Abstract
1. Cutaneous nerve stimulation was used to study the excitability of the spino-olivocerebellar pathways (SOCPs) to the c2 zone of the paravermal cerebellar cortex in the cat. Non-noxious single-shock stimulation of the right and left superficial radial (SR) nerves via implanted cuff electrodes was used to evoke field potentials in the cerebellar cortex via the SOCPs. 2. The evoked potentials were recorded extracellularly either in lobule V of the anterior lobe (three cats) or within the paramedian lobule of the posterior lobe (one cat) with glass-coated tungsten microelectrodes. Measurement of the amplitudes of the responses was used to monitor transmission in the SOCPs in cats at rest and during walking. 3. A total of eleven c2 recording sites were investigated in detail. At seven of these sites, responses were recorded both during locomotion and at rest. For all seven sites responses during locomotion were smaller, more variable in amplitude and less securely evoked (average reduction 59%). 4. At five out of the eleven recording sites (45%) the mean amplitude of responses elicited during different tenths of the step cycle fluctuated sufficiently that the largest response was more than twice the smallest. In the majority of these cases (4/5) the responses were largest in either mid-stance or late swing. These fluctuations in response size occurred without parallel fluctuation in the amplitude of the peripheral nerve volley. At the remaining sites fluctuation of the cerebellar field size was less and in some cases practically absent. 5. At six recording sites it was possible to record the climbing fibre potentials evoked by stimulation of both the ipsilateral and contralateral superficial radial nerves. In all six cases the fluctuations in size of the response during locomotion occurred in phase, despite the fact that the two limbs move out of phase. 6. The probability that an individual stimulus would evoke any cerebellar response also varied between the different tenths of the step cycle and such variations occurred in parallel with the fluctuations in response size. This shows that the SOCP regulatory mechanism(s) must, at least in part, operate at a precerebellar level.
