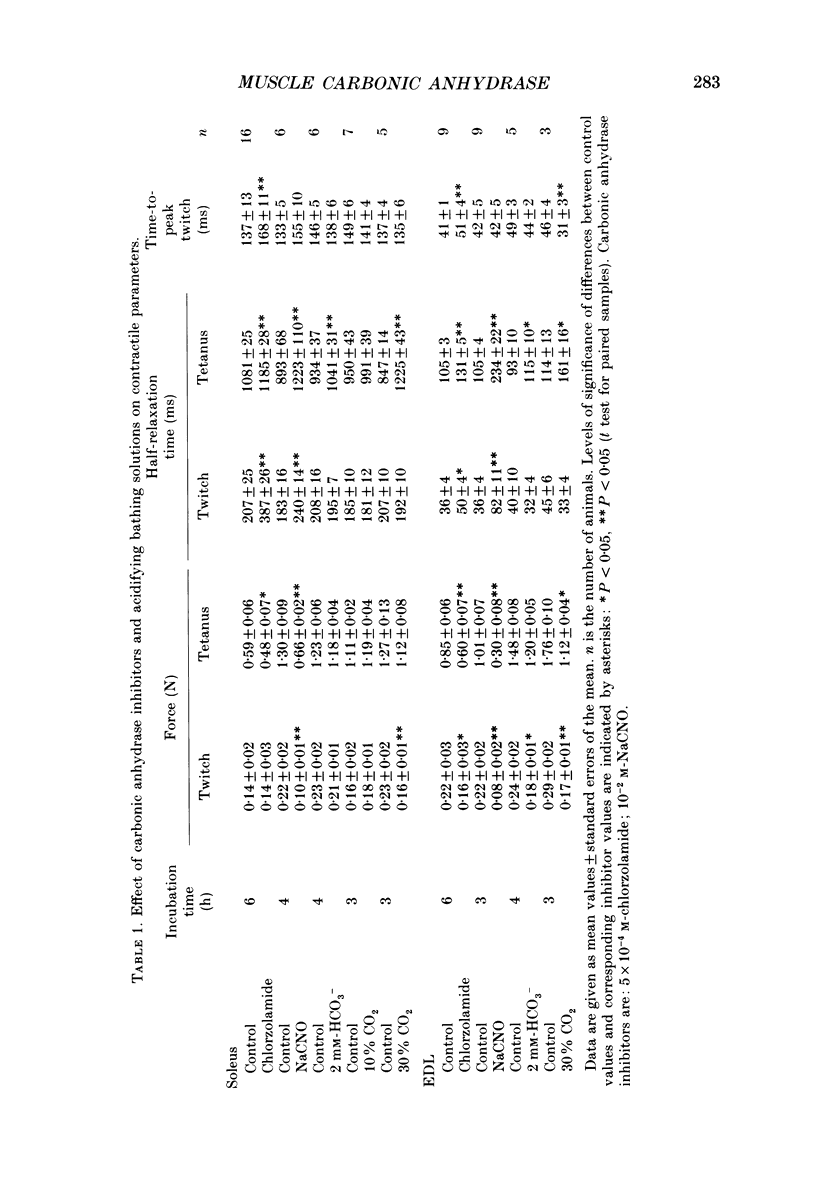
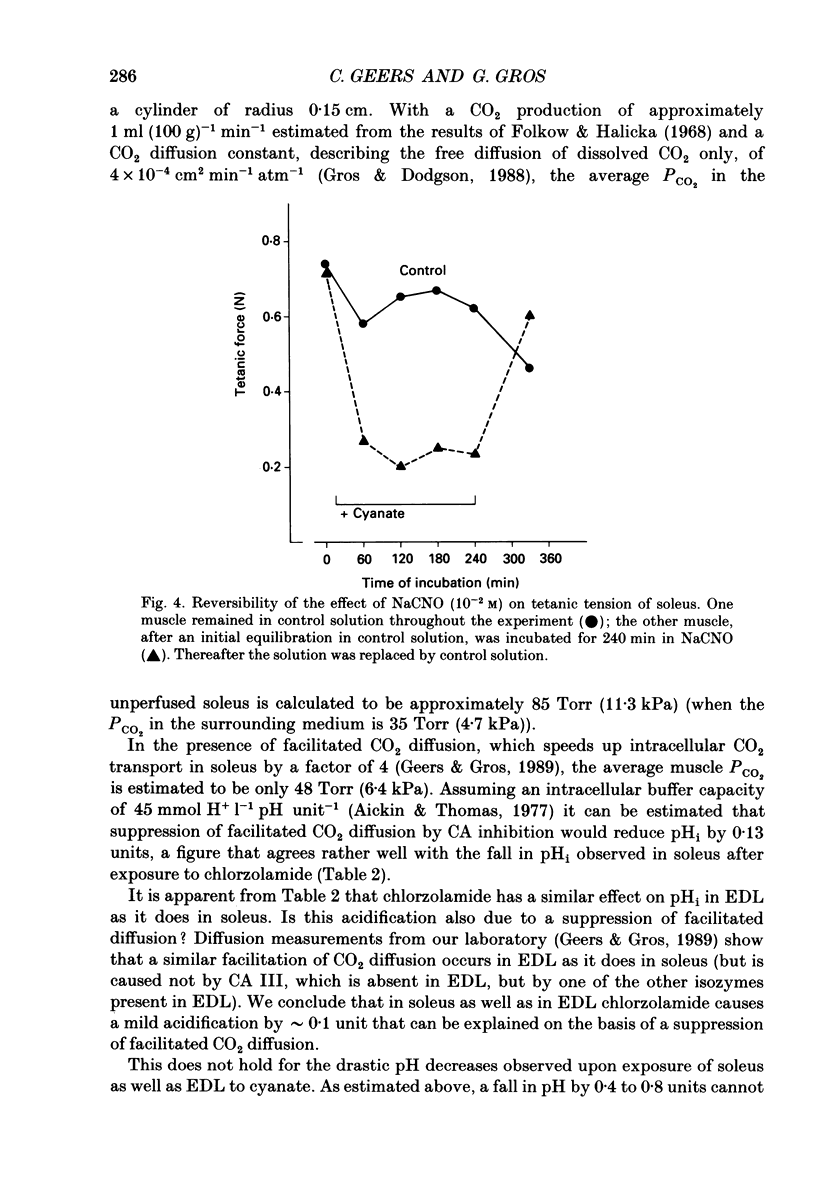
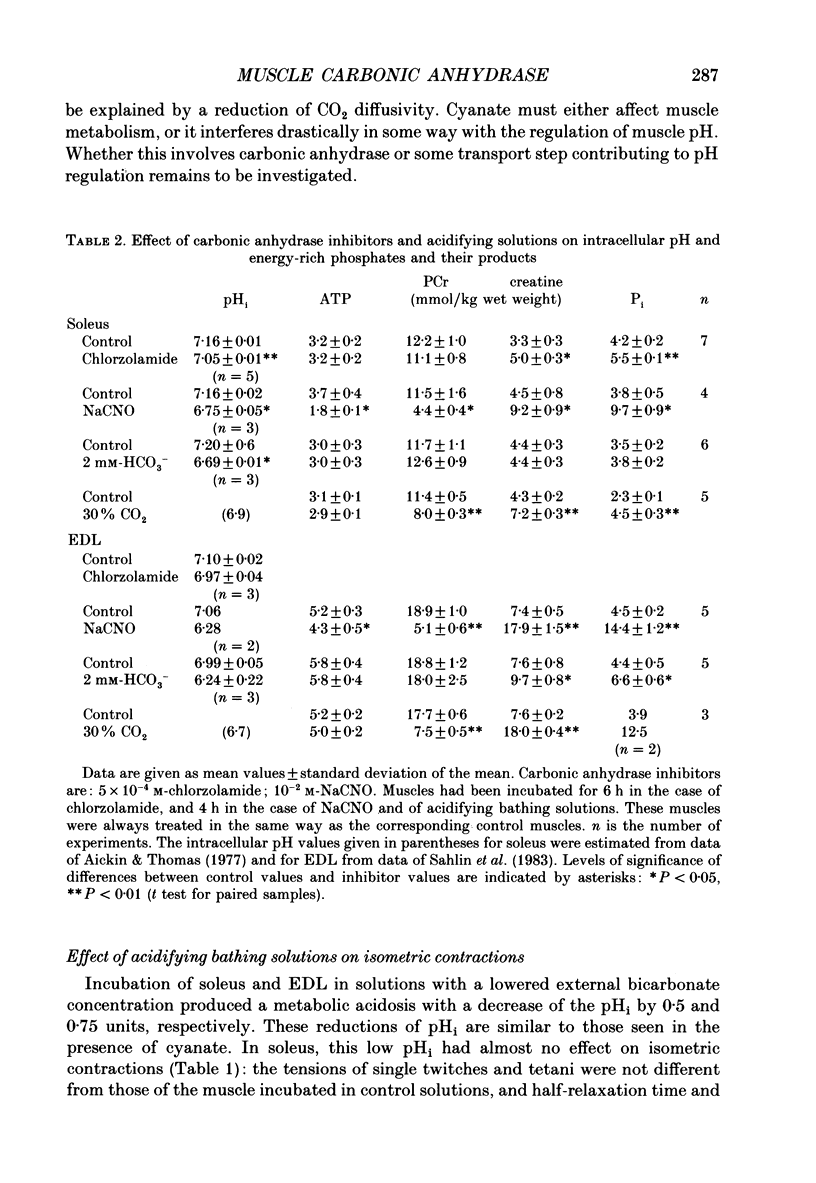
1. The effects of carbonic anhydrase inhibitors on contractile parameters, intracellular pH (pHi) and energy-rich phosphates were studied in isolated rat soleus and extensor digitorum longus (EDL) muscles. 2. The muscles were incubated either in Ringer solutions (95% O2/5% CO2 = control) or in solutions to which one of the inhibitors, 5 X 10(-4) M-chlorzolamide or 10(-2) M-NaCNO, had been added. Muscles were stimulated directly and contracted under isometric conditions. 3. Compared with control muscles, both inhibitor-treated muscles showed a significantly decreased tetanic force