Abstract
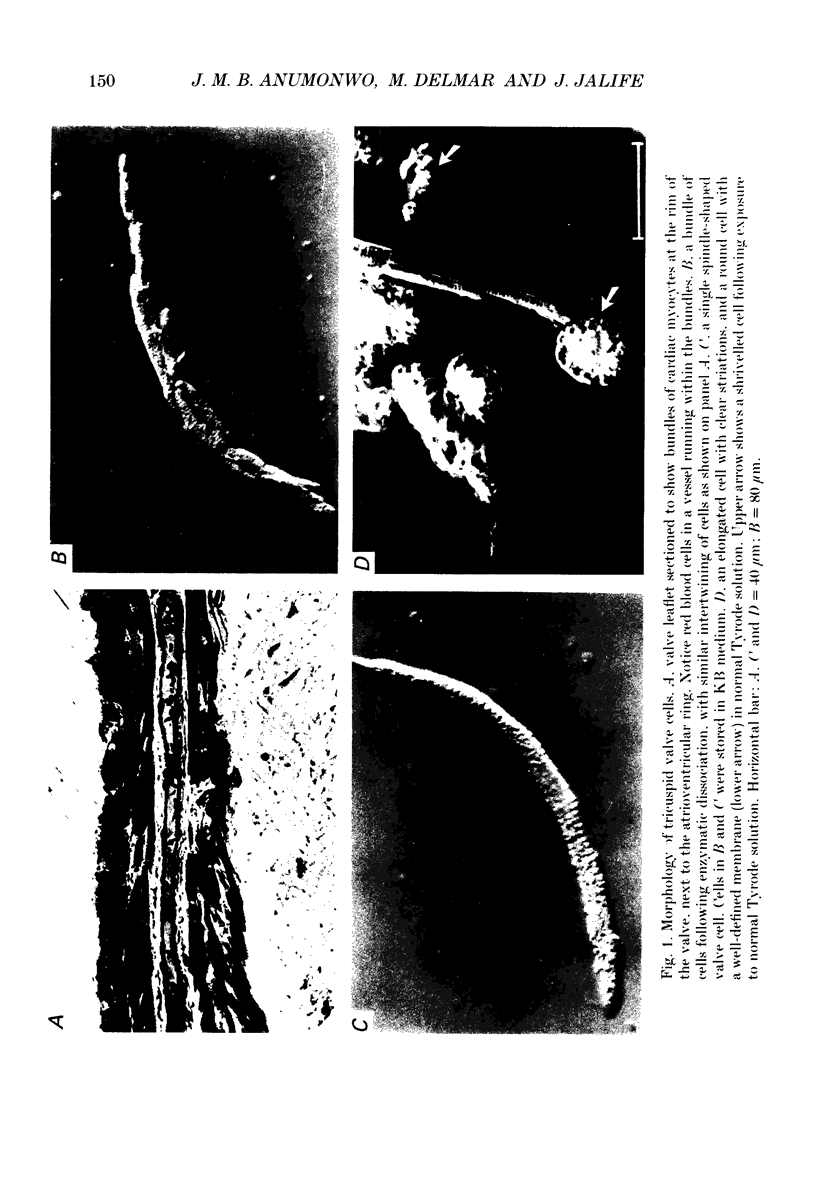
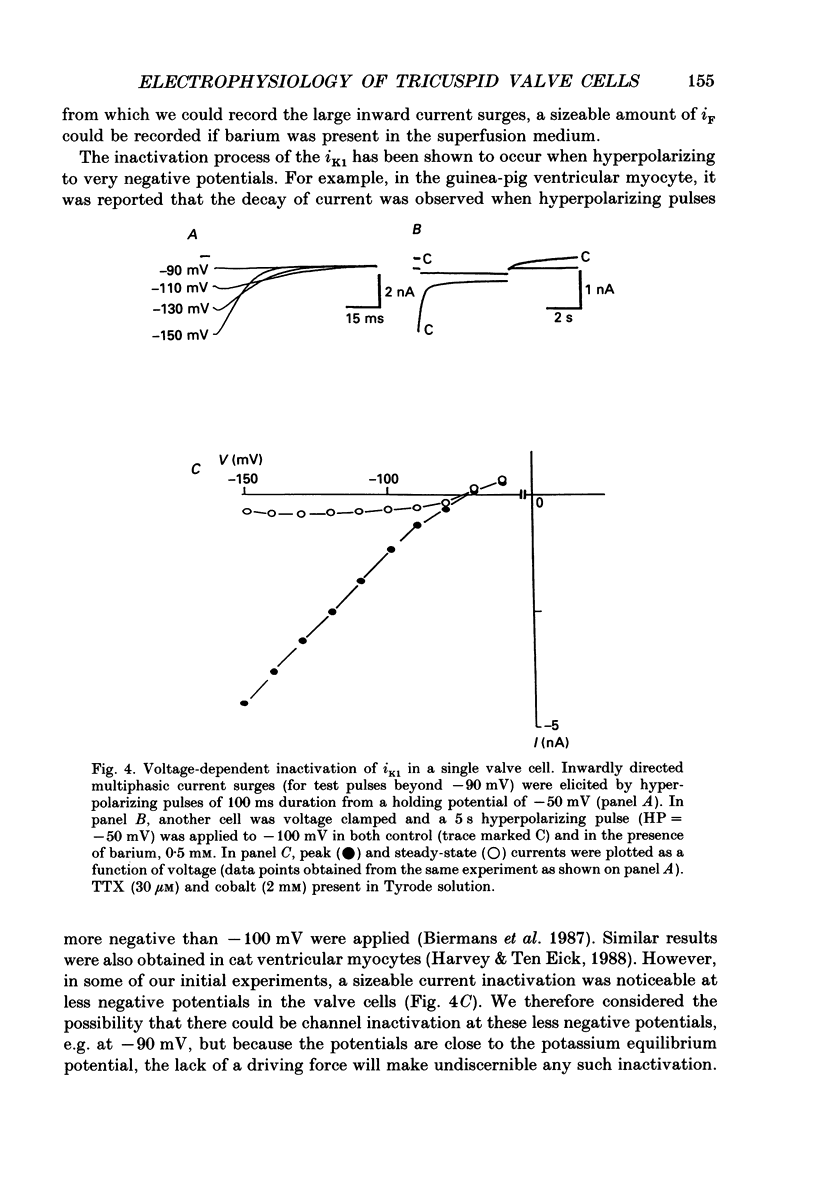
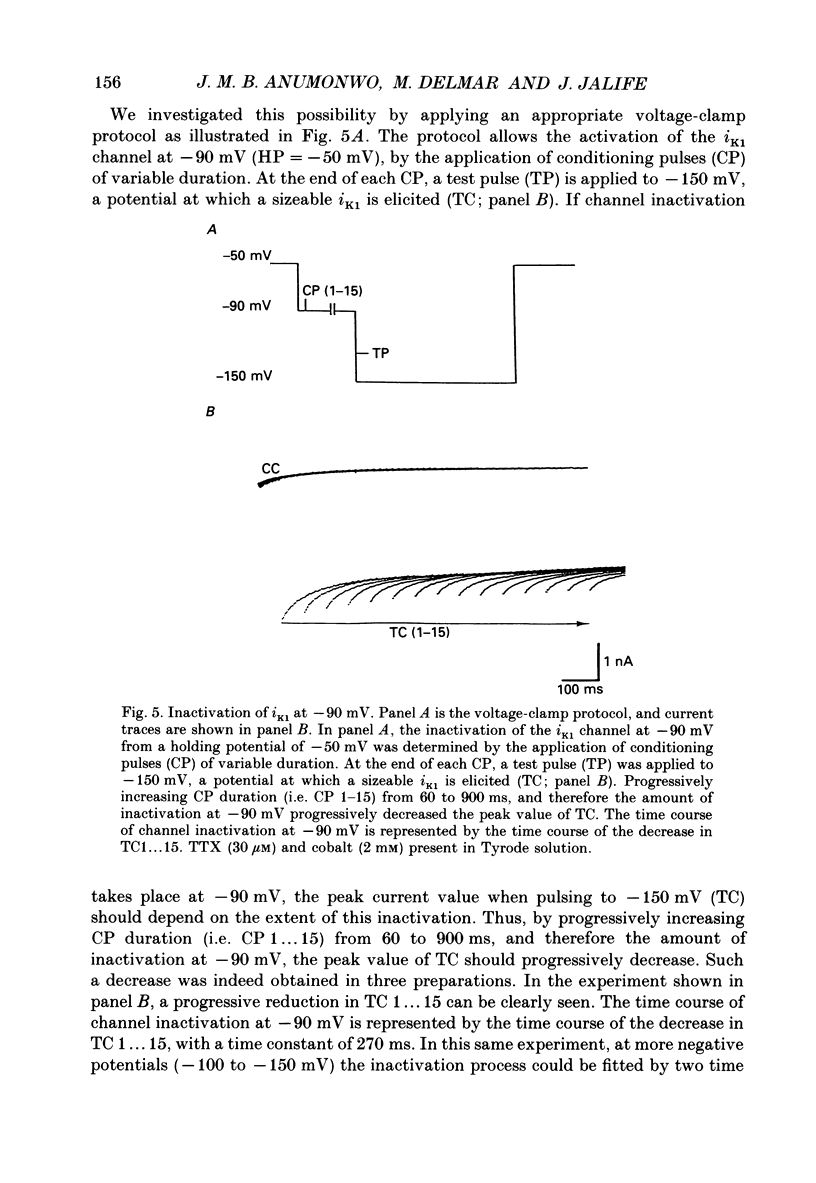
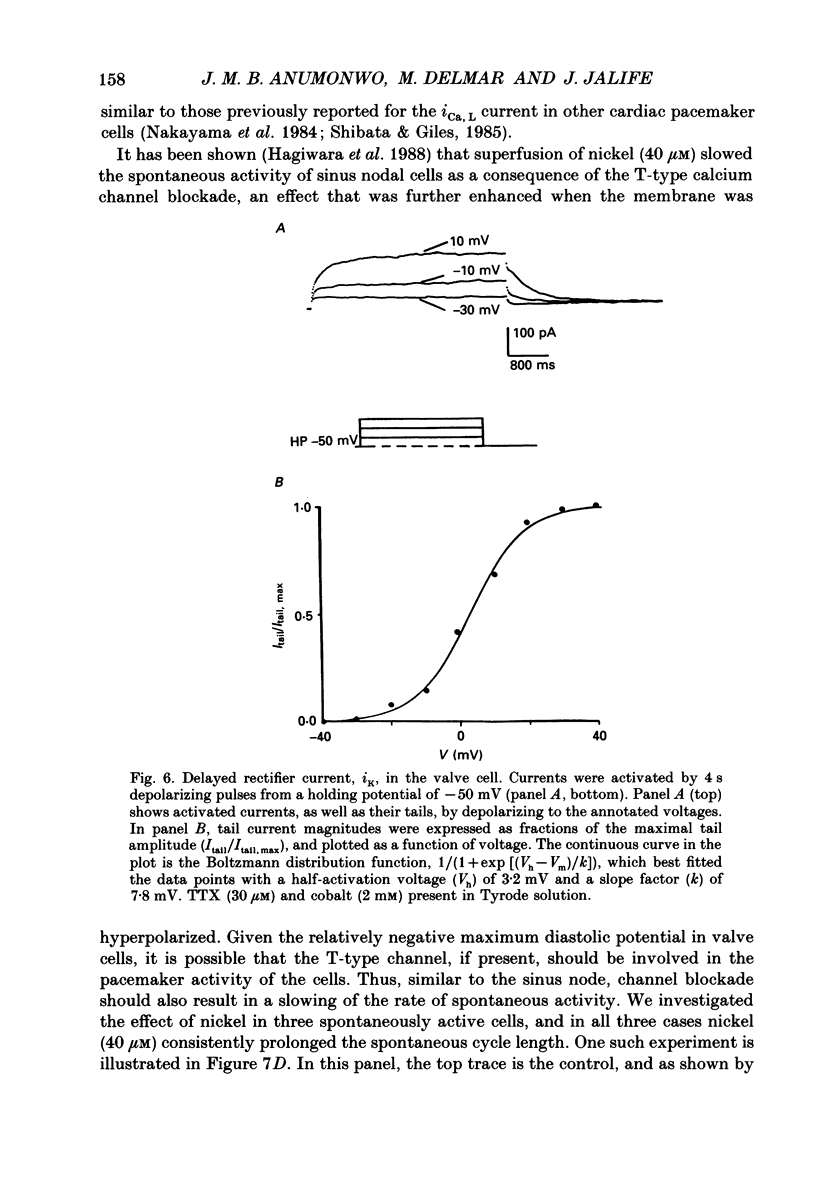
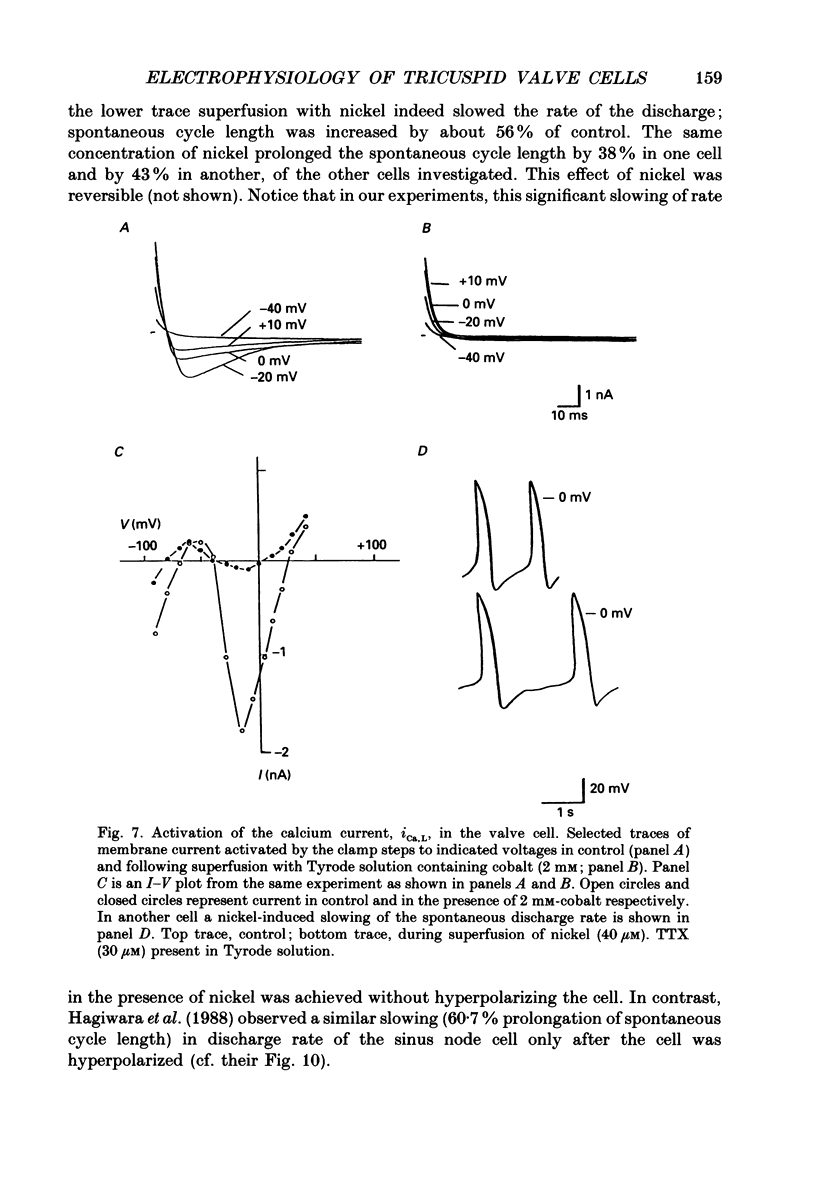
1. The electrophysiology of single myocytes isolated from the rabbit tricuspid valve was studied using the patch-clamp method (whole-cell configuration). Cell dispersion was achieved by collagenase treatment, using the Langendorff retrograde perfusion procedure. 2. After isolation, and while incubating in the recovery (Kraftbrühe) solution, cells had clear striations and were mostly spindle-shaped, or rod-like (less than 10%), with length varying from 35 microns to over 150 microns, and diameter from 3 to 10 microns. 3. Upon exposure to Tyrode solution, the calcium-tolerant cells were mostly rounded with smooth surfaces and well-defined borders. The mean diameter of these cells was 15 +/- 5 microns (S.D., n = 9). A smaller percentage (about 30%) retained the original elongated shape. 