Full text
PDF







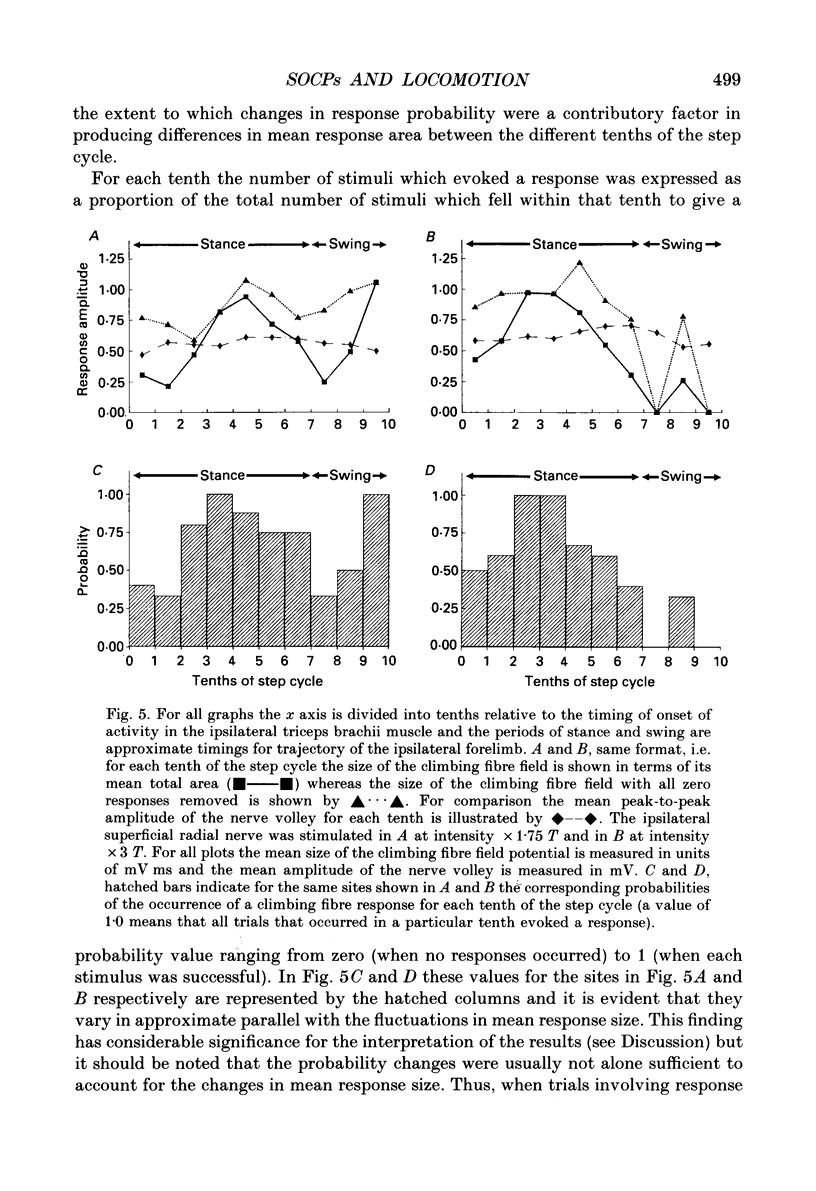
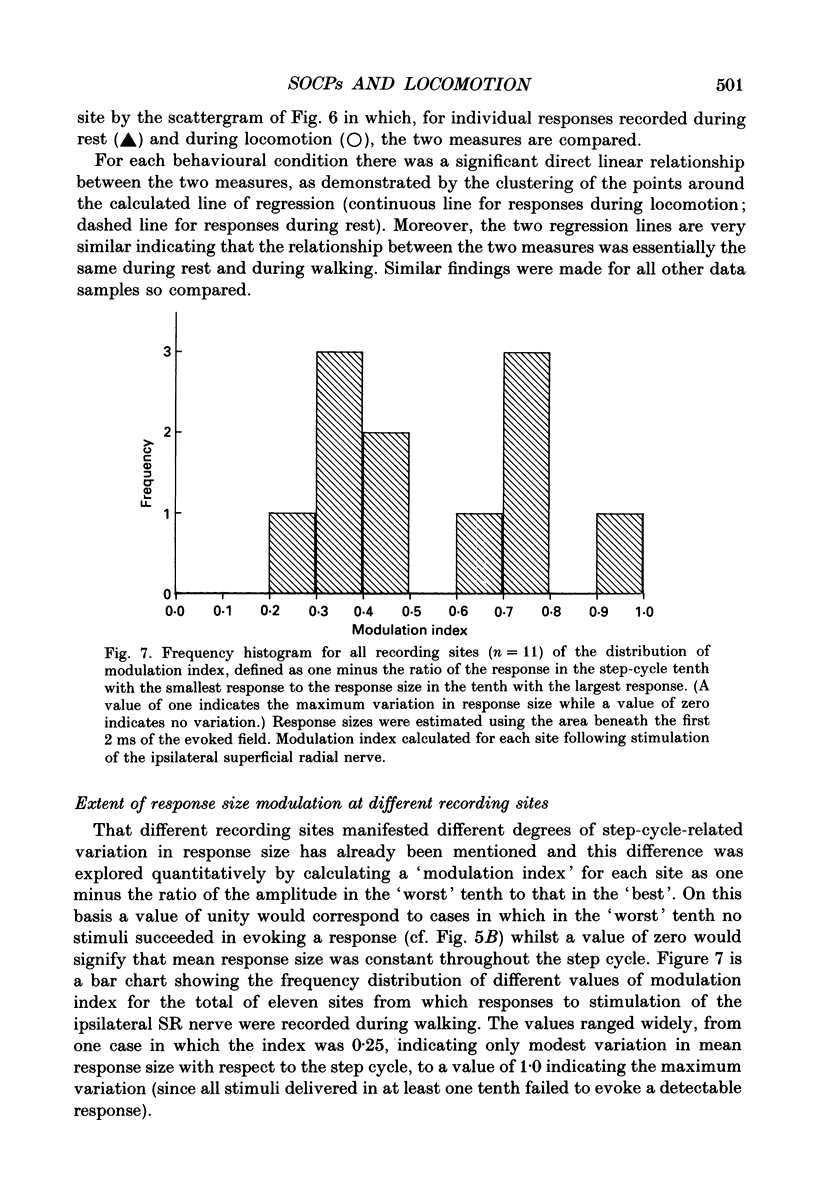

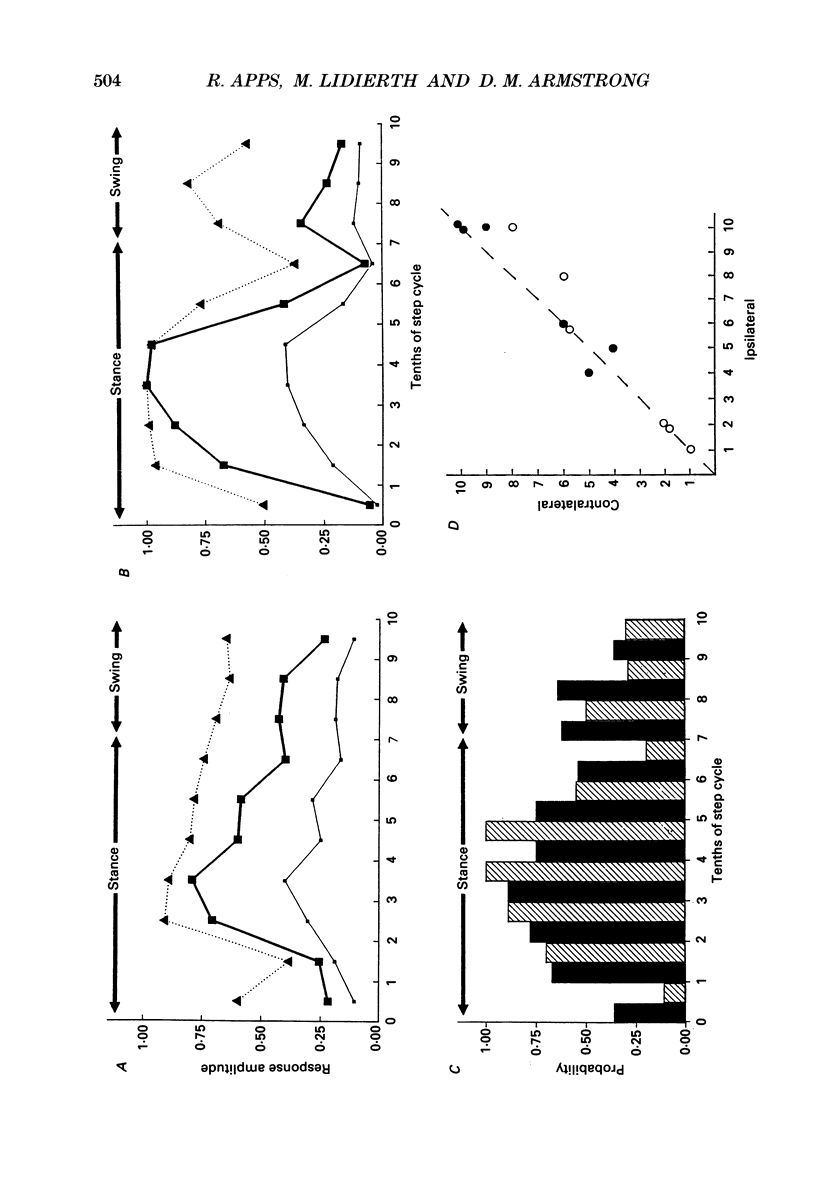








Selected References
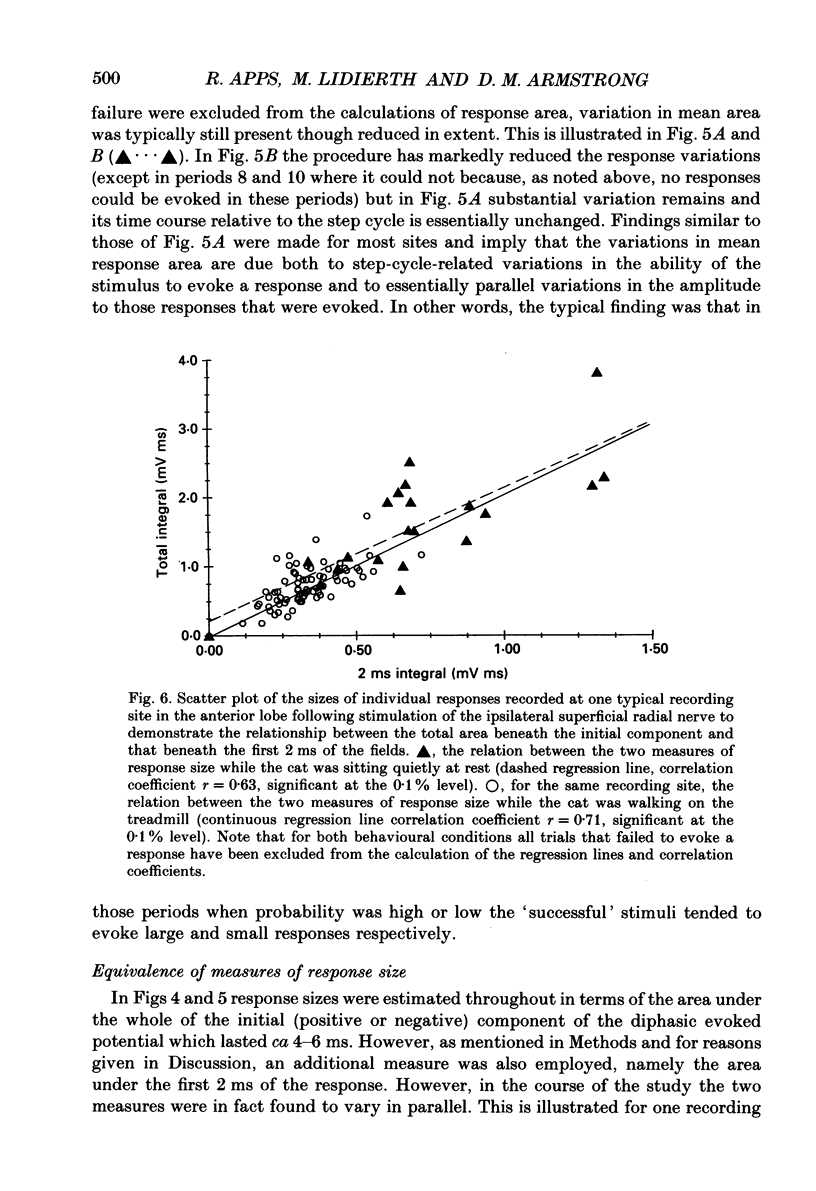
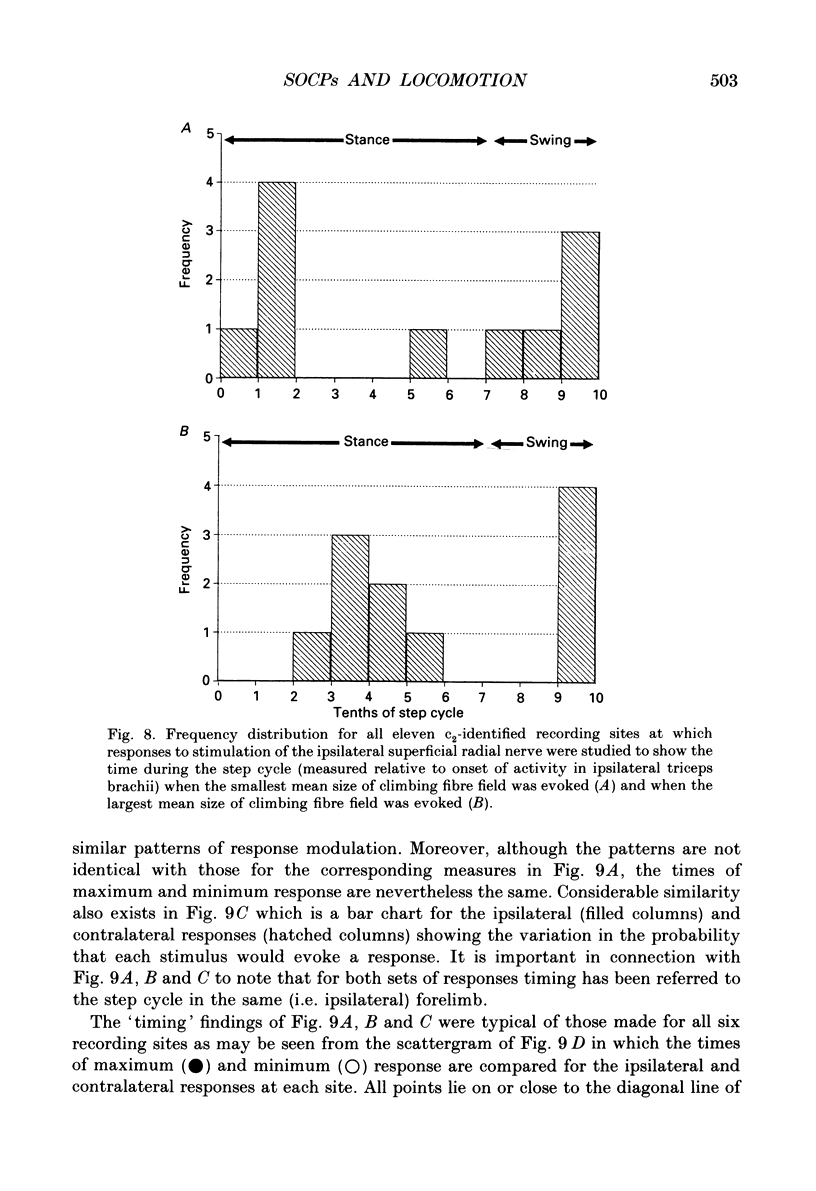
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen P., Etholm B., Gordon G. Presynaptic and post-synaptic inhibition elicited in the cat's dorsal column nuclei by mechanical stimulation of skin. J Physiol. 1970 Sep;210(2):433–455. doi: 10.1113/jphysiol.1970.sp009219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson G., Armstrong D. M. Complex spikes in Purkinje cells in the lateral vermis (b zone) of the cat cerebellum during locomotion. J Physiol. 1987 Apr;385:107–134. doi: 10.1113/jphysiol.1987.sp016487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson G., Sjölund B. The ventral spino-olivocerebellar system in the cat. IV. Spinal transmission after administration of clonidine and L-dopa. Exp Brain Res. 1978 Oct 13;33(2):227–240. doi: 10.1007/BF00238062. [DOI] [PubMed] [Google Scholar]
- Armstrong D. M., Drew T. Discharges of pyramidal tract and other motor cortical neurones during locomotion in the cat. J Physiol. 1984 Jan;346:471–495. doi: 10.1113/jphysiol.1984.sp015036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong D. M., Eccles J. C., Harvey R. J., Matthews P. B. Responses in the dorsal accessory olive of the cat to stimulation of hind limb afferents. J Physiol. 1968 Jan;194(1):125–145. doi: 10.1113/jphysiol.1968.sp008398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong D. M., Edgley S. A. Discharges of Purkinje cells in the paravermal part of the cerebellar anterior lobe during locomotion in the cat. J Physiol. 