Abstract
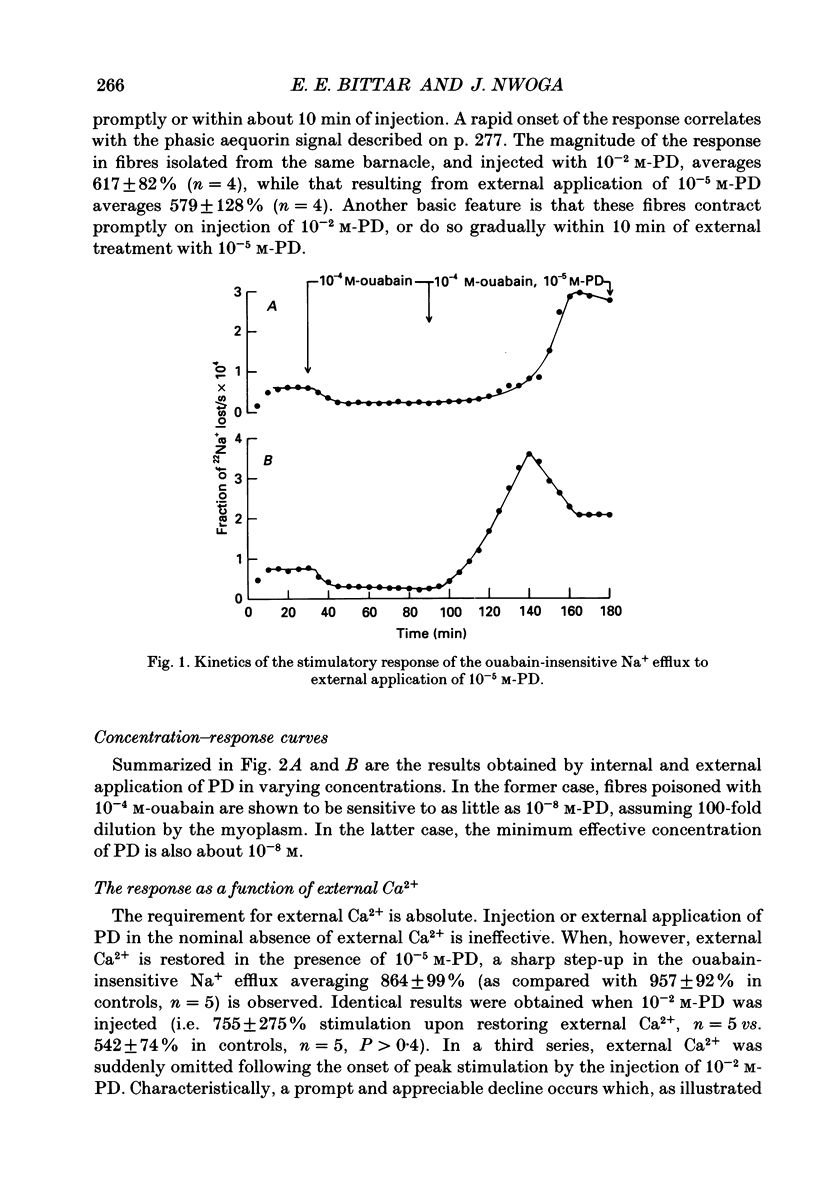
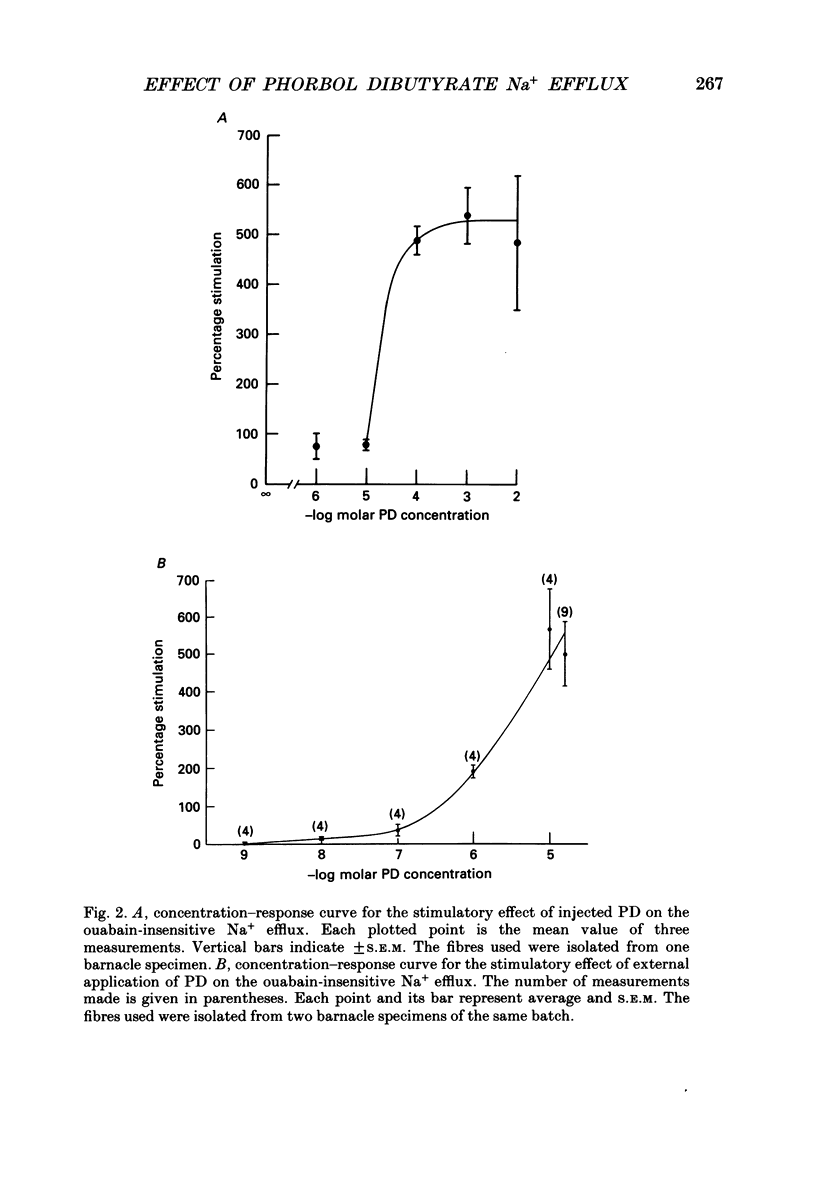
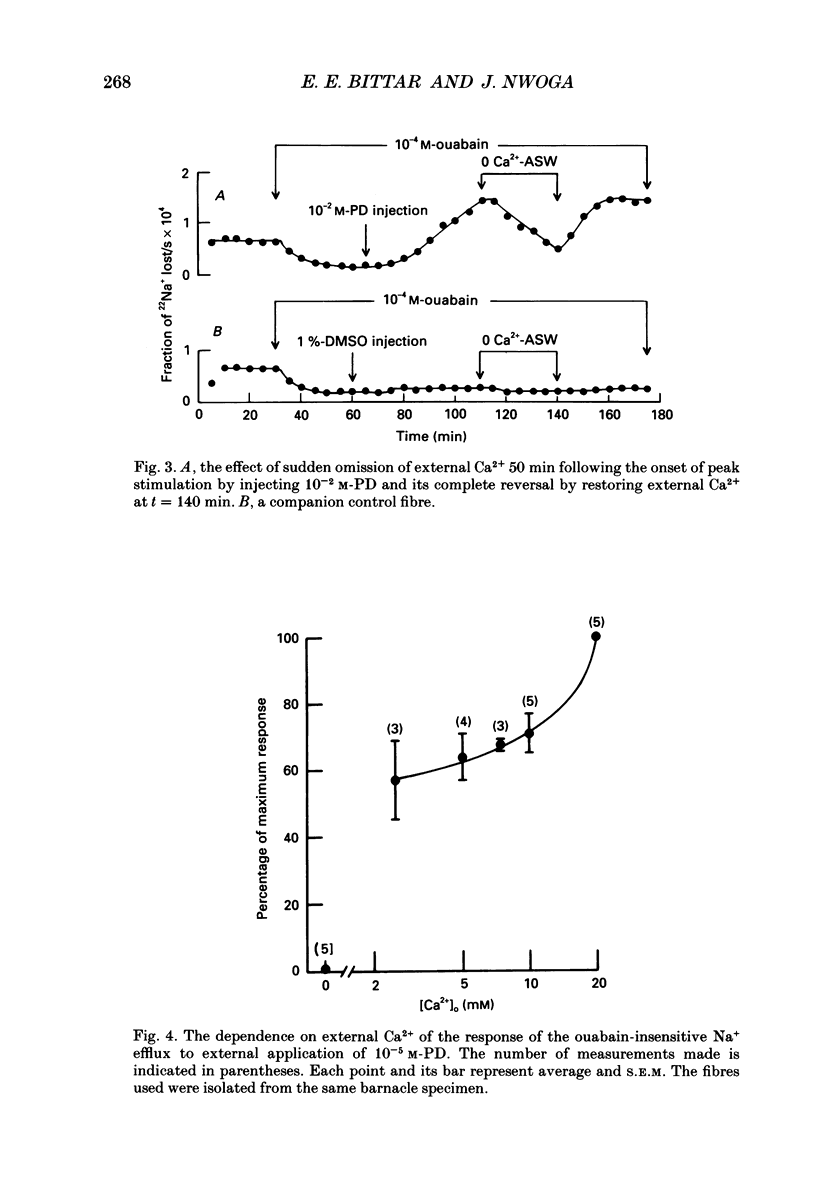
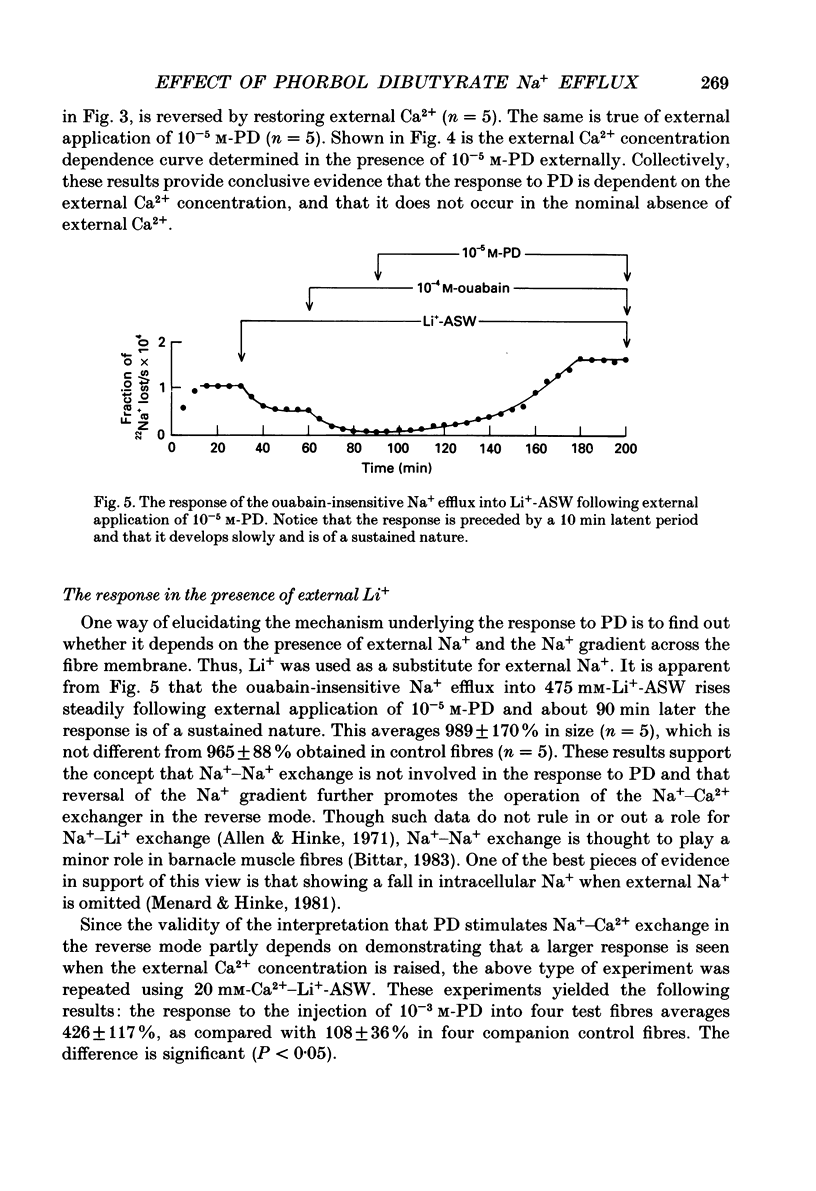
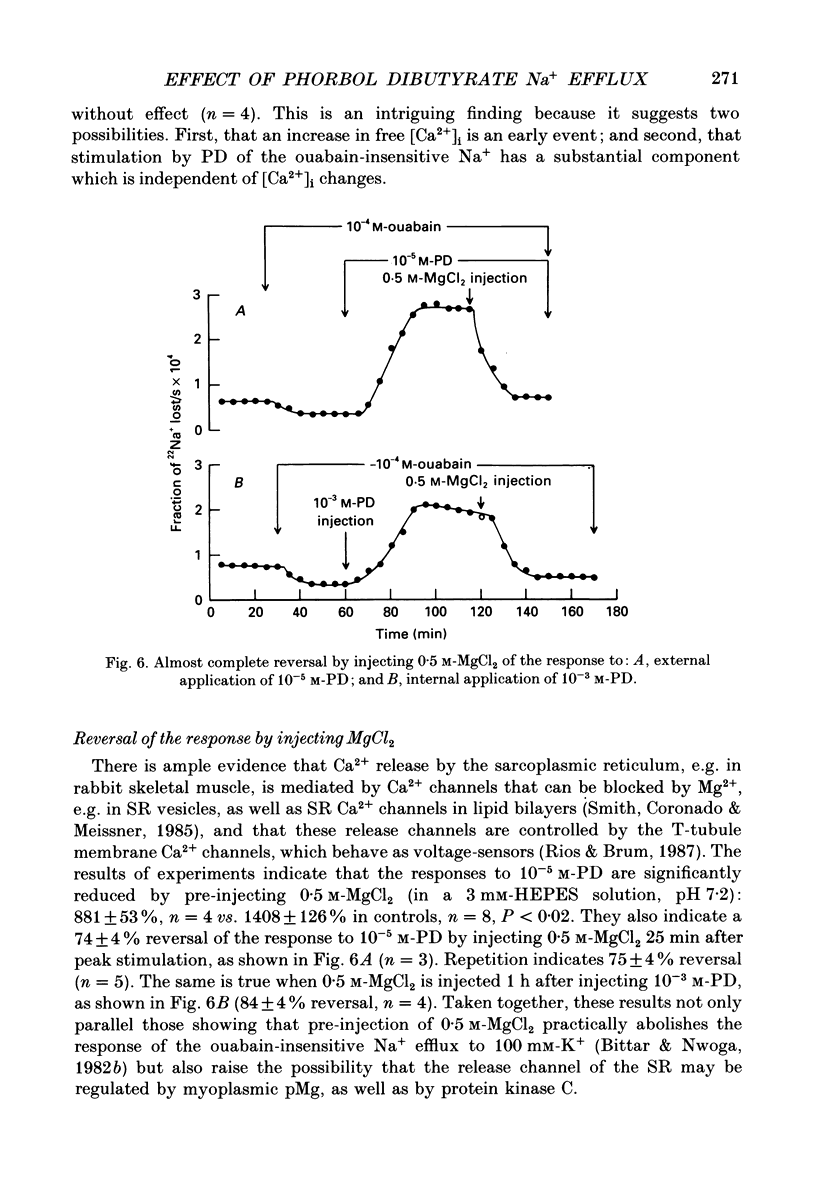
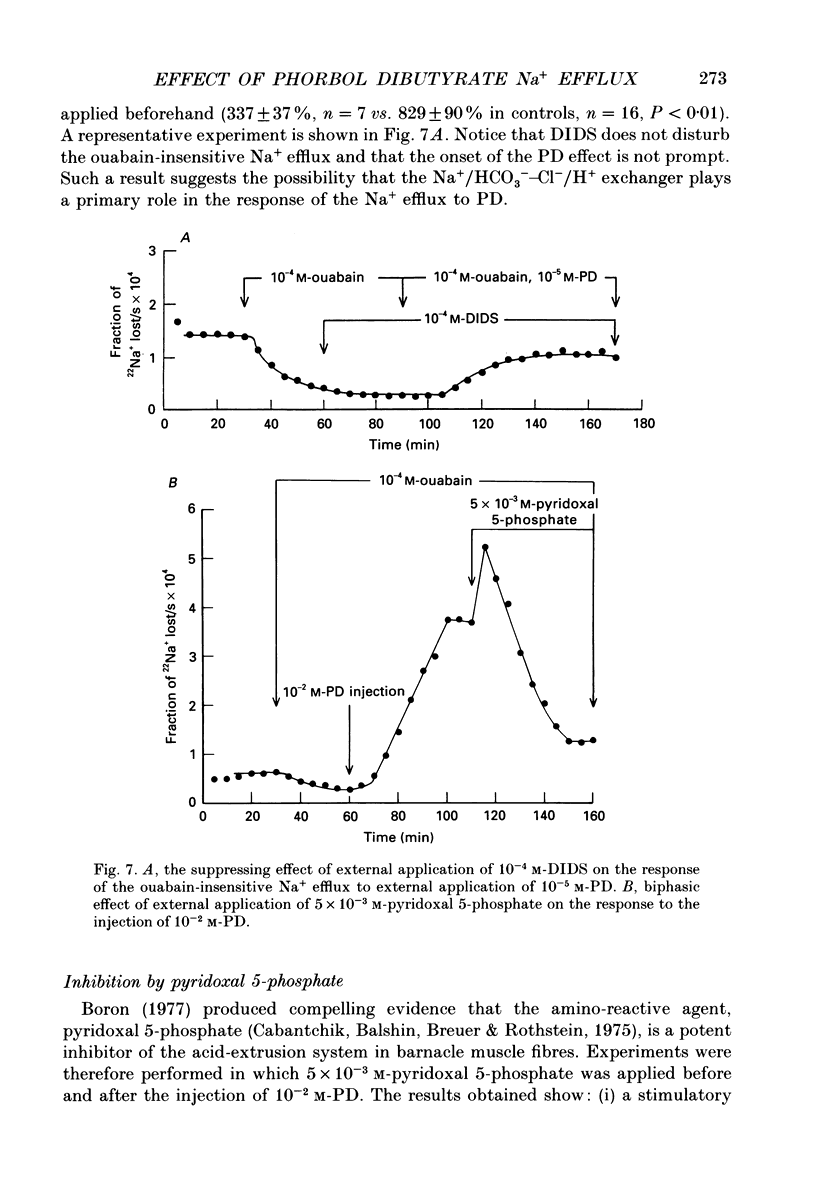
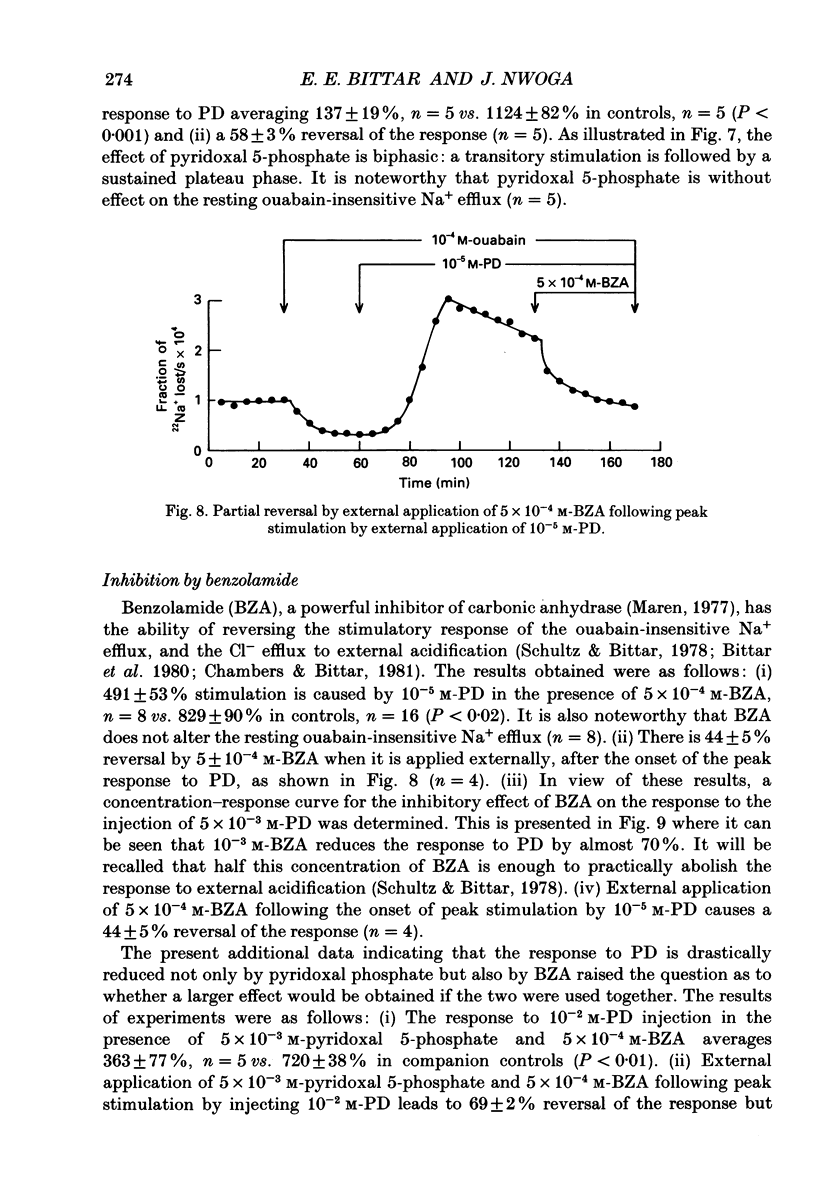
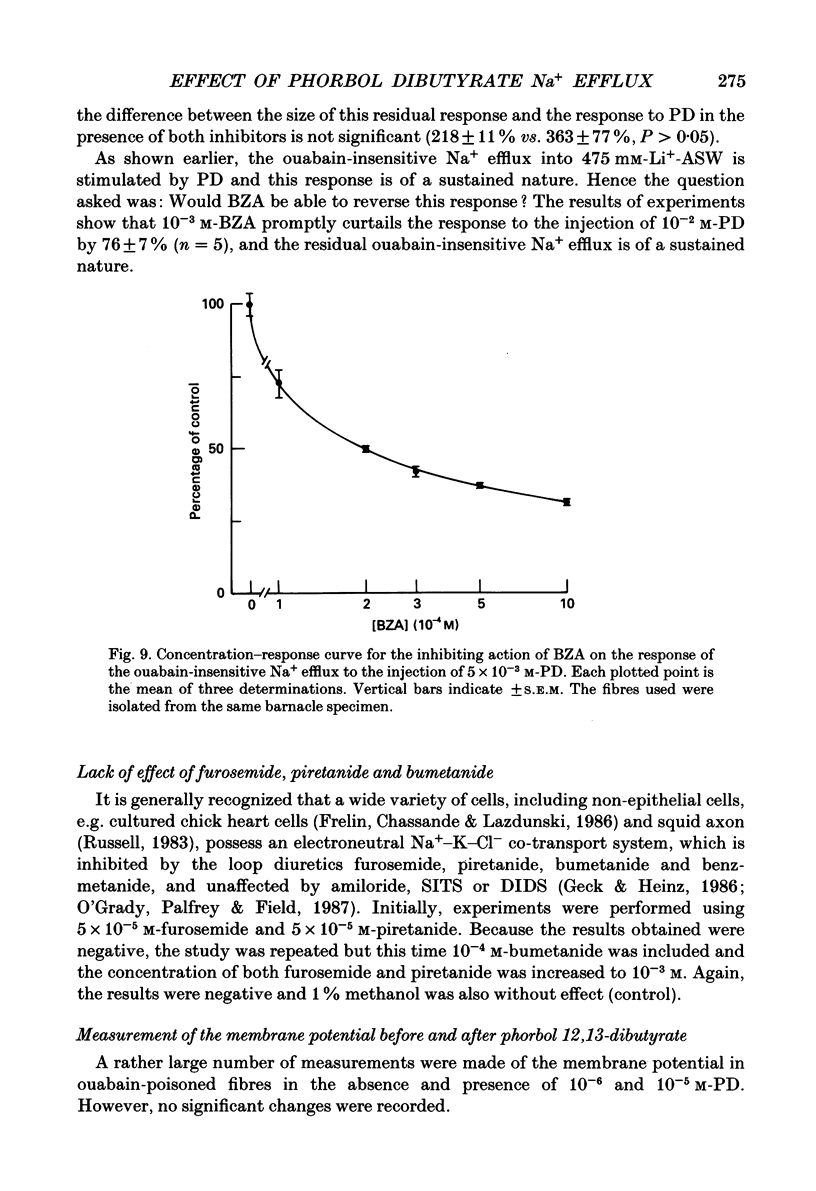
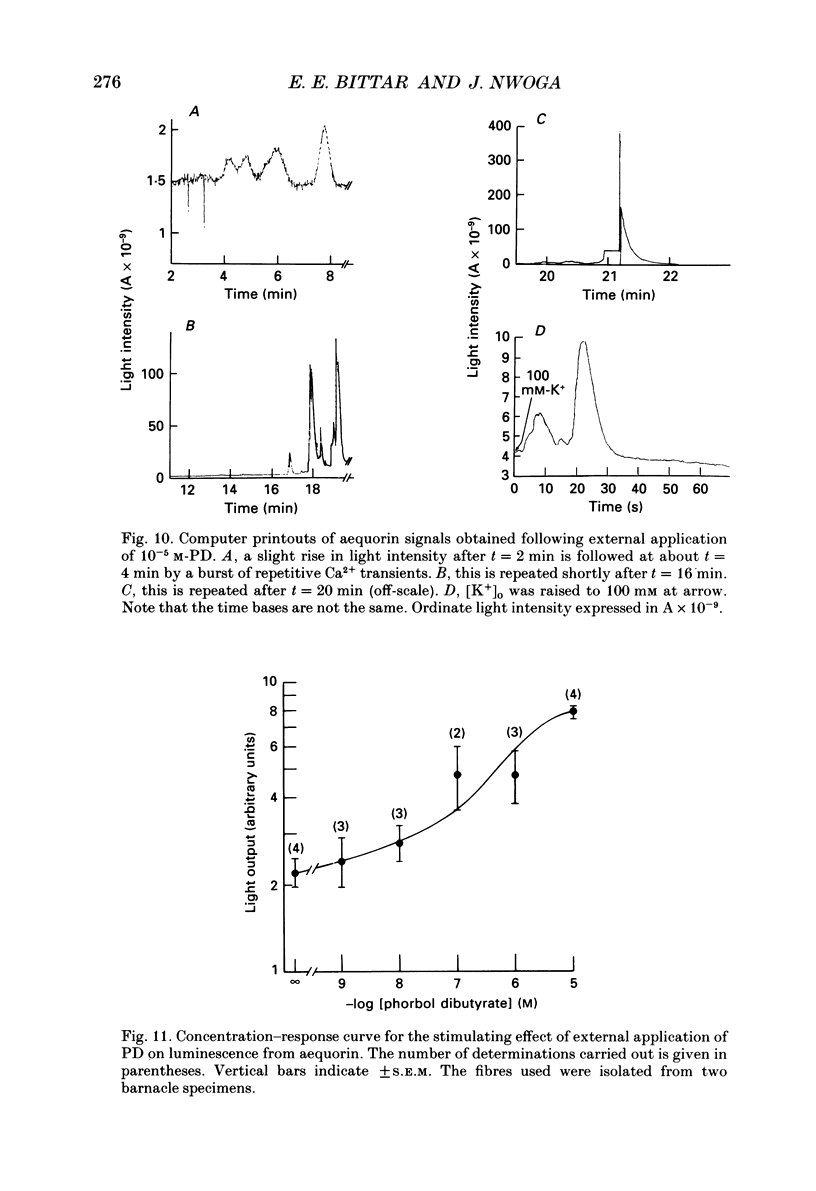
1. The resting ouabain-insensitive Na+ efflux in muscle fibres isolated from the barnacle, Balanus nubilus, is stimulated by external or internal application of phorbol 12,13-dibutyrate (PD). The response occurs fairly promptly and may not decay at all, or more commonly, decay rather slowly. The magnitude of the response to external or internal application of PD is dose-dependent, the minimum effective concentration being about 10(-8) M. 2. The response to PD fails to occur in the nominal absence of external Ca2+. Sudden removal of external Ca subsequent to peak stimulation by PD leads to almost complete reversal of the response. The response to PD of fibres suspended in Li(+)-ASW (artificial sea water) is similar in magnitude to that of fibres suspended in Na(+)-ASW. However, it differs in that it is of a sustained nature. 3. Calcium channel blockers, e.g. verapamil, completely prevent the response to PD from occurring. Both Cd2+ and Co2+ are less effective than verapamil. 4. Pre- but not post-injection of EGTA reduces the response to PD. Pre- or post-injection of Mg2+ reduces the response considerably. 