4. Patch pipette recordings showed the presence of spontaneous activity in about 30% of round cells, and less frequently in elongated cells. Maximum diastolic potentials (MDPs) in the round cells averaged -82 +/- 6 mV, with a take-off potential of -56 +/- 3 mV (n = 9), and an average maximum upstroke velocity (Vmax) value of 6.3 +/- 0.6 V/s (n = 4). In quiescent cells, the mean resting potential was 69 +/- 12 mV (n = 43). 5. Voltage clamp ramps revealed a steady-state I-V relation with a negative slope region. The mean input resistance value was 25 +/- 9 M omega (n = 16) for the elongated, and 883 +/- 481 M omega (n = 8) for the round cells. 6. Hyperpolarizing 5 s pulses (holding potential = -50 mV) occasionally revealed a slow, time-dependent inward current whose peak increased progressively as a function of clamp potential. The slowly activating current was sensitive to caesium 2 mM), indicating its similarity to the so-called 'pacemaker current' (iF). In alternate voltage- and current-clamp experiments, blocking of iF did not stop pacemaker activity, but there was up to a fourfold increase in pacemaker cycle length. 7. In some cells, 5 s hyperpolarizing steps from a holding potential of -40 or -50 mV produced large, inwardly directed and voltage-dependent current surges that decayed rapidly with time, similar to the inactivation described for the inward rectifier current, iK1. The current was very prominent at voltages more negative than -100 mV, and its decay process was best fitted by two time constants, one fast and one slow. For example, at -150 mV the time constants were 61 and 634 ms. The inward current was blocked by barium (1 mM).(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF














Images in this article
Selected References
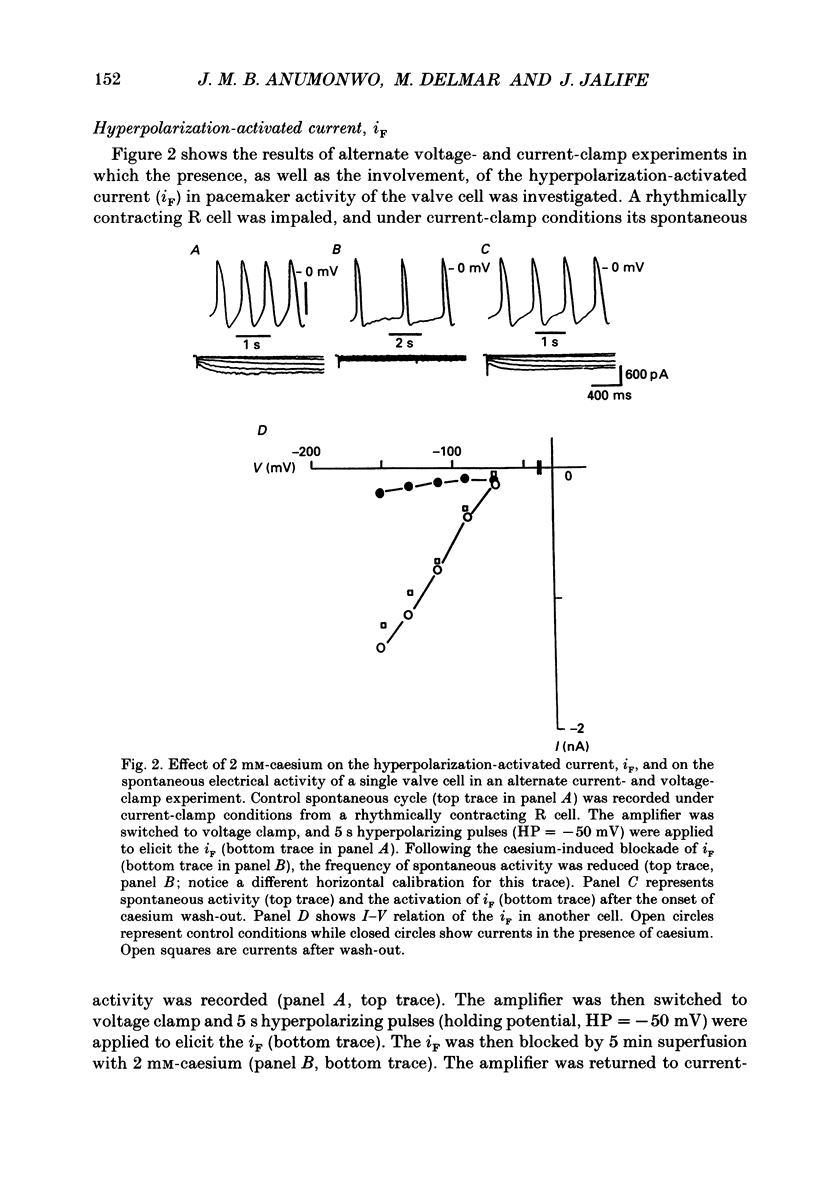
These references are in PubMed. This may not be the complete list of references from this article.
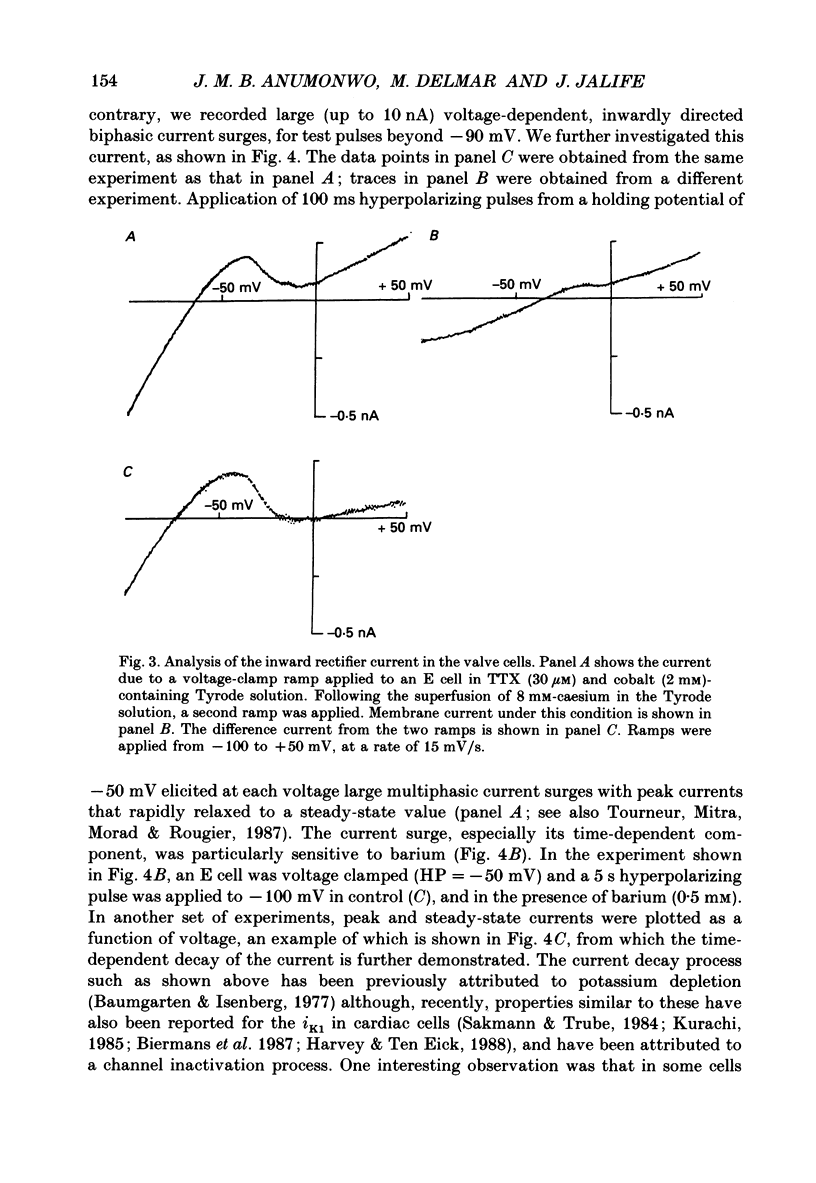
- Anderson R. H., Janse M. J., van Capelle F. J., Billette J., Becker A. E., Durrer D. A combined morphological and electrophysiological study of the atrioventricular node of the rabbit heart. Circ Res. 1974 Dec;35(6):909–922. doi: 10.1161/01.res.35.6.909. [DOI] [PubMed] [Google Scholar]