1984 Jul;352:403–424. doi: 10.1113/jphysiol.1984.sp015300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong D. M., Edgley S. A. Discharges of interpositus and Purkinje cells of the cat cerebellum during locomotion under different conditions. J Physiol. 1988 Jun;400:425–445. doi: 10.1113/jphysiol.1988.sp017130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong D. M., Edgley S. A., Lidierth M. Complex spikes in Purkinje cells of the paravermal part of the anterior lobe of the cat cerebellum during locomotion. J Physiol. 1988 Jun;400:405–414. doi: 10.1113/jphysiol.1988.sp017128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong D. M. Functional significance of connections of the inferior olive. Physiol Rev. 1974 Apr;54(2):358–417. doi: 10.1152/physrev.1974.54.2.358. [DOI] [PubMed] [Google Scholar]
- Armstrong D. M., Harvey R. J. Responses to a spino-olivo-cerebellar pathway in the cat. J Physiol. 1968 Jan;194(1):147–168. doi: 10.1113/jphysiol.1968.sp008399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong D. M., Harvey R. J., Schild R. F. Spino-olivocerebellar pathways to the posterior lobe of the cat cerebellum. Exp Brain Res. 1973 Aug 31;18(1):1–18. doi: 10.1007/BF00236553. [DOI] [PubMed] [Google Scholar]
- Armstrong D. M., Rawson J. A. Activity patterns of cerebellar cortical neurones and climbing fibre afferents in the awake cat. J Physiol. 1979 Apr;289:425–448. doi: 10.1113/jphysiol.1979.sp012745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodal A., Kawamura K. Olivocerebellar projection: a review. Adv Anat Embryol Cell Biol. 1980;64:IVIII, 1-140. [PubMed] [Google Scholar]
- Campbell N. C., Ekerot C. F., Hesslow G. Interaction between responses in Purkinje cells evoked by climbing fibre impulses and parallel fibre volleys in the cat. J Physiol. 1983 Jul;340:225–238. doi: 10.1113/jphysiol.1983.sp014760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carli G., Diete-Spiff K., Pompeiano O. Cerebellar responses evoked by somatic afferent volleys during sleep and waking. Arch Ital Biol. 1967 Nov;105(4):499–528. [PubMed] [Google Scholar]