and an increased half-relaxation time of twitches and tetani. Chlorzolamide increased time-to-peak in both muscles. Cyanate decreased isometric twitch force in both muscles. 4. Both inhibitors decreased pHi in both muscles; chlorzolamide by 0.1 unit, cyanate by 0.4 unit in soleus and by 0.8 unit in EDL. 5. Chlorzolamide increased the concentrations of creatine and inorganic phosphate (Pi) in soleus (the effect of chlorzolamide was not studied in EDL). Cyanate caused these same changes in soleus as well as EDL and in addition decreased the concentrations of ATP and phosphocreatine in soleus and EDL. 6. In muscles acidified by either low external HCO3- (2 mM) or by elevated PCO2 (30% CO2 in the gas phase) in the bath, decreases in isometric force and increases in half-relaxation time of tetani were observed. In addition there were increases in muscle Pi. These effects were more pronounced with 30% CO2 than with 2 mM-HCO3-. 7. Neither acidifying solutions prolonged either half-relaxation time or time-to-peak of twitches. 8. We conclude that carbonic anhydrase inhibition exerts its effect (a) on isometric tension at least partly via an elevated Pi (perhaps in combination with lowered pHi); (b) on the half-relaxation time of tetani by means of lowered pHi and elevated concentration of Pi; (c) on relaxation and time-to-peak of twitches by some unknown mechanism, neither directly by a change in pHi nor in Pi.