5. Fibres pre-injected with GTP show a reduced response to PD. Fibres pre-injected with PD show a reduced response to GTP. Pre-injection of protein kinase inhibitor is without effect on the response to PD. 6. Furosemide, piretanide and bumetanide are without effect on the response to PD. 7. DIDS (4,4'-diisothiocyanostilbene-2,2-disulphonic acid) is a potent inhibitor of the response to PD but not amiloride. Pyridoxal 5-phosphate and benzolamide are also powerful inhibitors. Pyridoxal 5-phosphate in combination with benzolamide fails to completely abolish or reverse the response to PD. 8. Luminescence from aequorin is promptly increased by PD in a dose-dependent manner, the minimal effective concentration being in the nanomolar range. The signal is monophasic or multiphasic in shape, and is often less than 5 min in duration. Not infrequently, however, the aequorin response fails to completely decay and the new level of resting glow remains above the original baseline level. 9. Collectively, these observations accord with a tentative general hypothesis stating that the stimulatory response of the ouabain-insensitive Na+ efflux to PD is triggered by two mechanisms. One involves a rise in myoplasmic free [Ca2+] resulting from the entry of external Ca2+ via opened Ca2+ channels which is followed by the operation of the Na(+)-Ca2+ exchanger in the reverse mode.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alkon D. L., Rasmussen H. A spatial-temporal model of cell activation. Science. 1988 Feb 26;239(4843):998–1005. doi: 10.1126/science.2830669. [DOI] [PubMed] [Google Scholar]
- Allen R. D., Hinke J. A. Na + -Li + exchange in single muscle fibers of the giant barnacle. Can J Physiol Pharmacol. 1971 Oct;49(10):862–866. doi: 10.1139/y71-120. [DOI] [PubMed] [Google Scholar]
- Anderson W. B., Estival A., Tapiovaara H., Gopalakrishna R. Altered subcellular distribution of protein kinase C (a phorbol ester receptor). Possible role in tumor promotion and the regulation of cell growth: relationship to changes in adenylate cyclase activity. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1985;19:287–306. [PubMed] [Google Scholar]
