Abstract
1. N-methyl-D-aspartate (NMDA)-, quisqualate- and kainate-induced currents were recorded in cultured rat hippocampal neurones using the whole-cell voltage-clamp technique. To isolate the inward currents carried by Ca2+ and other divalent cations (Sr2+, Ba2+, Mn2+ and Mg2+), both Na+ and K+ in the control external solution were replaced with the impermeant cation N-methylglucamine (NMG). 2. Replacement of Na+, K+ and Ca2+ with NMG abolished NMDA-, quisqualate- and kinate-induced inward currents. In Na(+)-, K(+)-free (abbreviated simply as Na(+)-free) solution containing 10 mM-Ca2+ NMDA caused prominent inward currents at -60 mV. In this solution with the internal solution containing 165 mM-Cs+, the reversal potential of the NMDA-induced current was -5.0 +/- 0.7 mV (n = 36), indicating a value of PCa/PCs = 6.2 for the ratio of the permeability coefficients of Ca2+ and Cs+ according to the constant-field equation. 3. NMDA elicited inward current responses at -60 mV in Na(+)-, Ca2(+)-free solution containing 10 mM-Sr2+, Ba2+, or Mn2+, but not in Na(+)-free, 10 mM-Mg2+ solution. On the basis of reversal potential measurements, the permeability sequence of NMDA receptor channels among the divalent cations was determined to be Ba2+ (1.2) greater than Ca2+ (1.0) greater than Sr2+ (0.8) greater than Mn2+ (0.3) much greater than Mg2+ (less than 0.02). 4. The reversal potential of the quisqualate-induced current was more negative than -80 mV in Na(+)-free, 10 mM-Ca2+ solution, indicating a value of PCa/PCs less than 0.18. 5. Kainate-induced current responses were classified into two types. In the type I response the reversal potential of the kainate-induced current was more negative than -80 mV in Na(+)-free, 10 mM-Ca2+ solution, indicating that the Ca2+ permeability of this type of kainate channel is as low as that of the quisqualate channel. In the neurones which showed a type I response, there was a tendency of outward rectification in the current-voltage plots of the kainate response in control solution. 