- Coulter J. D. Sensory transmission through lemniscal pathway during voluntary movement in the cat. J Neurophysiol. 1974 Sep;37(5):831–845. doi: 10.1152/jn.1974.37.5.831. [DOI] [PubMed] [Google Scholar]
- Crill W. E. Unitary multiple-spiked responses in cat inferior olive nucleus. J Neurophysiol. 1970 Mar;33(2):199–209. doi: 10.1152/jn.1970.33.2.199. [DOI] [PubMed] [Google Scholar]
- Eccles J. C., Llinás R., Sasaki K. The excitatory synaptic action of climbing fibres on the Purkinje cells of the cerebellum. J Physiol. 1966 Jan;182(2):268–296. doi: 10.1113/jphysiol.1966.sp007824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles J. C., Llinás R., Sasaki K., Voorhoeve P. E. Interaction experiments on the responses evoked in Purkinje cells by climbing fibres. J Physiol. 1966 Jan;182(2):297–315. doi: 10.1113/jphysiol.1966.sp007825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles J. C., Provini L., Strata P., Táboríková H. Topographical investigations on the climbing fiber inputs from forelimb and hindlimb afferents to the cerebellar anterior lobe. Exp Brain Res. 1968;6(3):195–215. doi: 10.1007/BF00235124. [DOI] [PubMed] [Google Scholar]
- Edgley S. A., Lidierth M. Step-related discharges of Purkinje cells in the paravermal cortex of the cerebellar anterior lobe in the cat. J Physiol. 1988 Jul;401:399–415. doi: 10.1113/jphysiol.1988.sp017169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekerot C. F., Gustavsson P., Oscarsson O., Schouenborg J. Climbing fibres projecting to cat cerebellar anterior lobe activated by cutaneous A and C fibres. J Physiol. 1987 May;386:529–538. doi: 10.1113/jphysiol.1987.sp016549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekerot C. F., Larson B. Correlation between sagittal projection zones of climbing and mossy fibre paths in cat cerebellar anterior lobe. Brain Res. 1973 Dec 21;64:446–450. doi: 10.1016/0006-8993(73)90203-5. [DOI] [PubMed] [Google Scholar]
