Abstract
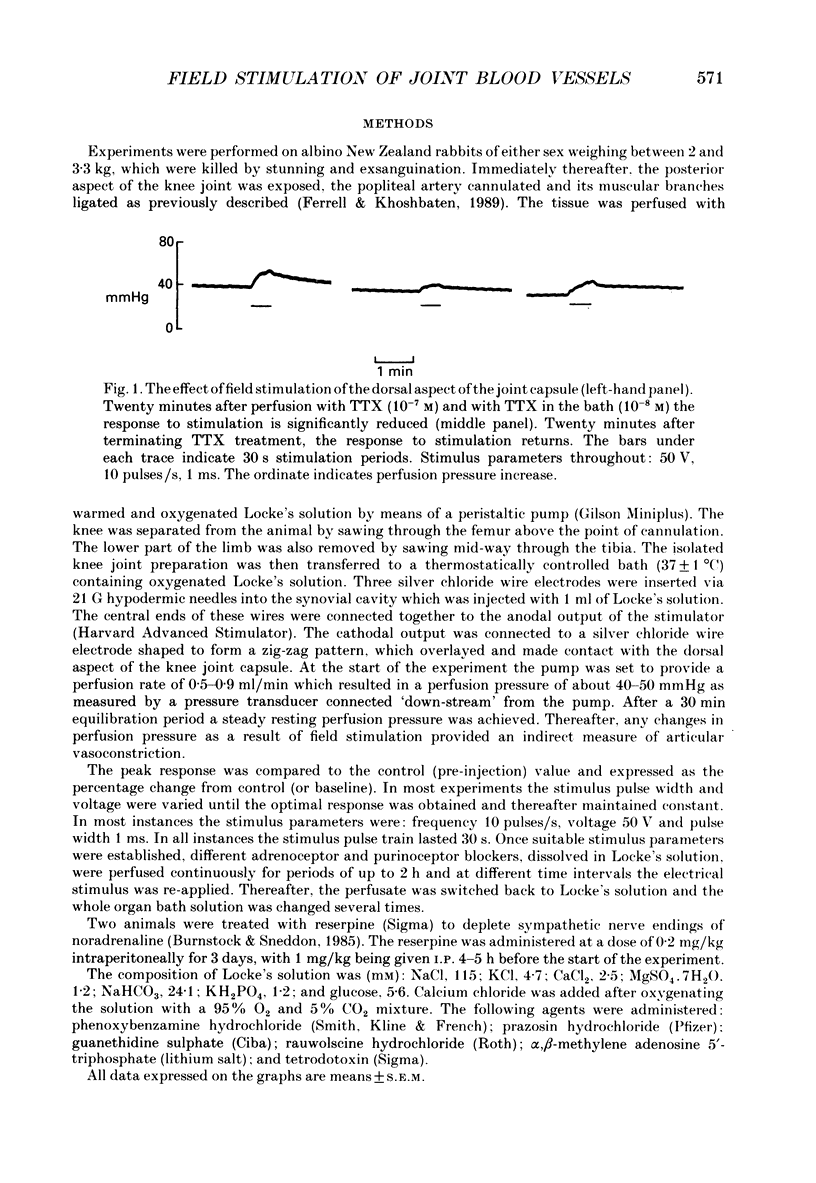
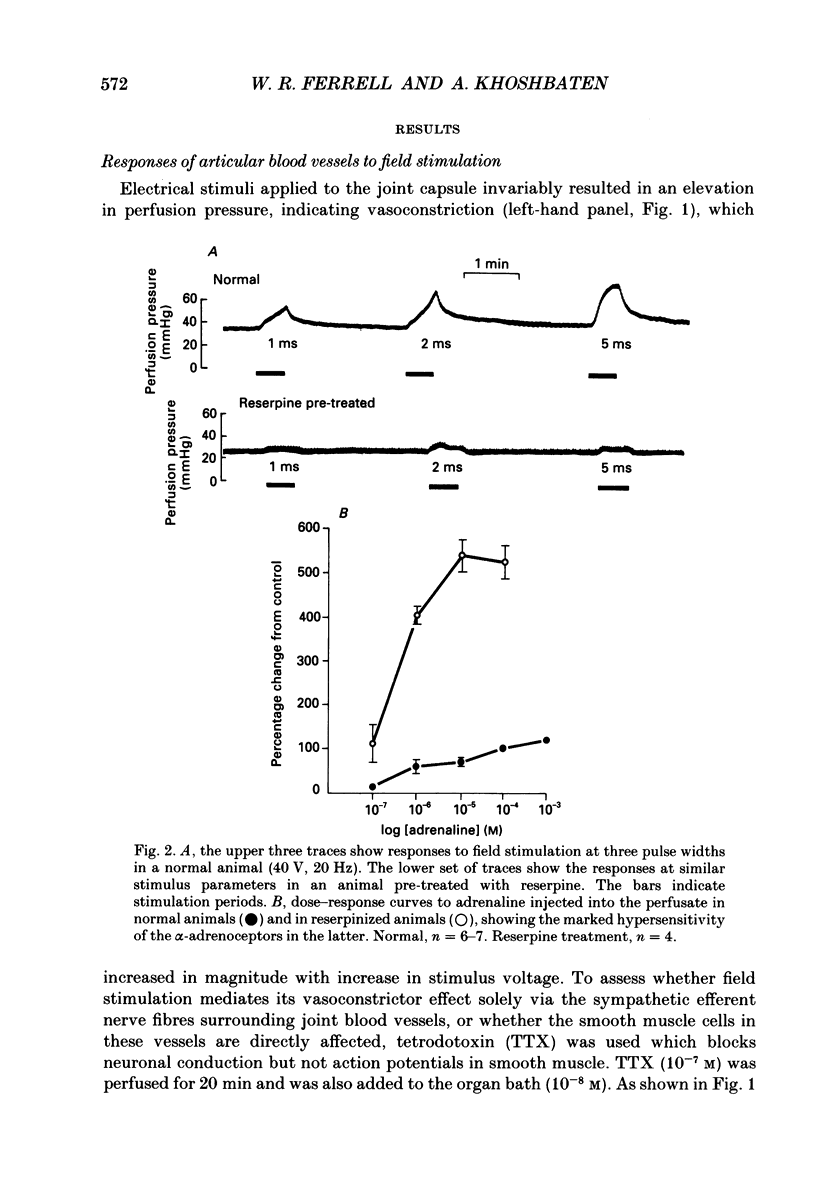
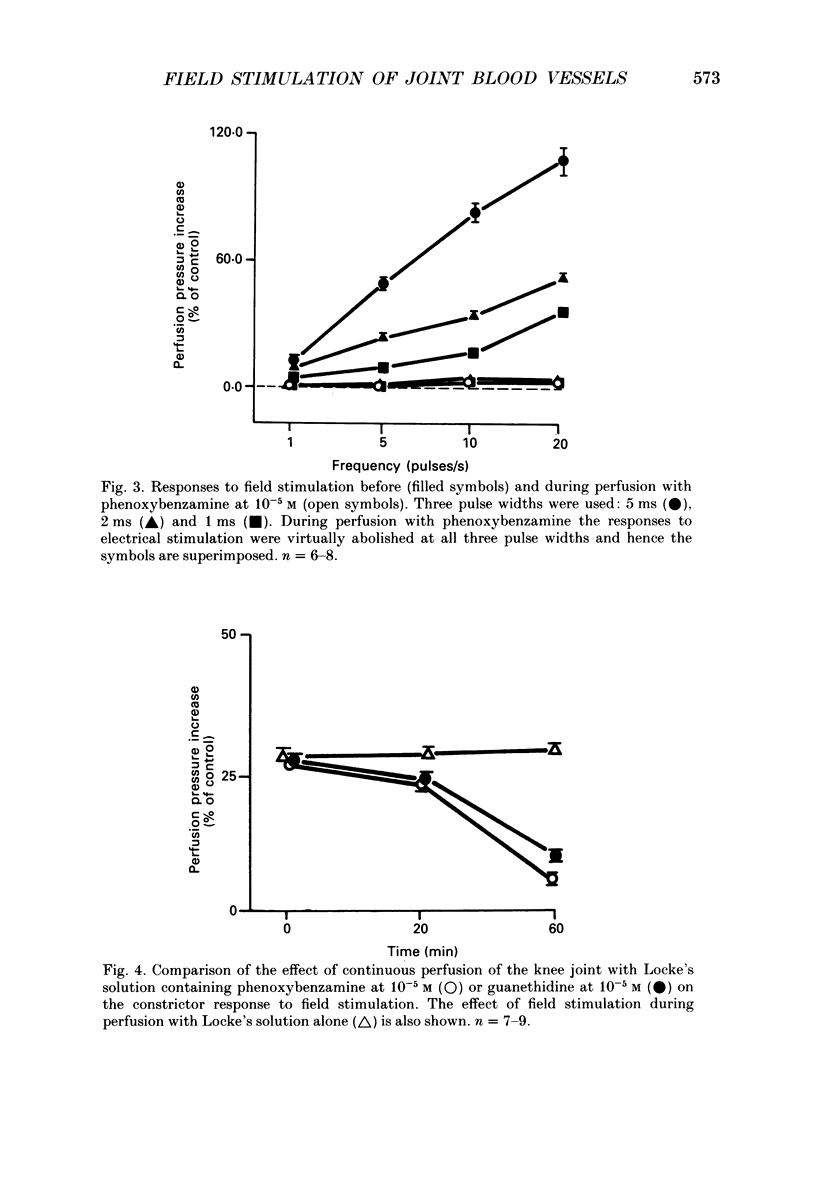
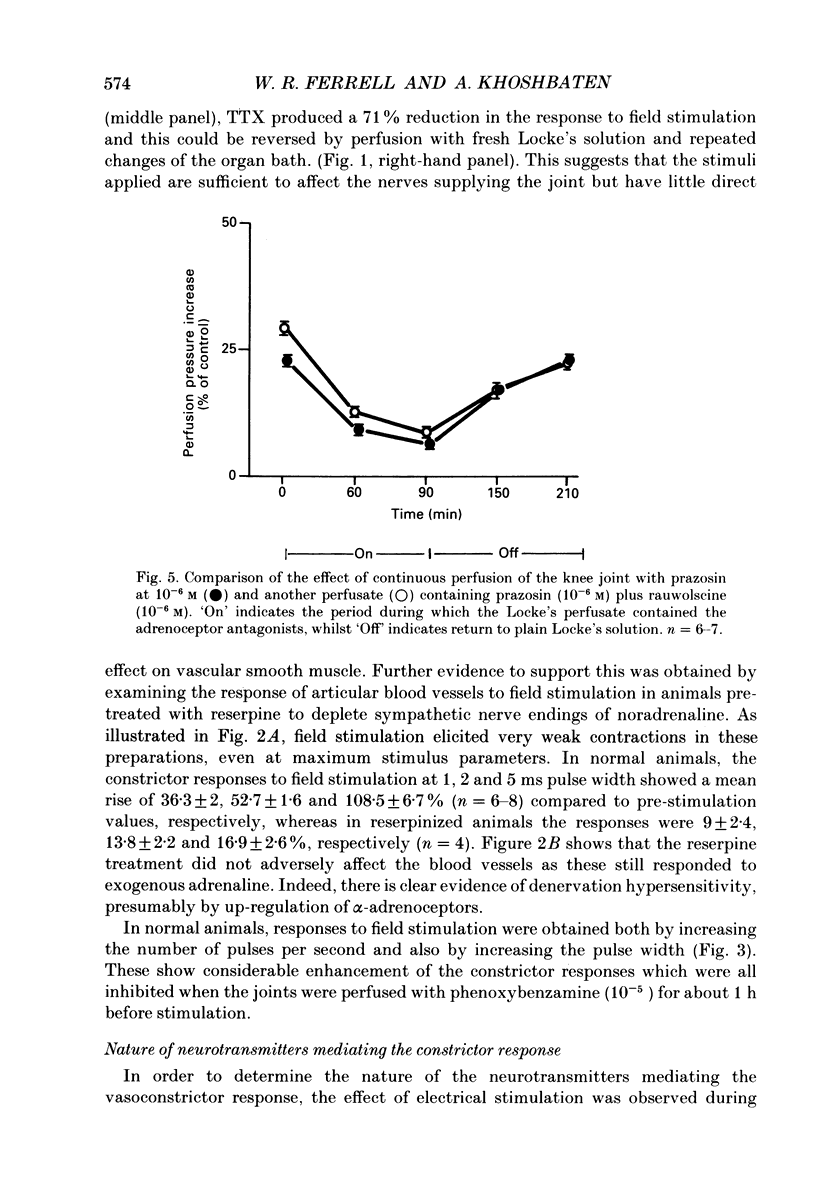
1. An in vitro preparation of the rabbit knee joint, perfused with oxygenated Locke's solution, was used to study the response of articular blood vessels to electrical stimulation of the joint capsule. 2. Using trains of stimulus pulses of different durations, frequency-response curves were obtained. Electrical stimulation always produced vasoconstriction of joint blood vessels, which increased as a function of both frequency and pulse width. 3. This vasoconstrictor response was neurally mediated as it was markedly inhibited after addition to both bath and perfusate of tetrodotoxin. In addition, the response to field stimulation of the capsule was virtually abolished in animals pretreated with reserpine which depletes sympathetic nerve endings of noradrenaline. 4. The response to electrical stimulation was substantially reduced by the alpha-adrenergic antagonist phenoxybenzamine (10(-5) M), the alpha 1-blocker prazosin (10(-6) M), and by guanethidine (10(-5) M) which inhibits the release of noradrenaline, ATP and neuropeptide Y from sympathetic nerve endings. 5. The attenuation of the vasoconstrictor response to field stimulation by prazosin (10(-6) M) was little altered by addition of the alpha 2-adrenoceptor blocker rauwolscine (10(-6) M) to the perfusate. 