- Aronson P. S., Nee J., Suhm M. A. Modifier role of internal H+ in activating the Na+-H+ exchanger in renal microvillus membrane vesicles. Nature. 1982 Sep 9;299(5879):161–163. doi: 10.1038/299161a0. [DOI] [PubMed] [Google Scholar]
- Ashley C. C., Ellory J. C., Lea T. J., Ramos M. The effects of inhibitors on 36Cl efflux from barnacle muscle fibres [proceedings]. J Physiol. 1978 Dec;285:52P–53P. [PubMed] [Google Scholar]
- Ashley C. C., Ridgway E. B. On the relationships between membrane potential, calcium transient and tension in single barnacle muscle fibres. J Physiol. 1970 Jul;209(1):105–130. doi: 10.1113/jphysiol.1970.sp009158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Carruthers A. Insulin regulation of sugar transport in giant muscle fibres of the barnacle. J Physiol. 1983 Mar;336:397–431. doi: 10.1113/jphysiol.1983.sp014588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazzi M. D., Nelsestuen G. L. Properties of membrane-inserted protein kinase C. Biochemistry. 1988 Oct 4;27(20):7589–7593. doi: 10.1021/bi00420a003. [DOI] [PubMed] [Google Scholar]
- Bittar E. E., Chambers G., Brown D. Some observations on the behaviour of the sodium efflux in barnacle muscle fibres towards lithium ions. Comp Biochem Physiol B. 1983;76(4):921–925. doi: 10.1016/0305-0491(83)90413-3. [DOI] [PubMed] [Google Scholar]
