Abstract
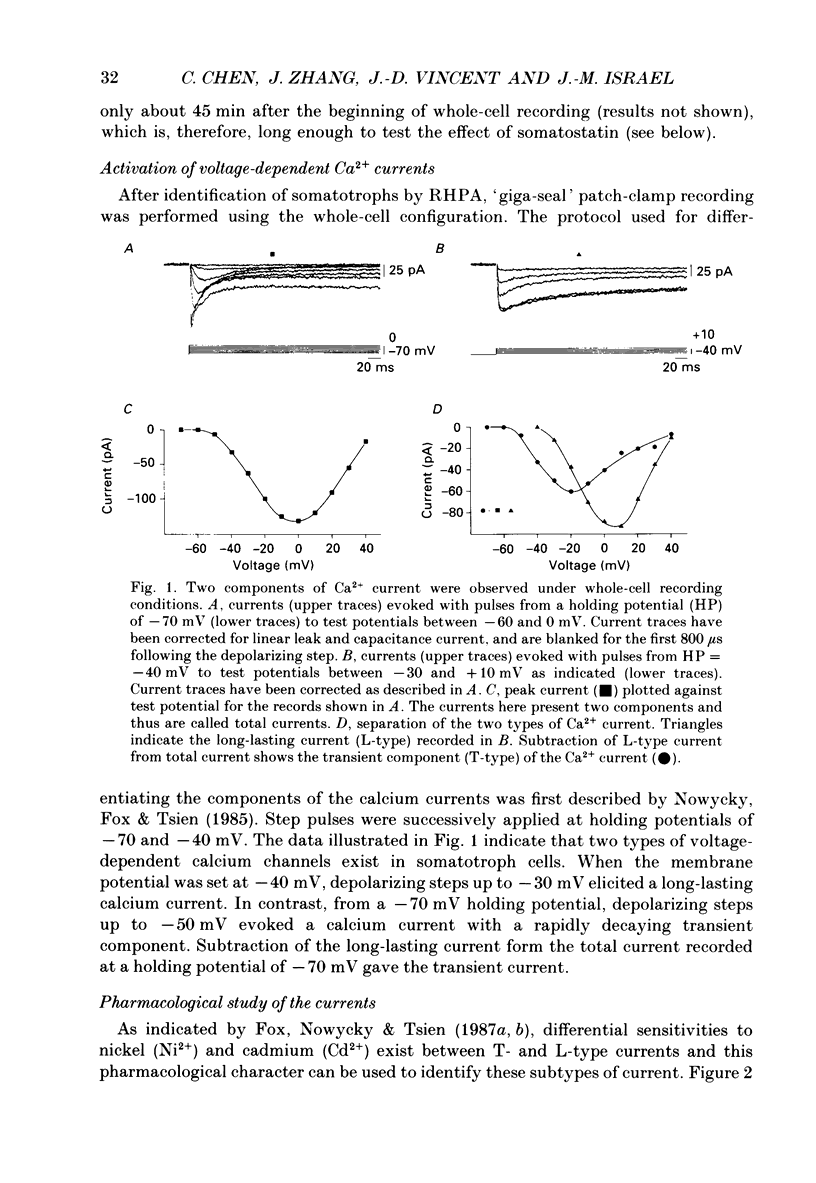
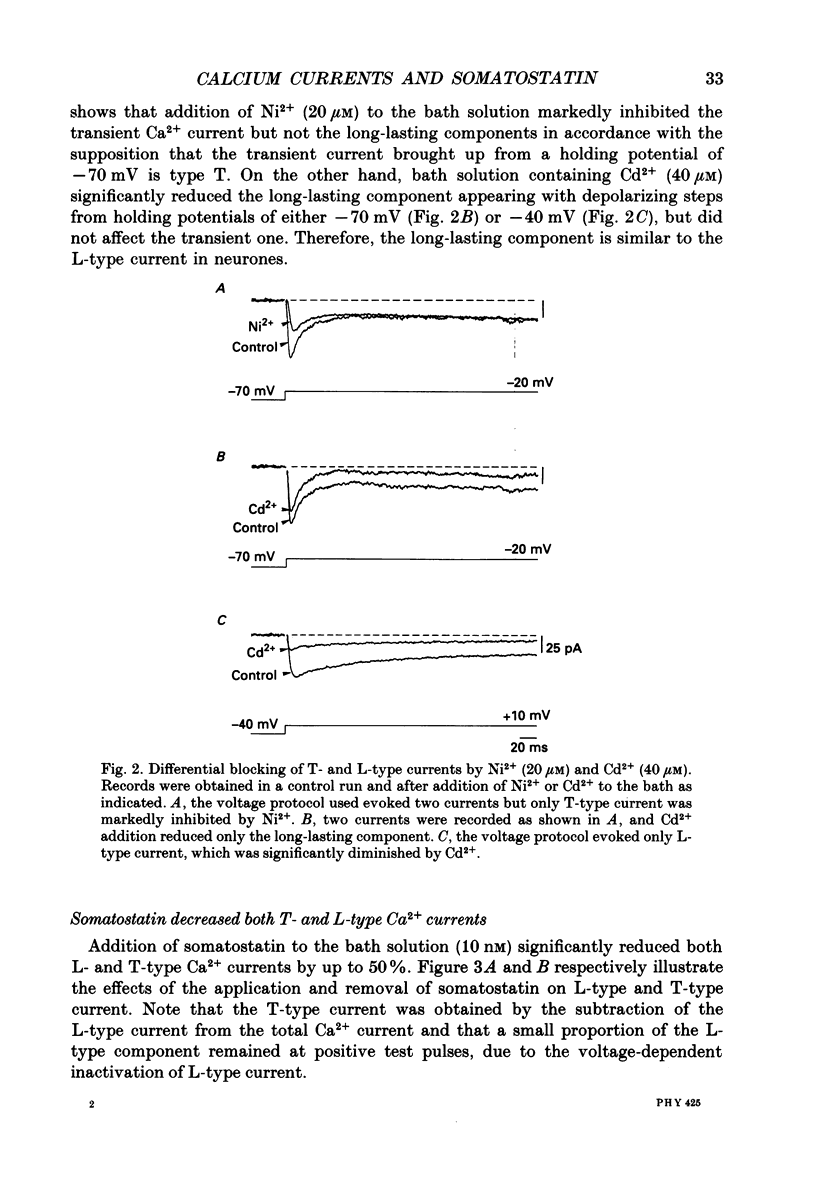
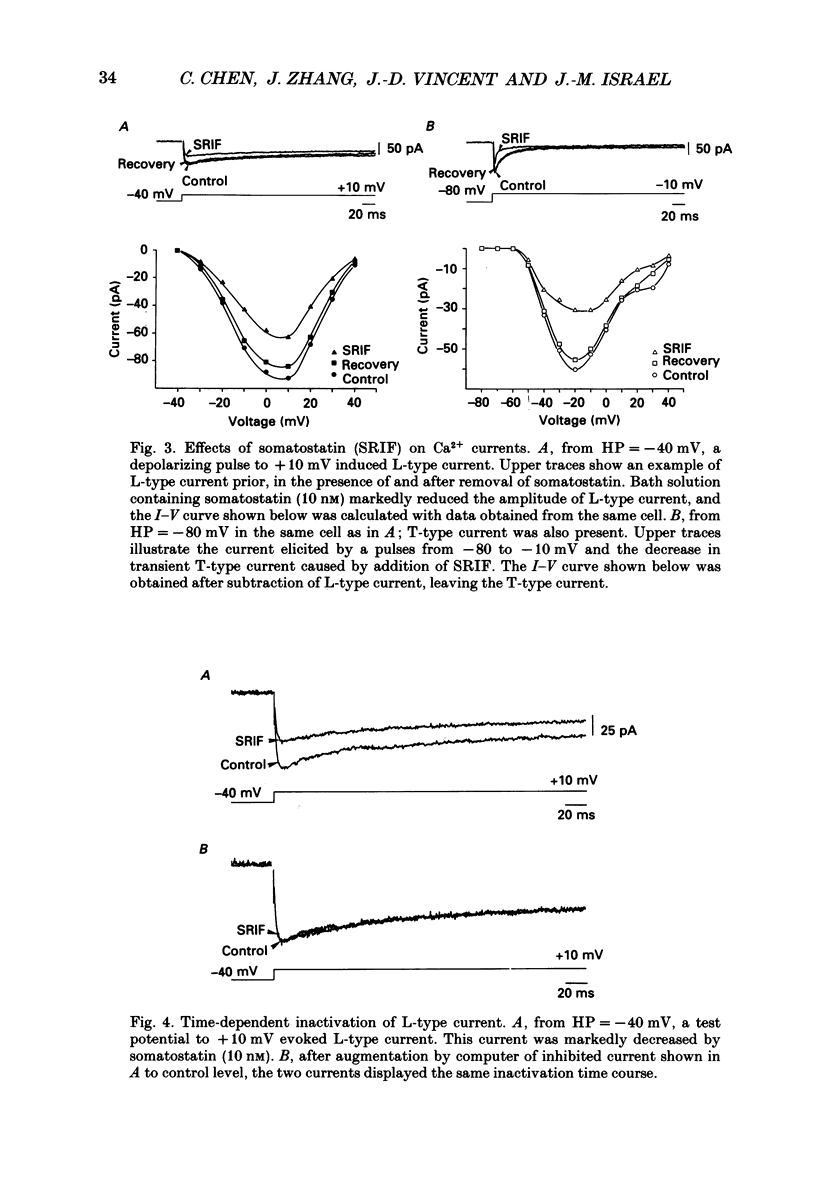
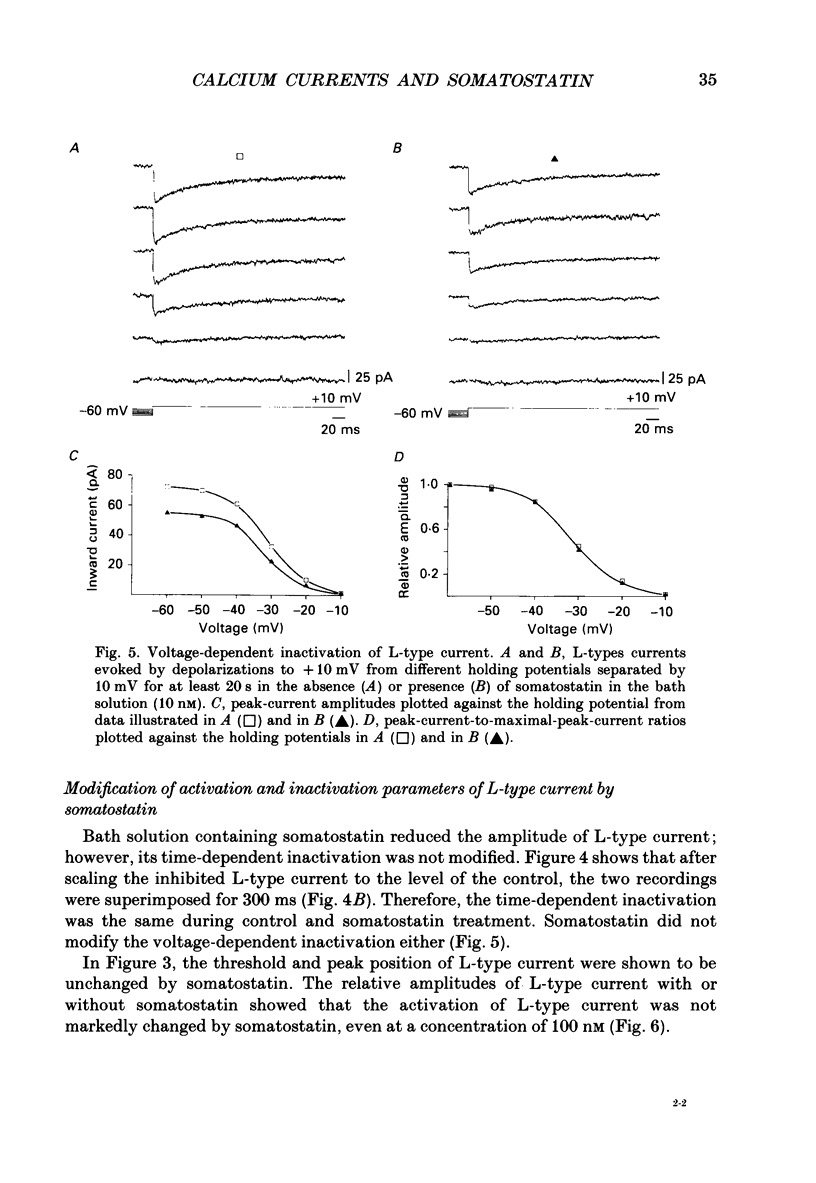
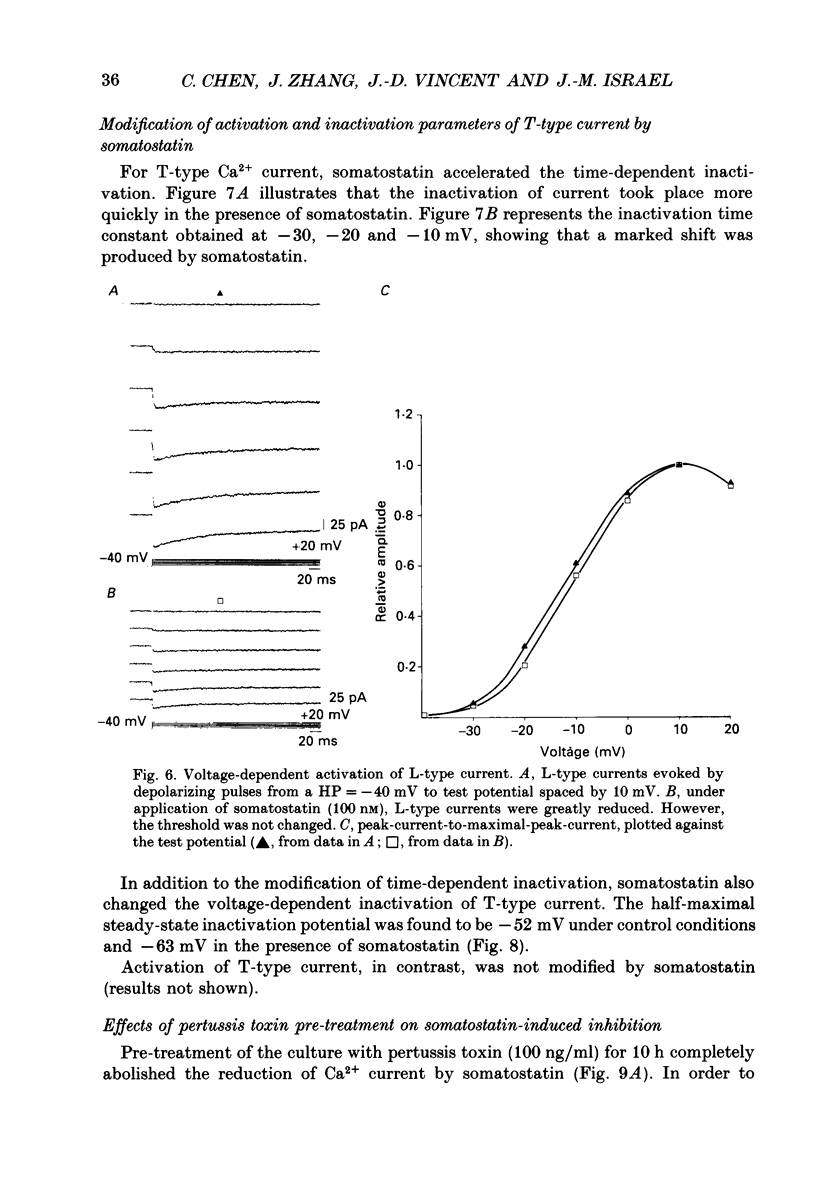
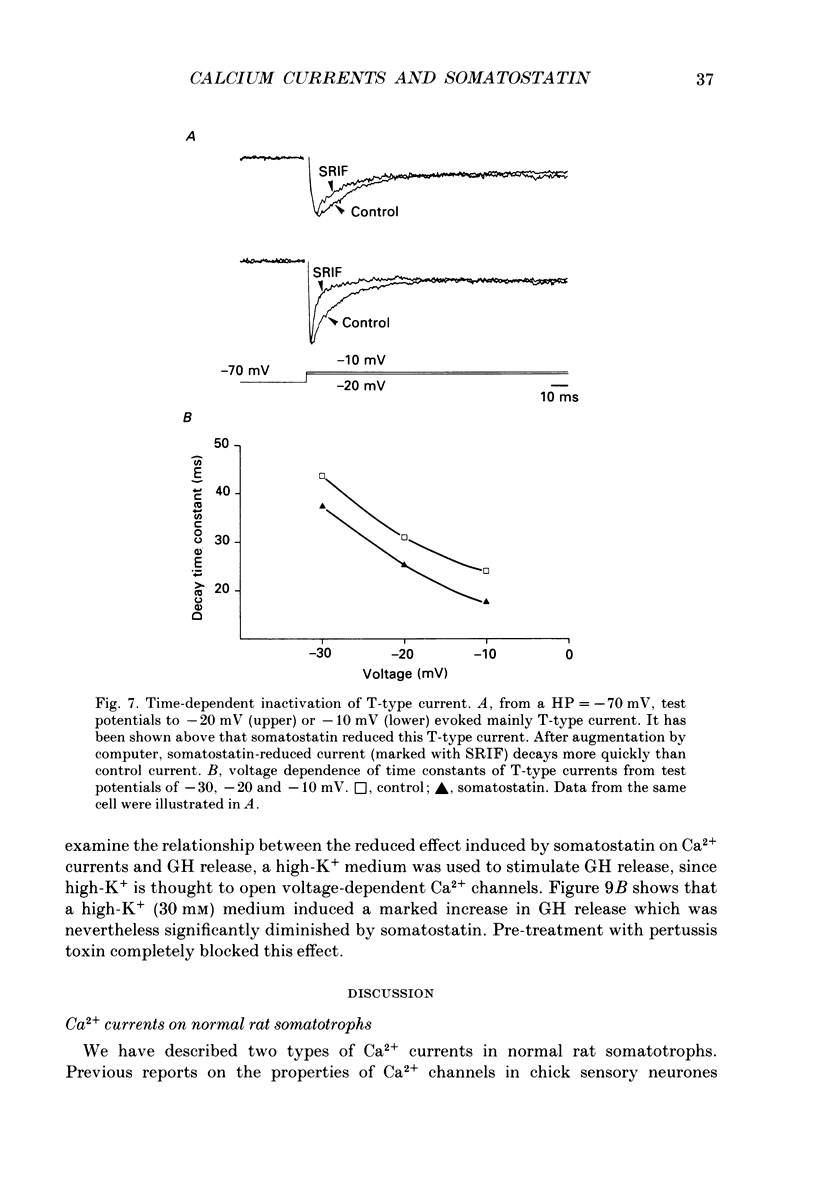
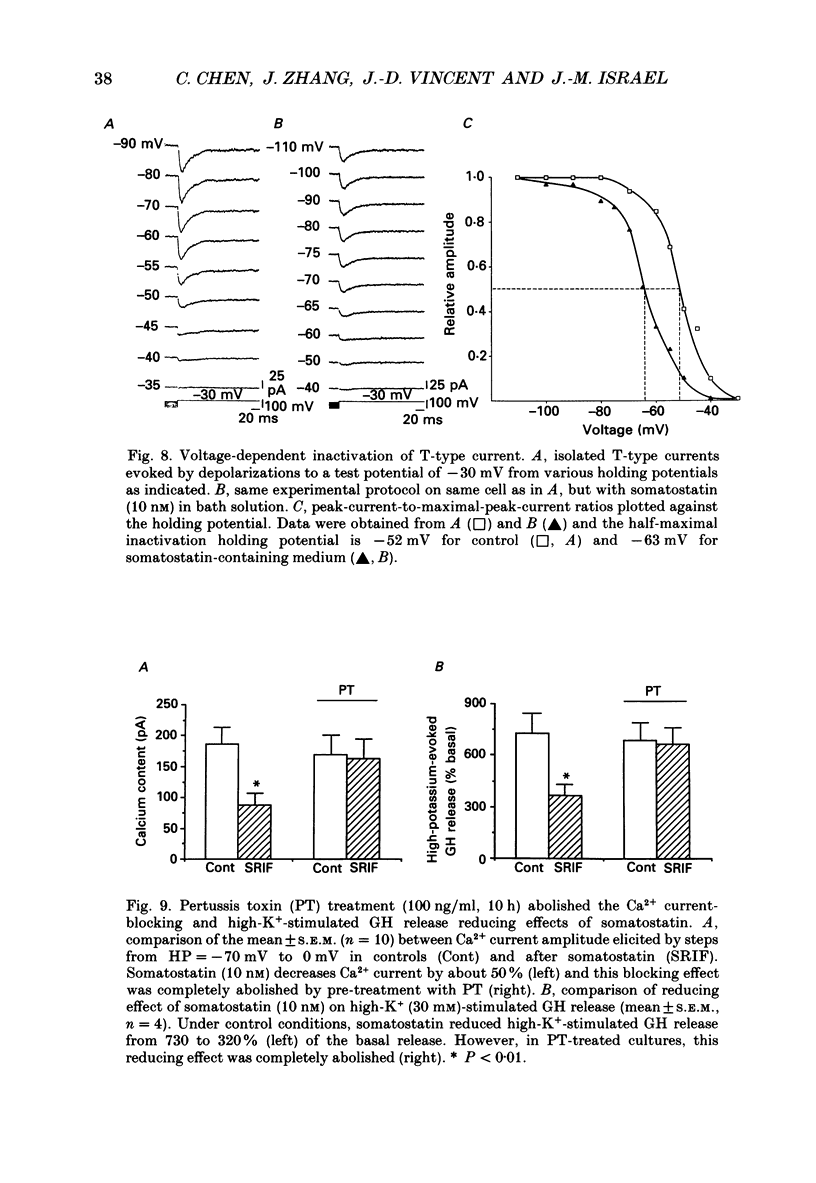
1. Somatotrophs were obtained from rat pituitary glands after dissociation, separation and enrichment on a continuous gradient of bovine serum albumin at unit gravity. Somatotrophs were enriched up to 85% in the heavy fractions (F8 and F9). 2. After identification by reverse hemolytic plaque assay, patch-clamp recording in the whole-cell mode was performed on somatotrophs. 3. Under voltage-clamp conditions, two types of Ca2+ currents were recorded. From a holding potential of -70 mV, depolarizing voltage steps to potentials more positive than -50 mV activated a current which rapidly inactivated and which was very sensitive to Ni2+ but not to Cd2+. This current corresponds to T-type current. Depolarizing steps to potentials more positive than -30 mV from a holding potential of -40 mV triggered a current which slowly inactivated and which was very sensitive to Cd2+ but not to Ni2+. This current corresponds to L-type current. 