6. alpha, beta-Methylene ATP (10(-6) M), a P2-purinoceptor desensitizer, had no effect on the vasoconstrictor response to electrical stimulation. 7. These results indicate that the vasoconstrictor response to electrical stimulation of the rabbit knee joint capsule is mediated via noradrenaline acting upon alpha 1-adrenoceptors.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURACK W. R., WEINER N., HAGEN P. B. The effect of reserpine on the catecholamine and adenine nucleotide contents of adrenal gland. J Pharmacol Exp Ther. 1960 Nov;130:245–250. [PubMed] [Google Scholar]
- Burnstock G., Campbell G., Satchell D., Smythe A. Evidence that adenosine triphosphate or a related nucleotide is the transmitter substance released by non-adrenergic inhibitory nerves in the gut. Br J Pharmacol. 1970 Dec;40(4):668–688. doi: 10.1111/j.1476-5381.1970.tb10646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G. Do some nerve cells release more than one transmitter? Neuroscience. 1976 Aug;1(4):239–248. doi: 10.1016/0306-4522(76)90054-3. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Evolution of the autonomic innervation of visceral and cardiovascular systems in vertebrates. Pharmacol Rev. 1969 Dec;21(4):247–324. [PubMed] [Google Scholar]
- COBBOLD A. F., LEWIS O. J. The nervous control of joint blood vessels. J Physiol. 1956 Aug 28;133(2):467–471. doi: 10.1113/jphysiol.1956.sp005601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Mey J., Burnstock G., Vanhoutte P. M. Modulation of the evoked release of noradrenaline in canine saphenous vein via presynaptic receptors for adenosine but not ATP. Eur J Pharmacol. 1979 May 15;55(4):401–405. doi: 10.1016/0014-2999(79)90115-8. [DOI] [PubMed] [Google Scholar]
- Ferrell W. R., Khoshbaten A. Adrenoceptor profile of blood vessels in the knee joint of the rabbit. J Physiol. 1989 Jul;414:377–383. doi: 10.1113/jphysiol.1989.sp017693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARDNER E. Physiology of movable joints. Physiol Rev. 1950 Apr;30(2):127–176. doi: 10.1152/physrev.1950.30.2.127. [DOI] [PubMed] [Google Scholar]
- Head R. J., Stitzel R. E., de la Lande I. S., Johnson S. M. Effect of chronic denervation on the activities of monoamine oxidase and catechol-O-methyl transferase and on the contents of noradrenaline and adenosine triphosphate in the rabbit ear artery. Blood Vessels. 1977;14(4):229–239. doi: 10.1159/000158131. [DOI] [PubMed] [Google Scholar]
- Hirst G. D., Neild T. O. Evidence for two populations of excitatory receptors for noradrenaline on arteriolar smooth muscle. Nature. 1980 Feb 21;283(5749):767–768. doi: 10.1038/283767a0. [DOI] [PubMed] [Google Scholar]
- Kasakov L., Burnstock G. The use of the slowly degradable analog, alpha, beta-methylene ATP, to produce desensitisation of the P2-purinoceptor: effect on non-adrenergic, non-cholinergic responses of the guinea-pig urinary bladder. Eur J Pharmacol. 1982 Dec 24;86(2):291–294. doi: 10.1016/0014-2999(82)90330-2. [DOI] [PubMed] [Google Scholar]
