Abstract
1. The purpose was to evaluate the hypothesis that neural expiration is composed of two phases: I, a post inspiratory period; and II, the period at which expiratory activities of spinal nerves reach peak values. We hypothesized that the discharge of pulmonary stretch receptors might differentially alter neural activities during these two phases. 2. Activities of the phrenic nerve, intercostal nerve and nerves innervating the thyroarytenoid muscle of the larynx and triangularis sterni muscle of the chest wall were recorded in decerebrate and paralysed cats. 3. The experimental animals were ventilated with a servo-respirator which produced changes in tracheal pressure, and lung volume, in parallel with alterations in integrated activity of the phrenic nerve. 4. In order to assess the influence of the discharge of slowly adapting pulmonary stretch receptors upon neural activities during expiration, lung volume was held at end-expiratory or end-inspiratory levels for individual respiratory cycles. 5. When pulmonary inflation was prevented, phrenic activity increased, as did activity of the thyroarytenoid nerve during early expiration. In contrast, activities of the triangularis sterni and intercostal nerves during mid- to late expiration declined. 6. Holding the lungs at end-inspiratory levels caused a reduction of thyroarytenoid activity and increases in peak triangularis sterni and intercostal activities. Neural expiration typically continued as long as the lungs were maintained at the end-inspiratory level. 7. Responses were qualitatively similar in hypocapnia, normocapnia and hypercapnia, but the magnitude of changes in neural activities was typically augmented with elevations in end-tidal fractional concentrations of CO2. 8. We conclude that the discharge of slowly adapting pulmonary stretch receptors inhibits neural activities during early expiration and augments activities during mid-to late expiration. Hence, our data support the concept that neural expiration is composed of two stages in which neural activities may be differentially controlled.
Full text
PDF


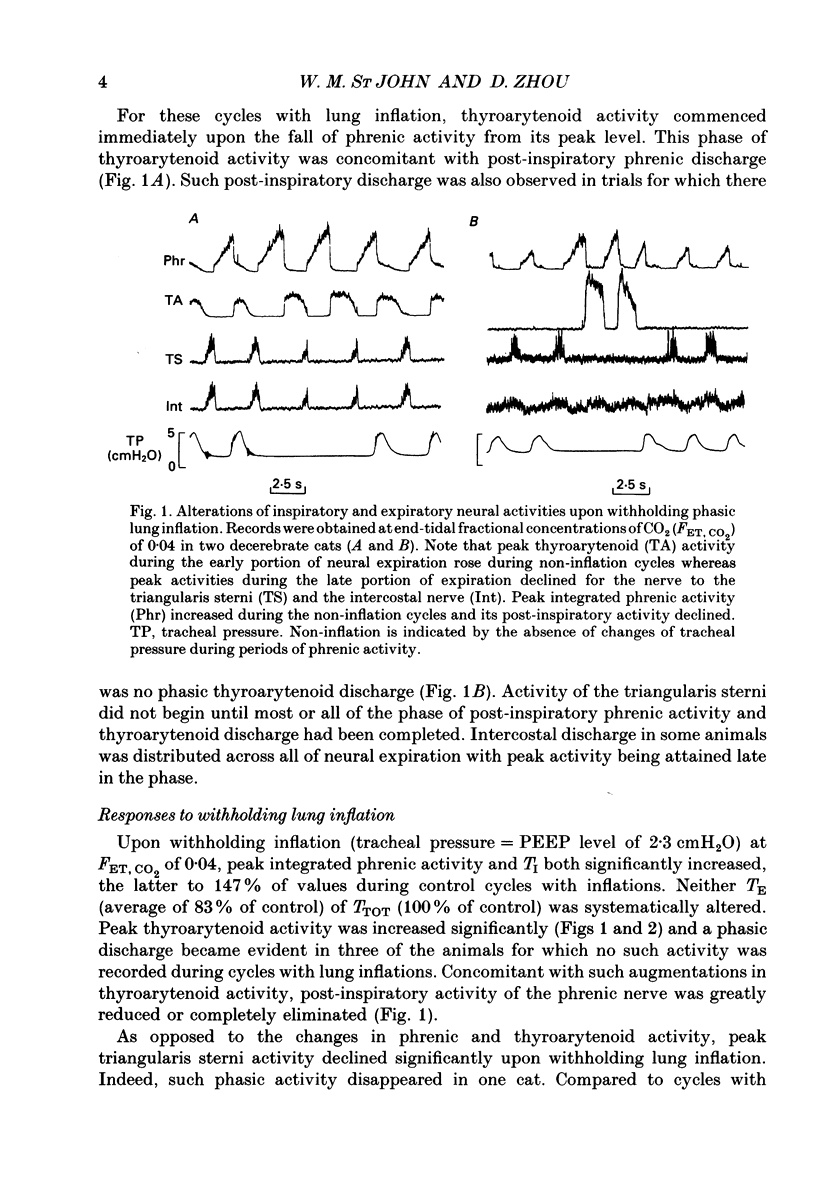
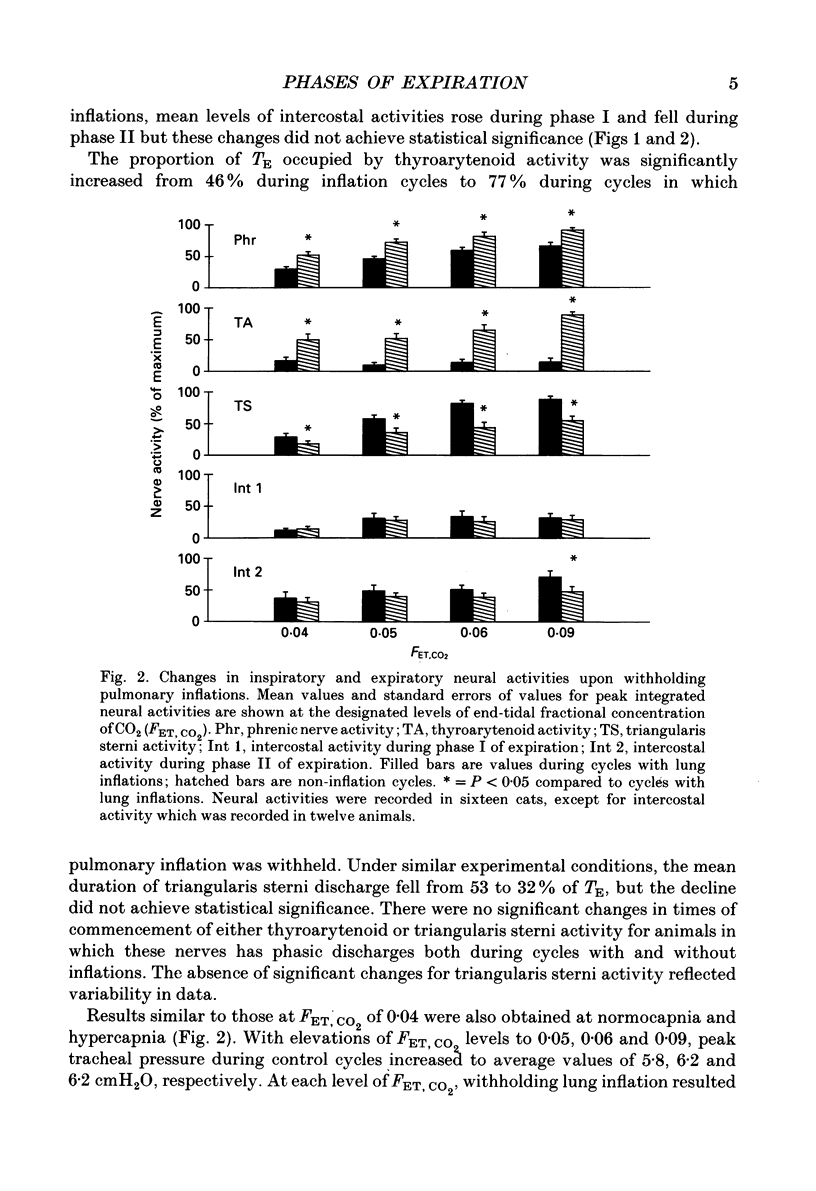
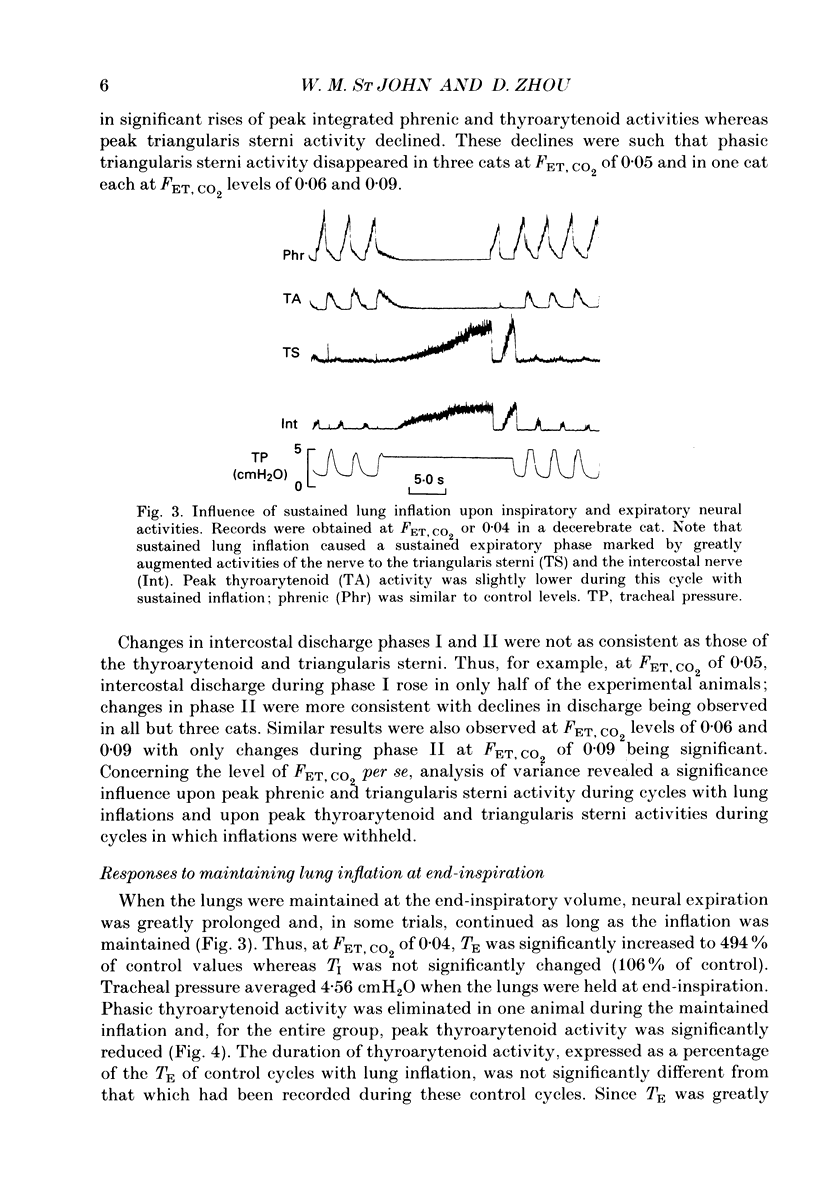
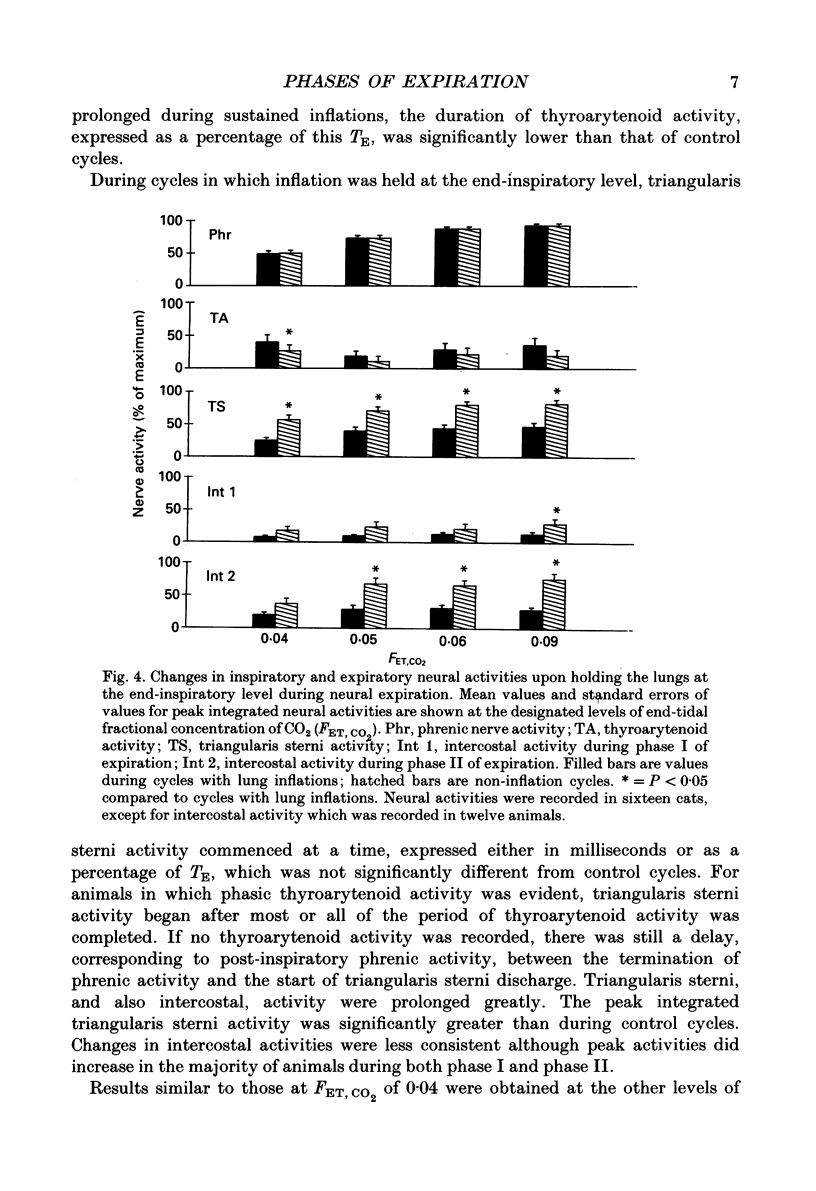





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ballantyne D., Richter D. W. Post-synaptic inhibition of bulbar inspiratory neurones in the cat. J Physiol. 