4. Application of somatostatin to the bath solution (10 nM) markedly reduced the amplitudes of both T- and L-type currents. Somatostatin decreased the conductance of L-type current without modifying its time- and voltage-dependent inactivation but its activation was not affected. However, somatostatin decreased the conductance of T-type currents, and also accelerated its time-dependent inactivation. Half-inactivation voltage of T-type current was shifted from -52 to -63 mV by somatostatin but no change was obtained in the current activation curve. 5. All these modifications in Ca2+ currents were abolished by a pre-treatment of the cultures with pertussis toxin (100 ng/ml, for 10 h). This pre-treatment also blocked the inhibitory effect of somatostatin on high-K(+)-stimulated growth hormone release. 6. Our results show that somatostatin acts on somatotrophs by attenuating the voltage-dependent Ca2+ currents. These effects may contribute to a somatostatin-induced reduction in [Ca2+]i and the subsequent decline in growth hormone release.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bokoch G. M., Katada T., Northup J. K., Ui M., Gilman A. G. Purification and properties of the inhibitory guanine nucleotide-binding regulatory component of adenylate cyclase. J Biol Chem. 1984 Mar 25;259(6):3560–3567. [PubMed] [Google Scholar]
- Chen C., Israel J. M., Vincent J. D. Electrophysiological responses of rat pituitary cells in somatotroph-enriched primary culture to human growth-hormone releasing factor. Neuroendocrinology. 1989 Dec;50(6):679–687. doi: 10.1159/000125299. [DOI] [PubMed] [Google Scholar]
- Chen C., Israel J. M., Vincent J. D. Electrophysiological responses to somatostatin of rat hypophysial cells in somatotroph-enriched primary cultures. J Physiol. 1989 Jan;408:493–510. doi: 10.1113/jphysiol.1989.sp017472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Zhang J., Vincent J. D., Israel J. M. Sodium and calcium currents in action potentials of rat somatotrophs: their possible functions in growth hormone secretion. Life Sci. 1990;46(14):983–989. doi: 10.1016/0024-3205(90)90021-i. [DOI] [PubMed] [Google Scholar]