- Bittar E. E., Demaille J., Fischer E. H., Schultz R. Mode of stimulation by injection of cyclic AMP and external acidification of the sodium efflux in barnacle muscle fibres. J Physiol. 1979 Nov;296:277–289. doi: 10.1113/jphysiol.1979.sp013005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittar E. E., Girard P. R. Evidence for the presence of protein kinase C in barnacle muscle fibers. Comp Biochem Physiol B. 1987;88(2):687–690. doi: 10.1016/0305-0491(87)90364-6. [DOI] [PubMed] [Google Scholar]
- Bittar E. E., Nwoga J. Further observations on the behaviour of ouabain-insensitive sodium efflux towards proctolin in barnacle muscle fibres. J Physiol. 1989 Dec;419:435–453. doi: 10.1113/jphysiol.1989.sp017879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittar E. E., Nwoga J. Some further observations on the stimulation by high external potassium of the sodium efflux in barnacle muscle fibers. Pflugers Arch. 1982 Dec;395(4):318–325. doi: 10.1007/BF00580796. [DOI] [PubMed] [Google Scholar]
- Bittar E. E., Nwoga J. Stimulation by injected guanosine triphosphate of the sodium efflux in barnacle muscle fibres. J Physiol. 1982 Jan;322:389–397. doi: 10.1113/jphysiol.1982.sp014043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittar E. E., Schultz R., Tesar J. Chloride efflux in single barnacle muscle fibres. J Physiol. 1980 Apr;301:317–336. doi: 10.1113/jphysiol.1980.sp013208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittar E. E. The barnacle muscle fibre as a model system for the investigation of the ouabain-insensitive sodium efflux and hormonal actions. Prog Neurobiol. 1983;20(1-2):1–54. doi: 10.1016/0301-0082(83)90009-6. [DOI] [PubMed] [Google Scholar]
- Blinks J. R., Wier W. G., Hess P., Prendergast F. G. Measurement of Ca2+ concentrations in living cells. Prog Biophys Mol Biol. 1982;40(1-2):1–114. doi: 10.1016/0079-6107(82)90011-6. [DOI] [PubMed] [Google Scholar]