- Bassett A. L., Fenoglio J. J., Jr, Wit A. L., Myerburg R. J., Gelband H. Eelctrophysiologic and ultrastructural characteristics of the canine tricuspid valve. Am J Physiol. 1976 May;230(5):1366–1373. doi: 10.1152/ajplegacy.1976.230.5.1366. [DOI] [PubMed] [Google Scholar]
- Baumgarten C. M., Isenberg G. Depletion and accumulation of potassium in the extracellular clefts of cardiac Purkinje fibers during voltage clamp hyperpolarization and depolarization. Pflugers Arch. 1977 Mar 11;368(1-2):19–31. doi: 10.1007/BF01063450. [DOI] [PubMed] [Google Scholar]
- Beeler G. W., McGuigan J. A. Voltage clamping of multicellular myocardial preparations: capabilities and limitations of existing methods. Prog Biophys Mol Biol. 1978;34(3):219–254. doi: 10.1016/0079-6107(79)90019-1. [DOI] [PubMed] [Google Scholar]
- Biermans G., Vereecke J., Carmeliet E. The mechanism of the inactivation of the inward-rectifying K current during hyperpolarizing steps in guinea-pig ventricular myocytes. Pflugers Arch. 1987 Dec;410(6):604–613. doi: 10.1007/BF00581320. [DOI] [PubMed] [Google Scholar]