Full text
PDF














Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albers C., Usinger W., Spaich P. Effect of temperature on the intracellular CO2 dissociation curve and pH. Respir Physiol. 1971 Jan;11(2):211–222. doi: 10.1016/0034-5687(71)90025-9. [DOI] [PubMed] [Google Scholar]
- Barclay J. K. Carbonic anhydrase III inhibition in normocapnic and hypercapnic contracting mouse soleus. Can J Physiol Pharmacol. 1987 Jan;65(1):100–104. doi: 10.1139/y87-020. [DOI] [PubMed] [Google Scholar]
- Barlogie B., Hasselbach W., Makinose M. Activation of calcium efflux by ADP and inorganic phosphate. FEBS Lett. 1971 Jan 30;12(5):267–268. doi: 10.1016/0014-5793(71)80194-1. [DOI] [PubMed] [Google Scholar]
- Blinks J. R., Rüdel R., Taylor S. R. Calcium transients in isolated amphibian skeletal muscle fibres: detection with aequorin. J Physiol. 1978 Apr;277:291–323. doi: 10.1113/jphysiol.1978.sp012273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruns W., Dermietzel R., Gros G. Carbonic anhydrase in the sarcoplasmic reticulum of rabbit skeletal muscle. J Physiol. 1986 Feb;371:351–364. doi: 10.1113/jphysiol.1986.sp015980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cammer W. Release of mitochondrial respiratory control by cyanate salts. Biochim Biophys Acta. 1982 Feb 17;679(2):343–346. doi: 10.1016/0005-2728(82)90305-x. [DOI] [PubMed] [Google Scholar]
- Cooke R., Bialek W. Contraction of glycerinated muscle fibers as a function of the ATP concentration. Biophys J. 1979 Nov;28(2):241–258. doi: 10.1016/S0006-3495(79)85174-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke R., Franks K., Luciani G. B., Pate E. The inhibition of rabbit skeletal muscle contraction by hydrogen ions and phosphate. J Physiol. 1988 Jan;395:77–97. doi: 10.1113/jphysiol.1988.sp016909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke R., Pate E. The effects of ADP and phosphate on the contraction of muscle fibers. Biophys J. 1985 Nov;48(5):789–798. doi: 10.1016/S0006-3495(85)83837-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousin J. L., Motais R. The role of carbonic anhydrase inhibitors on anion permeability into ox red blood cells. J Physiol. 1976 Mar;256(1):61–80. doi: 10.1113/jphysiol.1976.sp011311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson M. J., Gadian D. G., Wilkie D. R. Mechanical relaxation rate and metabolism studied in fatiguing muscle by phosphorus nuclear magnetic resonance. J Physiol. 1980 Feb;299:465–484. doi: 10.1113/jphysiol.1980.sp013137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson M. J., Gadian D. G., Wilkie D. R. Muscular fatigue investigated by phosphorus nuclear magnetic resonance. Nature. 1978 Aug 31;274(5674):861–866. doi: 10.1038/274861a0. [DOI] [PubMed] [Google Scholar]
- Donaldson S. K., Hermansen L., Bolles L. Differential, direct effects of H+ on Ca2+ -activated force of skinned fibers from the soleus, cardiac and adductor magnus muscles of rabbits. Pflugers Arch. 1978 Aug 25;376(1):55–65. doi: 10.1007/BF00585248. [DOI] [PubMed] [Google Scholar]