- Ekerot C. F., Larson B. The dorsal spino-olivocerebellar system in the cat. I. Functional organization and termination in the anterior lobe. Exp Brain Res. 1979 Jul 2;36(2):201–217. doi: 10.1007/BF00238905. [DOI] [PubMed] [Google Scholar]
- Forssberg H. Stumbling corrective reaction: a phase-dependent compensatory reaction during locomotion. J Neurophysiol. 1979 Jul;42(4):936–953. doi: 10.1152/jn.1979.42.4.936. [DOI] [PubMed] [Google Scholar]
- Gellman R., Gibson A. R., Houk J. C. Inferior olivary neurons in the awake cat: detection of contact and passive body displacement. J Neurophysiol. 1985 Jul;54(1):40–60. doi: 10.1152/jn.1985.54.1.40. [DOI] [PubMed] [Google Scholar]
- Ghez C., Lenzi G. L. Modulation of sensory transmission in cat lemniscal system during voluntary movement. Pflugers Arch. 1971;323(3):273–278. doi: 10.1007/BF00586390. [DOI] [PubMed] [Google Scholar]
- Groenewegen H. J., Voogd J., Freedman S. L. The parasagittal zonation within the olivocerebellar projection. II. Climbing fiber distribution in the intermediate and hemispheric parts of cat cerebellum. J Comp Neurol. 1979 Feb 1;183(3):551–601. doi: 10.1002/cne.901830307. [DOI] [PubMed] [Google Scholar]
- Groenewegen H. J., Voogd J. The parasagittal zonation within the olivocerebellar projection. I. Climbing fiber distribution in the vermis of cat cerebellum. J Comp Neurol. 1977 Aug 1;174(3):417–488. doi: 10.1002/cne.901740304. [DOI] [PubMed] [Google Scholar]
- Jabbur S. J., Banna N. R. Widespread cutaneous inhibition in dorsal column nuclei. J Neurophysiol. 1970 Sep;33(5):616–624. doi: 10.1152/jn.1970.33.5.616. [DOI] [PubMed] [Google Scholar]
- Kim J. H., Wang J. J., Ebner T. J. Climbing fiber afferent modulation during treadmill locomotion in the cat. J Neurophysiol. 1987 Mar;57(3):787–802. doi: 10.1152/jn.1987.57.3.787. [DOI] [PubMed] [Google Scholar]