- Cota G. Calcium channel currents in pars intermedia cells of the rat pituitary gland. Kinetic properties and washout during intracellular dialysis. J Gen Physiol. 1986 Jul;88(1):83–105. doi: 10.1085/jgp.88.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorflinger L. J., Schonbrunn A. Somatostatin inhibits basal and vasoactive intestinal peptide-stimulated hormone release by different mechanisms in GH pituitary cells. Endocrinology. 1983 Nov;113(5):1551–1558. doi: 10.1210/endo-113-5-1551. [DOI] [PubMed] [Google Scholar]
- Epelbaum J., Enjalbert A., Krantic S., Musset F., Bertrand P., Rasolonjanahary R., Shu C., Kordon C. Somatostatin receptors on pituitary somatotrophs, thyrotrophs, and lactotrophs: pharmacological evidence for loose coupling to adenylate cyclase. Endocrinology. 1987 Dec;121(6):2177–2185. doi: 10.1210/endo-121-6-2177. [DOI] [PubMed] [Google Scholar]
- Fox A. P., Nowycky M. C., Tsien R. W. Kinetic and pharmacological properties distinguishing three types of calcium currents in chick sensory neurones. J Physiol. 1987 Dec;394:149–172. doi: 10.1113/jphysiol.1987.sp016864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox A. P., Nowycky M. C., Tsien R. W. Single-channel recordings of three types of calcium channels in chick sensory neurones. J Physiol. 1987 Dec;394:173–200. doi: 10.1113/jphysiol.1987.sp016865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Holl R. W., Thorner M. O., Leong D. A. Intracellular calcium concentration and growth hormone secretion in individual somatotropes: effects of growth hormone-releasing factor and somatostatin. Endocrinology. 1988 Jun;122(6):2927–2932. doi: 10.1210/endo-122-6-2927. [DOI] [PubMed] [Google Scholar]
- Ikeda S. R., Schofield G. G. Somatostatin blocks a calcium current in rat sympathetic ganglion neurones. J Physiol. 1989 Feb;409:221–240. doi: 10.1113/jphysiol.1989.sp017494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue M., Nakajima S., Nakajima Y. Somatostatin induces an inward rectification in rat locus coeruleus neurones through a pertussis toxin-sensitive mechanism. J Physiol. 