- Langer S. Z., Pinto J. E. Possible involvement of a transmitter different from norepinephrine in the residual responses to nerve stimulation of the cat nictitating membrane after pretreatment with reserpine. J Pharmacol Exp Ther. 1976 Mar;196(3):697–713. [PubMed] [Google Scholar]
- Langford L. A., Schmidt R. F. Afferent and efferent axons in the medial and posterior articular nerves of the cat. Anat Rec. 1983 May;206(1):71–78. doi: 10.1002/ar.1092060109. [DOI] [PubMed] [Google Scholar]
- Lundberg J. M., Anggård A., Theodorsson-Norheim E., Pernow J. Guanethidine-sensitive release of neuropeptide Y-like immunoreactivity in the cat spleen by sympathetic nerve stimulation. Neurosci Lett. 1984 Nov 23;52(1-2):175–180. doi: 10.1016/0304-3940(84)90370-7. [DOI] [PubMed] [Google Scholar]
- Moylan R. D., Westfall T. C. Effect of adenosine on adrenergic neurotransmission in the superfused rat portal vein. Blood Vessels. 1979;16(6):302–310. doi: 10.1159/000158220. [DOI] [PubMed] [Google Scholar]
- Muramatsu I., Fujiwara M., Miura A., Sakakibara Y. Possible involvement of adenine nucleotides in sympathetic neuroeffector mechanisms of dog basilar artery. J Pharmacol Exp Ther. 1981 Feb;216(2):401–409. [PubMed] [Google Scholar]