- Boron W. F. Intracellular pH transients in giant barnacle muscle fibers. Am J Physiol. 1977 Sep;233(3):C61–C73. doi: 10.1152/ajpcell.1977.233.3.C61. [DOI] [PubMed] [Google Scholar]
- Cabantchik I. Z., Balshin M., Breuer W., Rothstein A. Pyridoxal phosphate. An anionic probe for protein amino groups exposed on the outer and inner surfaces of intact human red blood cells. J Biol Chem. 1975 Jul 10;250(13):5130–5136. [PubMed] [Google Scholar]
- Cabantchik Z. I., Rothstein A. Membrane proteins related to anion permeability of human red blood cells. I. Localization of disulfonic stilbene binding sites in proteins involved in permeation. J Membr Biol. 1974;15(3):207–226. doi: 10.1007/BF01870088. [DOI] [PubMed] [Google Scholar]
- Caroni P., Carafoli E. The regulation of the Na+ -Ca2+ exchanger of heart sarcolemma. Eur J Biochem. 1983 May 16;132(3):451–460. doi: 10.1111/j.1432-1033.1983.tb07383.x. [DOI] [PubMed] [Google Scholar]
- Castagna M., Takai Y., Kaibuchi K., Sano K., Kikkawa U., Nishizuka Y. Direct activation of calcium-activated, phospholipid-dependent protein kinase by tumor-promoting phorbol esters. J Biol Chem. 1982 Jul 10;257(13):7847–7851. [PubMed] [Google Scholar]
- Frelin C., Chassande O., Lazdunski M. Biochemical characterization of the Na+/K+/Cl- co-transport in chick cardiac cells. Biochem Biophys Res Commun. 1986 Jan 14;134(1):326–331. doi: 10.1016/0006-291x(86)90566-8. [DOI] [PubMed] [Google Scholar]
- Geck P., Heinz E. The Na-K-2Cl cotransport system. J Membr Biol. 1986;91(2):97–105. doi: 10.1007/BF01925787. [DOI] [PubMed] [Google Scholar]
- Grinstein S., Rothstein A. Mechanisms of regulation of the Na+/H+ exchanger. J Membr Biol. 1986;90(1):1–12. doi: 10.1007/BF01869680. [DOI] [PubMed] [Google Scholar]