- Brown H. F. Electrophysiology of the sinoatrial node. Physiol Rev. 1982 Apr;62(2):505–530. doi: 10.1152/physrev.1982.62.2.505. [DOI] [PubMed] [Google Scholar]
- Callewaert G., Carmeliet E., Vereecke J. Single cardiac Purkinje cells: general electrophysiology and voltage-clamp analysis of the pace-maker current. J Physiol. 1984 Apr;349:643–661. doi: 10.1113/jphysiol.1984.sp015179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denyer J., Brown H. A method for isolating rabbit sinoatrial node cells which maintains their natural shape. Jpn J Physiol. 1987;37(5):963–965. doi: 10.2170/jjphysiol.37.963. [DOI] [PubMed] [Google Scholar]
- DiFrancesco D. Block and activation of the pace-maker channel in calf purkinje fibres: effects of potassium, caesium and rubidium. J Physiol. 1982 Aug;329:485–507. doi: 10.1113/jphysiol.1982.sp014315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFrancesco D., Ferroni A., Mazzanti M., Tromba C. Properties of the hyperpolarizing-activated current (if) in cells isolated from the rabbit sino-atrial node. J Physiol. 1986 Aug;377:61–88. doi: 10.1113/jphysiol.1986.sp016177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerr T., Denger R., Trautwein W. Calcium currents in single SA nodal cells of the rabbit heart studied with action potential clamp. Pflugers Arch. 1989 Apr;413(6):599–603. doi: 10.1007/BF00581808. [DOI] [PubMed] [Google Scholar]
- Ellison J. P., Hibbs R. G. The atrioventricular valves of the guinea-pig. I. A light microscopic study. Am J Anat. 1973 Nov;138(3):331–345. doi: 10.1002/aja.1001380304. [DOI] [PubMed] [Google Scholar]
- Fenoglio J. J., Jr, Tuan-Duc-Pham, Wit A. L., Bassett A. L., Wagner B. M. Canine mitral complex. Ultrastructure and electromechanical properties. Circ Res. 1972 Sep;31(3):417–430. doi: 10.1161/01.res.31.3.417. [DOI] [PubMed] [Google Scholar]
- Frelin C., Cognard C., Vigne P., Lazdunski M. Tetrodotoxin-sensitive and tetrodotoxin-resistant Na+ channels differ in their sensitivity to Cd2+ and Zn2+. Eur J Pharmacol. 1986 Mar 18;122(2):245–250. doi: 10.1016/0014-2999(86)90109-3. [DOI] [PubMed] [Google Scholar]