1988 Dec;407:177–198. doi: 10.1113/jphysiol.1988.sp017409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis D. L., Weight F. F., Luini A. A guanine nucleotide-binding protein mediates the inhibition of voltage-dependent calcium current by somatostatin in a pituitary cell line. Proc Natl Acad Sci U S A. 1986 Dec;83(23):9035–9039. doi: 10.1073/pnas.83.23.9035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason W. T., Rawlings S. R. Whole-cell recordings of ionic currents in bovine somatotrophs and their involvement in growth hormone secretion. J Physiol. 1988 Nov;405:577–593. doi: 10.1113/jphysiol.1988.sp017349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matteson D. R., Armstrong C. M. Properties of two types of calcium channels in clonal pituitary cells. J Gen Physiol. 1986 Jan;87(1):161–182. doi: 10.1085/jgp.87.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihara S., North R. A., Surprenant A. Somatostatin increases an inwardly rectifying potassium conductance in guinea-pig submucous plexus neurones. J Physiol. 1987 Sep;390:335–355. doi: 10.1113/jphysiol.1987.sp016704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollard P., Vacher P., Dufy B., Barker J. L. Somatostatin blocks Ca2+ action potential activity in prolactin-secreting pituitary tumor cells through coordinate actions on K+ and Ca2+ conductances. Endocrinology. 