6. In the type II response kainate caused prominent inward currents at -60 mV in Na(+)-free, 10 mM-Ca2+ solution. The reversal potential was -23.3 +/- 5.6 mV (n = 17), indicating a permeability ratio PCa/PCs = 2.3. In the neurones which showed a type II response, a remarkable inward rectification was observed in the current-voltage plots of the kainate response in control solution. 7. Type II kainate channels showed relatively poor selectivity among divalent cations.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF











Selected References
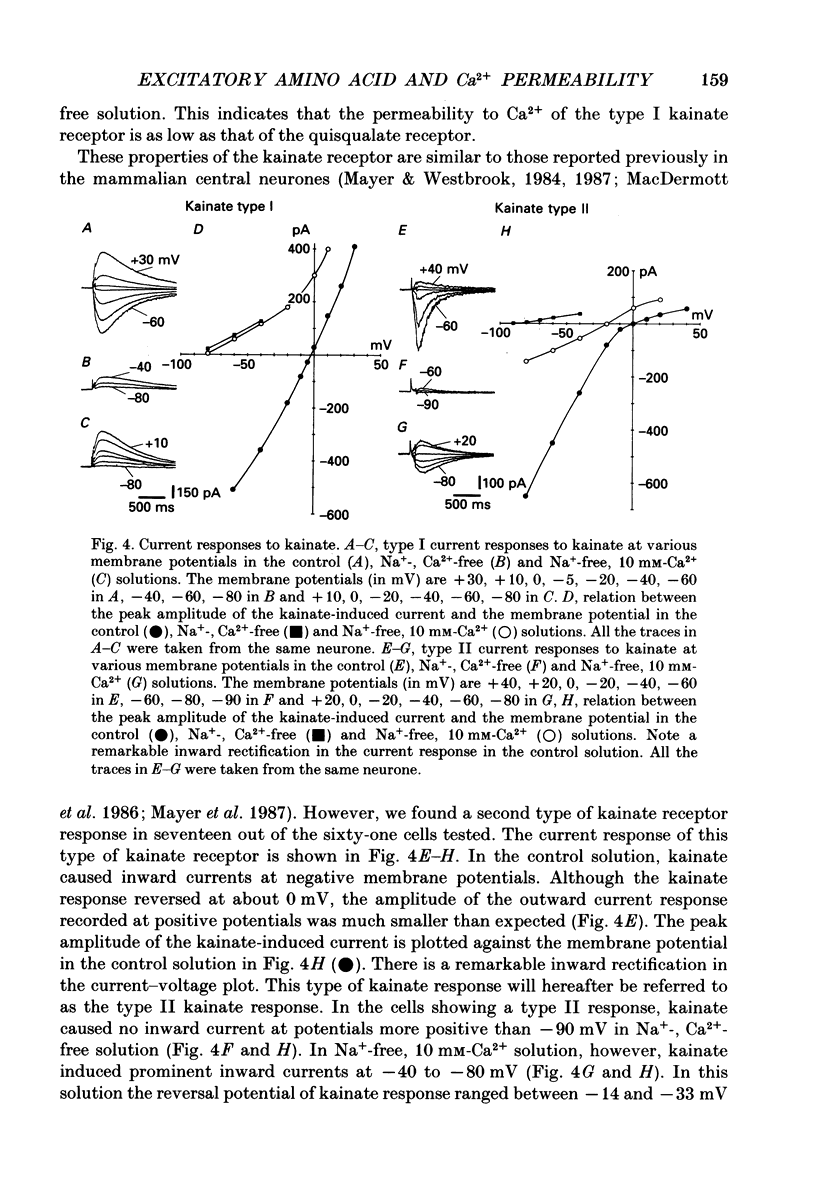
These references are in PubMed. This may not be the complete list of references from this article.
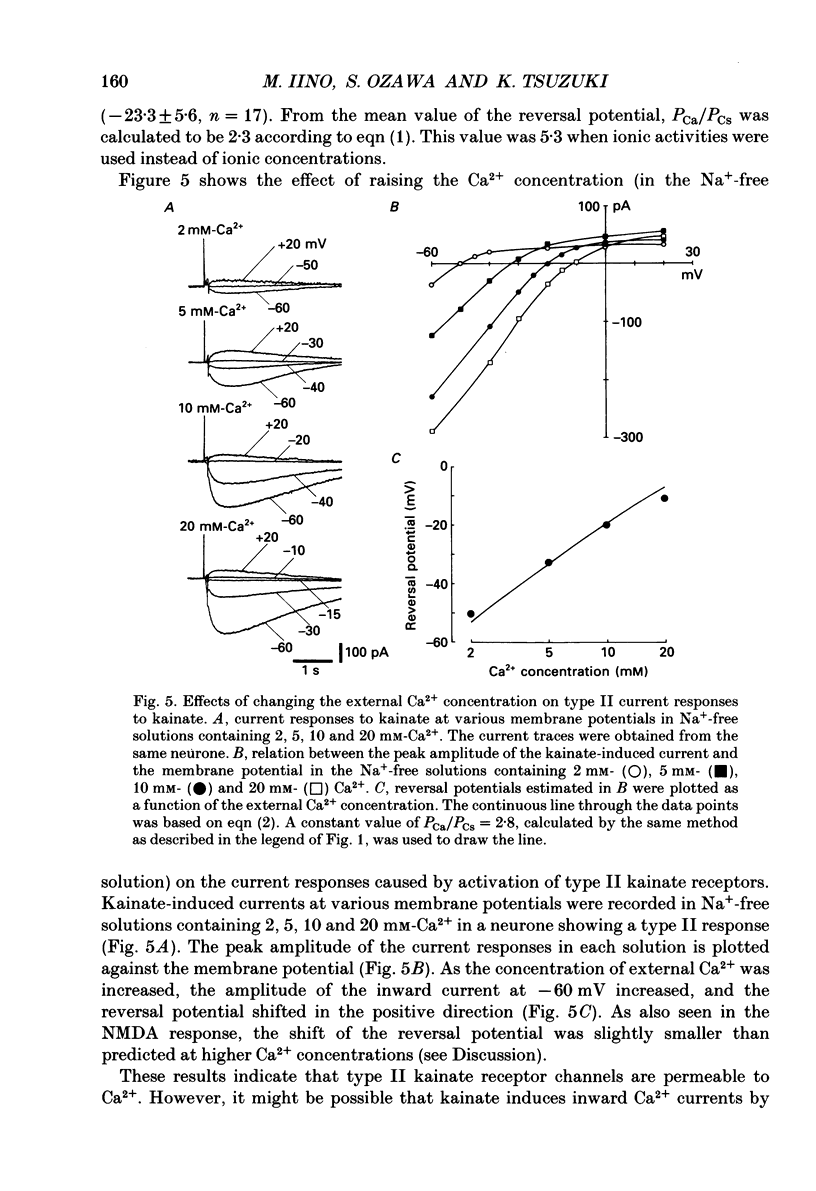
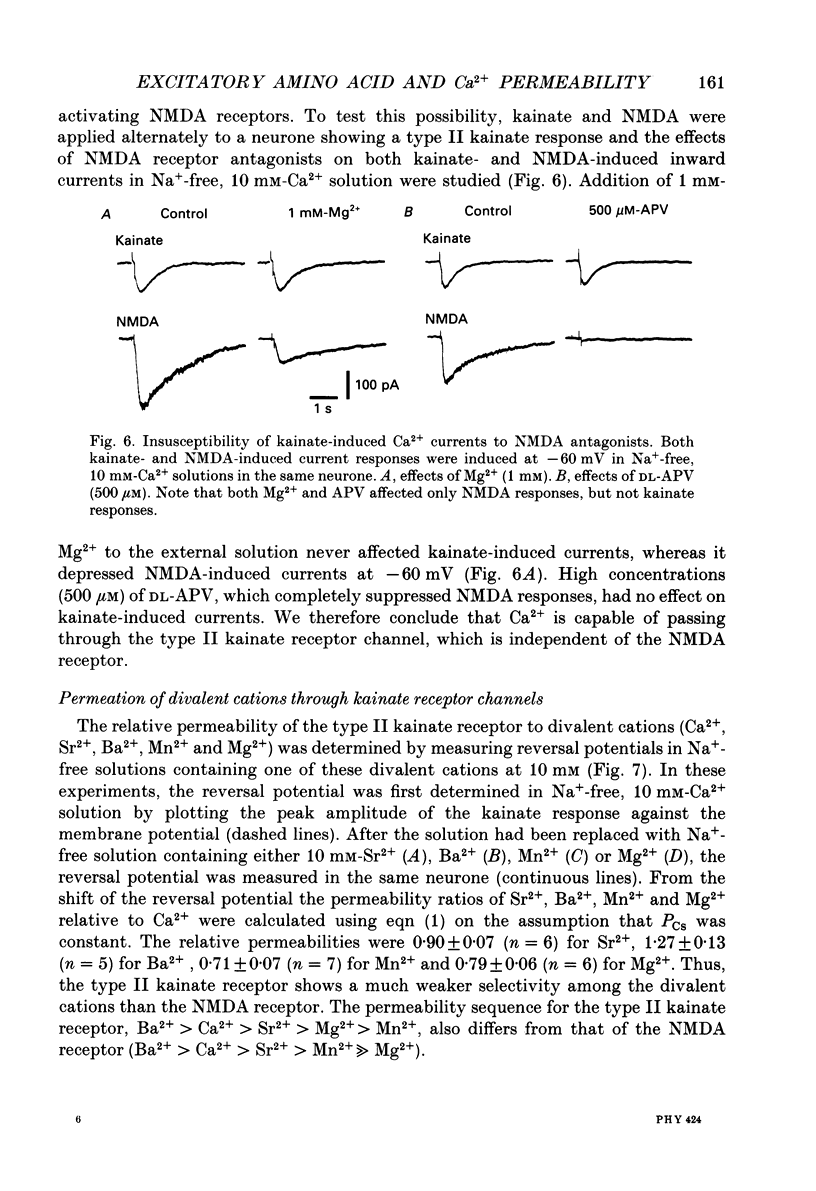
- Adams D. J., Dwyer T. M., Hille B. The permeability of endplate channels to monovalent and divalent metal cations. J Gen Physiol. 1980 May;75(5):493–510. doi: 10.1085/jgp.75.5.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascher P., Nowak L. The role of divalent cations in the N-methyl-D-aspartate responses of mouse central neurones in culture. J Physiol. 1988 May;399:247–266. doi: 10.1113/jphysiol.1988.sp017078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banker G. A., Cowan W. M. Rat hippocampal neurons in dispersed cell culture. Brain Res. 1977 May 13;126(3):397–342. doi: 10.1016/0006-8993(77)90594-7. [DOI] [PubMed] [Google Scholar]
- Cull-Candy S. G., Howe J. R., Ogden D. C. Noise and single channels activated by excitatory amino acids in rat cerebellar granule neurones. J Physiol. 1988 Jun;400:189–222. doi: 10.1113/jphysiol.1988.sp017117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cull-Candy S. G., Usowicz M. M. Multiple-conductance channels activated by excitatory amino acids in cerebellar neurons. Nature. 1987 Feb 5;325(6104):525–528. doi: 10.1038/325525a0. [DOI] [PubMed] [Google Scholar]