- Gros G., Dodgson S. J. Velocity of CO2 exchange in muscle and liver. Annu Rev Physiol. 1988;50:669–694. doi: 10.1146/annurev.ph.50.030188.003321. [DOI] [PubMed] [Google Scholar]
- Hinkle P. M., Kinsella P. A., Osterhoudt K. C. Cadmium uptake and toxicity via voltage-sensitive calcium channels. J Biol Chem. 1987 Dec 5;262(34):16333–16337. [PubMed] [Google Scholar]
- Katada T., Gilman A. G., Watanabe Y., Bauer S., Jakobs K. H. Protein kinase C phosphorylates the inhibitory guanine-nucleotide-binding regulatory component and apparently suppresses its function in hormonal inhibition of adenylate cyclase. Eur J Biochem. 1985 Sep 2;151(2):431–437. doi: 10.1111/j.1432-1033.1985.tb09120.x. [DOI] [PubMed] [Google Scholar]
- Knakal R. C., Summers W. C., Cragoe E. J., Jr, Boron W. F. Expression of a mammalian Na-H exchanger in muscle fibres of the giant barnacle. 1985 Jun 27-Jul 3Nature. 315(6022):756–758. doi: 10.1038/315756a0. [DOI] [PubMed] [Google Scholar]
- Kuo J. F., Andersson R. G., Wise B. C., Mackerlova L., Salomonsson I., Brackett N. L., Katoh N., Shoji M., Wrenn R. W. Calcium-dependent protein kinase: widespread occurrence in various tissues and phyla of the animal kingdom and comparison of effects of phospholipid, calmodulin, and trifluoperazine. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7039–7043. doi: 10.1073/pnas.77.12.7039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren T. H. Use of inhibitors in physiological studies of carbonic anhydrase. Am J Physiol. 1977 Apr;232(4):F291–F297. doi: 10.1152/ajprenal.1977.232.4.F291. [DOI] [PubMed] [Google Scholar]
- Mason-Sharp D., Bittar E. E. Stimulation by high external potassium of the sodium efflux in barnacle muscle fibers. J Membr Biol. 1981 Feb 28;58(3):213–226. doi: 10.1007/BF01870907. [DOI] [PubMed] [Google Scholar]