- LARSELL O. The cerebellum of the cat and the monkey. J Comp Neurol. 1953 Aug;99(1):135–199. doi: 10.1002/cne.900990110. [DOI] [PubMed] [Google Scholar]
- Larson B., Miller S., Oscarsson O. A spinocerebellar climbing fibre path activated by the flexor reflex afferents from all four limbs. J Physiol. 1969 Aug;203(3):641–649. doi: 10.1113/jphysiol.1969.sp008883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson B., Miller S., Oscarsson O. Termination and functional organization of the dorsolateral spino-olivocerebellar path. J Physiol. 1969 Aug;203(3):611–640. doi: 10.1113/jphysiol.1969.sp008882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinas R., Baker R., Sotelo C. Electrotonic coupling between neurons in cat inferior olive. J Neurophysiol. 1974 May;37(3):560–571. doi: 10.1152/jn.1974.37.3.560. [DOI] [PubMed] [Google Scholar]
- Llinás R., Yarom Y. Electrophysiology of mammalian inferior olivary neurones in vitro. Different types of voltage-dependent ionic conductances. J Physiol. 1981 Jun;315:549–567. doi: 10.1113/jphysiol.1981.sp013763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Yarom Y. Properties and distribution of ionic conductances generating electroresponsiveness of mammalian inferior olivary neurones in vitro. J Physiol. 1981 Jun;315:569–584. doi: 10.1113/jphysiol.1981.sp013764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou J. S., Bloedel J. R. The responses of simultaneously recorded Purkinje cells to the perturbations of the step cycle in the walking ferret: a study using a new analytical method--the real-time postsynaptic response (RTPR). Brain Res. 1986 Feb 19;365(2):340–344. doi: 10.1016/0006-8993(86)91646-x. [DOI] [PubMed] [Google Scholar]
- Miller S., Nezlina N., Oscarsson O. Climbing fibre projection to cerebellar anterior lobe activated from structures in midbrain and from spinal cord. Brain Res. 1969 Jun;14(1):234–236. doi: 10.1016/0006-8993(69)90046-8. [DOI] [PubMed] [Google Scholar]