- Cull-Candy S. G., Usowicz M. M. On the multiple-conductance single channels activated by excitatory amino acids in large cerebellar neurones of the rat. J Physiol. 1989 Aug;415:555–582. doi: 10.1113/jphysiol.1989.sp017736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekin M. S. Permeability changes induced by L-glutamate at the crayfish neuromuscular junction. J Physiol. 1983 Aug;341:105–125. doi: 10.1113/jphysiol.1983.sp014795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingledine R. N-methyl aspartate activates voltage-dependent calcium conductance in rat hippocampal pyramidal cells. J Physiol. 1983 Oct;343:385–405. doi: 10.1113/jphysiol.1983.sp014899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox A. P., Nowycky M. C., Tsien R. W. Kinetic and pharmacological properties distinguishing three types of calcium currents in chick sensory neurones. J Physiol. 1987 Dec;394:149–172. doi: 10.1113/jphysiol.1987.sp016864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KATZ B. The effect of sodium ions on the electrical activity of giant axon of the squid. J Physiol. 1949 Mar 1;108(1):37–77. doi: 10.1113/jphysiol.1949.sp004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Ohmori H. Studies of calcium channels in rat clonal pituitary cells with patch electrode voltage clamp. J Physiol. 1982 Oct;331:231–252. doi: 10.1113/jphysiol.1982.sp014371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Jahr C. E., Stevens C. F. Glutamate activates multiple single channel conductances in hippocampal neurons. Nature. 1987 Feb 5;325(6104):522–525. doi: 10.1038/325522a0. [DOI] [PubMed] [Google Scholar]
- Lewis C. A. Ion-concentration dependence of the reversal potential and the single channel conductance of ion channels at the frog neuromuscular junction. J Physiol. 1979 Jan;286:417–445. doi: 10.1113/jphysiol.1979.sp012629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDermott A. B., Mayer M. L., Westbrook G. L., Smith S. J., Barker J. L. NMDA-receptor activation increases cytoplasmic calcium concentration in cultured spinal cord neurones. 1986 May 29-Jun 4Nature. 321(6069):519–522. doi: 10.1038/321519a0. [DOI] [PubMed] [Google Scholar]