1984 Mar;348:67–87. doi: 10.1113/jphysiol.1984.sp015100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barillot J. C., Dussardier M. Activité des motoneurones laryngés expiratoires. J Physiol (Paris) 1976 Jun;72(3):311–343. [PubMed] [Google Scholar]
- Bishop B., Bachofen H. Comparative influence of proprioceptors and chemoreceptors in the control of respiratory muscles. Acta Neurobiol Exp (Wars) 1973;33(1):381–390. [PubMed] [Google Scholar]
- Cohen M. I., Feldman J. L., Sommer D. Caudal medullary expiratory neurone and internal intercostal nerve discharges in the cat: effects of lung inflation. J Physiol. 1985 Nov;368:147–178. doi: 10.1113/jphysiol.1985.sp015851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daubenspeck J. A., Pichon D., Knuth K. V., Bartlett D., Jr, St John W. M. An inexpensive servo-respirator based upon regulation of a shunt resistance. Respir Physiol. 1988 Jul;73(1):87–96. doi: 10.1016/0034-5687(88)90129-6. [DOI] [PubMed] [Google Scholar]
- Huang Q., Zhou D., St John W. M., Bartlett D., Jr Influence of lung volume on activities of branches of the recurrent laryngeal nerve. J Appl Physiol (1985) 1989 Sep;67(3):1179–1184. doi: 10.1152/jappl.1989.67.3.1179. [DOI] [PubMed] [Google Scholar]