- Mazzei G. J., Girard P. R., Kuo J. F. Environmental pollutant Cd2+ biphasically and differentially regulates myosin light chain kinase and phospholipid/Ca2+-dependent protein kinase. FEBS Lett. 1984 Jul 23;173(1):124–128. doi: 10.1016/0014-5793(84)81030-3. [DOI] [PubMed] [Google Scholar]
- Menard M. R., Hinke J. A. The influence of "nonmyoplasmic" sodium on the measured value of the sodium efflux from barnacle muscle. Can J Physiol Pharmacol. 1981 Dec;59(12):1219–1227. doi: 10.1139/y81-191. [DOI] [PubMed] [Google Scholar]
- Nwoga J., Bittar E. E. Stimulation by proctolin of the ouabain-insensitive sodium efflux in single barnacle muscle fibers. Comp Biochem Physiol C. 1985;81(2):345–350. doi: 10.1016/0742-8413(85)90018-0. [DOI] [PubMed] [Google Scholar]
- O'Callahan C. M., Hosey M. M. Multiple phosphorylation sites in the 165-kilodalton peptide associated with dihydropyridine-sensitive calcium channels. Biochemistry. 1988 Aug 9;27(16):6071–6077. doi: 10.1021/bi00416a036. [DOI] [PubMed] [Google Scholar]
- O'Callahan C. M., Ptasienski J., Hosey M. M. Phosphorylation of the 165-kDa dihydropyridine/phenylalkylamine receptor from skeletal muscle by protein kinase C. J Biol Chem. 1988 Nov 25;263(33):17342–17349. [PubMed] [Google Scholar]
- O'Grady S. M., Palfrey H. C., Field M. Characteristics and functions of Na-K-Cl cotransport in epithelial tissues. Am J Physiol. 1987 Aug;253(2 Pt 1):C177–C192. doi: 10.1152/ajpcell.1987.253.2.C177. [DOI] [PubMed] [Google Scholar]
- Philipson K. D., Bersohn M. M., Nishimoto A. Y. Effects of pH on Na+-Ca2+ exchange in canine cardiac sarcolemmal vesicles. Circ Res. 1982 Feb;50(2):287–293. doi: 10.1161/01.res.50.2.287. [DOI] [PubMed] [Google Scholar]