- Mayer M. L., MacDermott A. B., Westbrook G. L., Smith S. J., Barker J. L. Agonist- and voltage-gated calcium entry in cultured mouse spinal cord neurons under voltage clamp measured using arsenazo III. J Neurosci. 1987 Oct;7(10):3230–3244. doi: 10.1523/JNEUROSCI.07-10-03230.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L., Guthrie P. B. Voltage-dependent block by Mg2+ of NMDA responses in spinal cord neurones. Nature. 1984 May 17;309(5965):261–263. doi: 10.1038/309261a0. [DOI] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L. Mixed-agonist action of excitatory amino acids on mouse spinal cord neurones under voltage clamp. J Physiol. 1984 Sep;354:29–53. doi: 10.1113/jphysiol.1984.sp015360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L. Permeation and block of N-methyl-D-aspartic acid receptor channels by divalent cations in mouse cultured central neurones. J Physiol. 1987 Dec;394:501–527. doi: 10.1113/jphysiol.1987.sp016883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak L., Bregestovski P., Ascher P., Herbet A., Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984 Feb 2;307(5950):462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- Ozawa S., Nakamura T., Yuzaki M. Cation permeability change caused by L-glutamate in cultured rat hippocampal neurons. Brain Res. 1988 Mar 8;443(1-2):85–94. doi: 10.1016/0006-8993(88)91601-0. [DOI] [PubMed] [Google Scholar]
- Pumain R., Heinemann U. Stimulus- and amino acid-induced calcium and potassium changes in rat neocortex. J Neurophysiol. 1985 Jan;53(1):1–16. doi: 10.1152/jn.1985.53.1.1. [DOI] [PubMed] [Google Scholar]
- Shatkay A. Individual activity of calcium ions in pure solutions of CaCl2 and in mixtures. Biophys J. 1968 Aug;8(8):912–919. doi: 10.1016/S0006-3495(68)86528-2. [DOI] [PMC free article] [PubMed] [Google Scholar]