1988 Aug;123(2):721–732. doi: 10.1210/endo-123-2-721. [DOI] [PubMed] [Google Scholar]
- Neill J. D., Frawley L. S. Detection of hormone release from individual cells in mixed populations using a reverse hemolytic plaque assay. Endocrinology. 1983 Mar;112(3):1135–1137. doi: 10.1210/endo-112-3-1135. [DOI] [PubMed] [Google Scholar]
- Nowycky M. C., Fox A. P., Tsien R. W. Three types of neuronal calcium channel with different calcium agonist sensitivity. Nature. 1985 Aug 1;316(6027):440–443. doi: 10.1038/316440a0. [DOI] [PubMed] [Google Scholar]
- Sheppard M. S., Moor B. C., Kraicer J. Release of growth hormone (GH) from purified somatotrophs: interaction of GH-releasing factor and somatostatin and role of adenosine 3',5'-monophosphate. Endocrinology. 1985 Dec;117(6):2364–2370. doi: 10.1210/endo-117-6-2364. [DOI] [PubMed] [Google Scholar]
- Sheppard M. S., Spence J. W., Kraicer J. Release of growth hormone from purified somatotrophs: role of adenosine 3',5'-monophosphate and guanosine 3',5'-monophosphate. Endocrinology. 1979 Jul;105(1):261–268. doi: 10.1210/endo-105-1-261. [DOI] [PubMed] [Google Scholar]
- Sikdar S. K., Waring D. W., Mason W. T. Voltage activated ionic currents in gonadotrophs of the ovine pars tuberalis. Neurosci Lett. 1986 Oct 30;71(1):95–100. doi: 10.1016/0304-3940(86)90263-6. [DOI] [PubMed] [Google Scholar]
- Swandulla D., Armstrong C. M. Calcium channel block by cadmium in chicken sensory neurons. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1736–1740. doi: 10.1073/pnas.86.5.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien R. W., Lipscombe D., Madison D. V., Bley K. R., Fox A. P. Multiple types of neuronal calcium channels and their selective modulation. Trends Neurosci. 1988 Oct;11(10):431–438. doi: 10.1016/0166-2236(88)90194-4. [DOI] [PubMed] [Google Scholar]
- Yamashita N., Shibuya N., Ogata E. Hyperpolarization of the membrane potential caused by somatostatin in dissociated human pituitary adenoma cells that secrete growth hormone. Proc Natl Acad Sci U S A. 1986 Aug;83(16):6198–6202. doi: 10.1073/pnas.83.16.6198. [DOI] [PMC free article] [PubMed] [Google Scholar]