- Miller S., Nezlina N., Oscarsson O. Projection and convergence patterns in climbing fibre paths to cerebellar anterior lobe activated from cerebral cortex and spinal cord. Brain Res. 1969 Jun;14(1):230–233. doi: 10.1016/0006-8993(69)90045-6. [DOI] [PubMed] [Google Scholar]
- Oscarsson O. Termination and functional organization of the dorsal spino-olivocerebellar path. J Physiol. 1969 Jan;200(1):129–149. doi: 10.1113/jphysiol.1969.sp008685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oscarsson O. Termination and functional organization of the ventral spino-olivocerebellar path. J Physiol. 1968 May;196(2):453–478. doi: 10.1113/jphysiol.1968.sp008518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer C. I., Marks W. B., Bak M. J. The responses of cat motor cortical units to electrical cutaneous stimulation during locomotion and during lifting, falling and landing. Exp Brain Res. 1985;58(1):102–116. doi: 10.1007/BF00238958. [DOI] [PubMed] [Google Scholar]
- Sjölund B. The ventral spino-olivocerebellar system in the cat. V. Supraspinal control of spinal transmission. Exp Brain Res. 1978 Nov 15;33(3-4):509–522. doi: 10.1007/BF00235571. [DOI] [PubMed] [Google Scholar]
- Trott J. R., Armstrong D. M. The cerebellar corticonuclear projection from lobule Vb/c of the cat anterior lobe: a combined electrophysiological and autoradiographic study. I. Projections from the intermediate region. Exp Brain Res. 1987;66(2):318–338. doi: 10.1007/BF00243308. [DOI] [PubMed] [Google Scholar]
- Trott J. R., Armstrong D. M. The cerebellar corticonuclear projection from lobule Vb/c of the cat anterior lobe: a combined electrophysiological and autoradiographic study. II. Projections from the vermis. Exp Brain Res. 1987;68(2):339–354. doi: 10.1007/BF00248800. [DOI] [PubMed] [Google Scholar]
- Udo M., Matsukawa K., Kamei H., Minoda K., Oda Y. Simple and complex spike activities of Purkinje cells during locomotion in the cerebellar vermal zones of decerebrate cats. Exp Brain Res. 1981;41(3-4):292–300. doi: 10.1007/BF00238886. [DOI] [PubMed] [Google Scholar]