- Remmers J. E., Richter D. W., Ballantyne D., Bainton C. R., Klein J. P. Reflex prolongation of stage I of expiration. Pflugers Arch. 1986 Aug;407(2):190–198. doi: 10.1007/BF00580675. [DOI] [PubMed] [Google Scholar]
- Richter D. W., Ballantyne D., Remmers J. E. The differential organization of medullary post-inspiratory activities. Pflugers Arch. 1987 Nov;410(4-5):420–427. doi: 10.1007/BF00586520. [DOI] [PubMed] [Google Scholar]
- Richter D. W. Generation and maintenance of the respiratory rhythm. J Exp Biol. 1982 Oct;100:93–107. doi: 10.1242/jeb.100.1.93. [DOI] [PubMed] [Google Scholar]
- St John W. M. Differential alteration by hypercapnia and hypoxia of the apneustic respiratory pattern in decerebrate cats. J Physiol. 1979 Feb;287:467–491. doi: 10.1113/jphysiol.1979.sp012671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St John W. M., Zhou D. Differing control of neural activities during various portions of expiration in the cat. J Physiol. 1989 Nov;418:189–204. doi: 10.1113/jphysiol.1989.sp017834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D., Huang Q., St John W. M., Bartlett D., Jr Respiratory activities of intralaryngeal branches of the recurrent laryngeal nerve. J Appl Physiol (1985) 1989 Sep;67(3):1171–1178. doi: 10.1152/jappl.1989.67.3.1171. [DOI] [PubMed] [Google Scholar]