- Edwards R. H., Hill D. K., Jones D. A. Metabolic changes associated with the slowing of relaxation in fatigued mouse muscle. J Physiol. 1975 Oct;251(2):287–301. doi: 10.1113/jphysiol.1975.sp011093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Effects of pH on the myofilaments and the sarcoplasmic reticulum of skinned cells from cardiace and skeletal muscles. J Physiol. 1978 Mar;276:233–255. doi: 10.1113/jphysiol.1978.sp012231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fretthold D. W., Garg L. C. The effect of acid--base changes on skeletal muscle twitch tension. Can J Physiol Pharmacol. 1978 Aug;56(4):543–549. doi: 10.1139/y78-087. [DOI] [PubMed] [Google Scholar]
- Geers C., Gros G. Carbonic anhydrase inhibition affects contraction of directly stimulated rat soleus. Life Sci. 1988;42(1):37–45. doi: 10.1016/0024-3205(88)90622-4. [DOI] [PubMed] [Google Scholar]
- Geers C., Gros G., Gärtner A. Extracellular carbonic anhydrase of skeletal muscle associated with the sarcolemma. J Appl Physiol (1985) 1985 Aug;59(2):548–558. doi: 10.1152/jappl.1985.59.2.548. [DOI] [PubMed] [Google Scholar]
- Godt R. E., Nosek T. M. Changes of intracellular milieu with fatigue or hypoxia depress contraction of skinned rabbit skeletal and cardiac muscle. J Physiol. 1989 May;412:155–180. doi: 10.1113/jphysiol.1989.sp017609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gros G., Dodgson S. J. Velocity of CO2 exchange in muscle and liver. Annu Rev Physiol. 1988;50:669–694. doi: 10.1146/annurev.ph.50.030188.003321. [DOI] [PubMed] [Google Scholar]
- HASSELBACH W., MAKINOSE M. ATP and active transport. Biochem Biophys Res Commun. 1962 Apr 3;7:132–136. doi: 10.1016/0006-291x(62)90161-4. [DOI] [PubMed] [Google Scholar]
- Hasselbach W. The reversibility of the sarcoplasmic calcium pump. Biochim Biophys Acta. 1978 Apr 10;515(1):23–53. doi: 10.1016/0304-4157(78)90007-2. [DOI] [PubMed] [Google Scholar]
- Hibberd M. G., Dantzig J. A., Trentham D. R., Goldman Y. E. Phosphate release and force generation in skeletal muscle fibers. Science. 1985 Jun 14;228(4705):1317–1319. doi: 10.1126/science.3159090. [DOI] [PubMed] [Google Scholar]
- Hoar P. E., Mahoney C. W., Kerrick W. G. MgADP- increases maximum tension and Ca2+ sensitivity in skinned rabbit soleus fibers. Pflugers Arch. 1987 Sep;410(1-2):30–36. doi: 10.1007/BF00581892. [DOI] [PubMed] [Google Scholar]
- Jeffery S., Carter N. D., Smith A. Immunocytochemical localization of carbonic anhydrase isozymes I, II, and III in rat skeletal muscle. J Histochem Cytochem. 1986 Apr;34(4):513–516. doi: 10.1177/34.4.2936798. [DOI] [PubMed] [Google Scholar]
- Kawai M., Güth K., Winnikes K., Haist C., Rüegg J. C. The effect of inorganic phosphate on the ATP hydrolysis rate and the tension transients in chemically skinned rabbit psoas fibers. Pflugers Arch. 1987 Jan;408(1):1–9. doi: 10.1007/BF00581833. [DOI] [PubMed] [Google Scholar]
- Kawashiro T., Scheid P. Measurement of Krogh's diffusion constant of CO2 in respiring muscle at various CO2 levels: evidence for facilitated diffusion. Pflugers Arch. 1976 Mar 30;362(2):127–133. doi: 10.1007/BF00583638. [DOI] [PubMed] [Google Scholar]
- Meyer R. A., Brown T. R., Kushmerick M. J. Phosphorus nuclear magnetic resonance of fast- and slow-twitch muscle. Am J Physiol. 1985 Mar;248(3 Pt 1):C279–C287. doi: 10.1152/ajpcell.1985.248.3.C279. [DOI] [PubMed] [Google Scholar]