- Nakanishi H., Takeda H. The possibility that adenosine triphosphate is an excitatory transmitter in guinea-pig seminal vesicle. Jpn J Pharmacol. 1972 Apr;22(2):269–270. doi: 10.1254/jjp.22.269. [DOI] [PubMed] [Google Scholar]
- Neild T. O., Zelcer E. Noradrenergic neuromuscular transmission with special reference to arterial smooth muscle. Prog Neurobiol. 1982;19(3):141–158. doi: 10.1016/0301-0082(82)90004-1. [DOI] [PubMed] [Google Scholar]
- Sneddon P., Burnstock G. ATP as a co-transmitter in rat tail artery. Eur J Pharmacol. 1984 Oct 30;106(1):149–152. doi: 10.1016/0014-2999(84)90688-5. [DOI] [PubMed] [Google Scholar]
- Su C. Modes of vasoconstrictor and vasodilator neurotransmission. Blood Vessels. 1978;15(1-3):183–189. doi: 10.1159/000158164. [DOI] [PubMed] [Google Scholar]
- Su C. Neurogenic release of purine compounds in blood vessels. J Pharmacol Exp Ther. 1975 Oct;195(1):159–166. [PubMed] [Google Scholar]
- Su C. Purinergic inhibition of adrenergic transmission in rabbit blood vessels. J Pharmacol Exp Ther. 1978 Feb;204(2):351–361. [PubMed] [Google Scholar]
- Wakade A. R., Wakade T. D. Inhibition of noradrenaline release by adenosine. J Physiol. 1978 Sep;282:35–49. doi: 10.1113/jphysiol.1978.sp012446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westfall D. P., Stitzel R. E., Rowe J. N. The postjunctional effects and neural release of purine compounds in the guinea-pig vas deferens. Eur J Pharmacol. 1978 Jul 1;50(1):27–38. doi: 10.1016/0014-2999(78)90250-9. [DOI] [PubMed] [Google Scholar]