- Rios E., Brum G. Involvement of dihydropyridine receptors in excitation-contraction coupling in skeletal muscle. Nature. 1987 Feb 19;325(6106):717–720. doi: 10.1038/325717a0. [DOI] [PubMed] [Google Scholar]
- Russell J. M., Brodwick M. S. Properties of chloride transport in barnacle muscle fibers. J Gen Physiol. 1979 Mar;73(3):343–368. doi: 10.1085/jgp.73.3.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell J. M., Brodwick M. S. The interaction of intracellular Mg2+ and pH on Cl- fluxes associated with intracellular pH regulation in barnacle muscle fibers. J Gen Physiol. 1988 Apr;91(4):495–513. doi: 10.1085/jgp.91.4.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell J. M. Cation-coupled chloride influx in squid axon. Role of potassium and stoichiometry of the transport process. J Gen Physiol. 1983 Jun;81(6):909–925. doi: 10.1085/jgp.81.6.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz R., Bittar E. E. Further studies of the mechanism of stimulation by external acidification of the sodium efflux in barnacle muscle fibers. Pflugers Arch. 1978 May 31;374(3):219–228. doi: 10.1007/BF00585598. [DOI] [PubMed] [Google Scholar]
- Smith J. S., Coronado R., Meissner G. Sarcoplasmic reticulum contains adenine nucleotide-activated calcium channels. Nature. 1985 Aug 1;316(6027):446–449. doi: 10.1038/316446a0. [DOI] [PubMed] [Google Scholar]
- Stefani E., Chiarandini D. J. Ionic channels in skeletal muscle. Annu Rev Physiol. 1982;44:357–372. doi: 10.1146/annurev.ph.44.030182.002041. [DOI] [PubMed] [Google Scholar]
- Strong J. A., Fox A. P., Tsien R. W., Kaczmarek L. K. Stimulation of protein kinase C recruits covert calcium channels in Aplysia bag cell neurons. Nature. 1987 Feb 19;325(6106):714–717. doi: 10.1038/325714a0. [DOI] [PubMed] [Google Scholar]