- Micro-electrode measurement of the intracellular pH and buffering power of mouse soleus muscle fibres. J Physiol. 1977 Jun;267(3):791–810. doi: 10.1113/jphysiol.1977.sp011838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannier J. L., Weyne J., Leusen I. Effects of PCO2, bicarbonate and lactate on the isometric contractions of isolated soleus muscle of the rat. Pflugers Arch. 1970;320(2):120–132. doi: 10.1007/BF00588547. [DOI] [PubMed] [Google Scholar]
- Petrofsky J. S., Weber C., Phillips C. A. Mechanical and electrical correlates of isometric muscle fatigue in skeletal muscle in the cat. Pflugers Arch. 1980 Aug;387(1):33–38. doi: 10.1007/BF00580841. [DOI] [PubMed] [Google Scholar]
- Ranatunga K. W. Effects of acidosis on tension development in mammalian skeletal muscle. Muscle Nerve. 1987 Jun;10(5):439–445. doi: 10.1002/mus.880100510. [DOI] [PubMed] [Google Scholar]
- Renaud J. M., Stein R. B., Gordon T. The effects of pH on force and stiffness development in mouse muscles. Can J Physiol Pharmacol. 1987 Aug;65(8):1798–1801. doi: 10.1139/y87-280. [DOI] [PubMed] [Google Scholar]
- Sahlin K., Edström L., Sjöholm H., Hultman E. Effects of lactic acid accumulation and ATP decrease on muscle tension and relaxation. Am J Physiol. 1981 Mar;240(3):C121–C126. doi: 10.1152/ajpcell.1981.240.3.C121. [DOI] [PubMed] [Google Scholar]
- Sanyal G., Pessah N. I., Maren T. H. Kinetics and inhibition of membrane-bound carbonic anhydrase from canine renal cortex. Biochim Biophys Acta. 1981 Jan 15;657(1):128–137. doi: 10.1016/0005-2744(81)90136-4. [DOI] [PubMed] [Google Scholar]
- Scheid P., Siffert W. Effects of inhibiting carbonic anhydrase on isometric contraction of frog skeletal muscle. J Physiol. 1985 Apr;361:91–101. doi: 10.1113/jphysiol.1985.sp015634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siffert W., Gros G. Carbonic anhydrase C in white-skeletal-muscle tissue. Biochem J. 1982 Sep 1;205(3):559–566. doi: 10.1042/bj2050559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somlyo A. V., Gonzalez-Serratos H. G., Shuman H., McClellan G., Somlyo A. P. Calcium release and ionic changes in the sarcoplasmic reticulum of tetanized muscle: an electron-probe study. J Cell Biol. 1981 Sep;90(3):577–594. doi: 10.1083/jcb.90.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spriet L. L., Matsos C. G., Peters S. J., Heigenhauser G. J., Jones N. L. Effects of acidosis on rat muscle metabolism and performance during heavy exercise. Am J Physiol. 1985 Mar;248(3 Pt 1):C337–C347. doi: 10.1152/ajpcell.1985.248.3.C337. [DOI] [PubMed] [Google Scholar]
- Stein R. B., Gordon T., Shriver J. Temperature dependence of mammalian muscle contractions and ATPase activities. Biophys J. 1982 Nov;40(2):97–107. doi: 10.1016/S0006-3495(82)84464-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veech R. L., Lawson J. W., Cornell N. W., Krebs H. A. Cytosolic phosphorylation potential. J Biol Chem. 1979 Jul 25;254(14):6538–6547. [PubMed] [Google Scholar]
- WADDELL W. J., BUTLER T. C. Calculation of intracellular pH from the distribution of 5,5-dimethyl-2,4-oxazolidinedione (DMO); application to skeletal muscle of the dog. J Clin Invest. 1959 May;38(5):720–729. doi: 10.1172/JCI103852. [DOI] [PMC free article] [PubMed] [Google Scholar]