- Giles W. R., van Ginneken A. C. A transient outward current in isolated cells from the crista terminalis of rabbit heart. J Physiol. 1985 Nov;368:243–264. doi: 10.1113/jphysiol.1985.sp015856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross L., Kugel M. A. Topographic Anatomy and Histology of the Valves in the Human Heart. Am J Pathol. 1931 Sep;7(5):445–474.7. [PMC free article] [PubMed] [Google Scholar]
- Hagiwara N., Irisawa H., Kameyama M. Contribution of two types of calcium currents to the pacemaker potentials of rabbit sino-atrial node cells. J Physiol. 1988 Jan;395:233–253. doi: 10.1113/jphysiol.1988.sp016916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Harvey R. D., Ten Eick R. E. Characterization of the inward-rectifying potassium current in cat ventricular myocytes. J Gen Physiol. 1988 Apr;91(4):593–615. doi: 10.1085/jgp.91.4.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isenberg G., Klockner U. Calcium tolerant ventricular myocytes prepared by preincubation in a "KB medium". Pflugers Arch. 1982 Oct;395(1):6–18. doi: 10.1007/BF00584963. [DOI] [PubMed] [Google Scholar]
- Johnson E. A., Lieberman M. Heart: excitation and contraction. Annu Rev Physiol. 1971;33:479–532. doi: 10.1146/annurev.ph.33.030171.002403. [DOI] [PubMed] [Google Scholar]
- Kurachi Y. Voltage-dependent activation of the inward-rectifier potassium channel in the ventricular cell membrane of guinea-pig heart. J Physiol. 1985 Sep;366:365–385. doi: 10.1113/jphysiol.1985.sp015803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama T., Kurachi Y., Noma A., Irisawa H. Action potential and membrane currents of single pacemaker cells of the rabbit heart. Pflugers Arch. 1984 Nov;402(3):248–257. doi: 10.1007/BF00585507. [DOI] [PubMed] [Google Scholar]
- Noma A., Kotake H., Irisawa H. Slow inward current and its role mediating the chronotropic effect of epinephrine in the rabbit sinoatrial node. Pflugers Arch. 1980 Oct;388(1):1–9. doi: 10.1007/BF00582621. [DOI] [PubMed] [Google Scholar]
- Noma A., Morad M., Irisawa H. Does the "pacemaker current" generate the diastolic depolarization in the rabbit SA node cells? Pflugers Arch. 1983 May;397(3):190–194. doi: 10.1007/BF00584356. [DOI] [PubMed] [Google Scholar]
- Noma A., Nakayama T., Kurachi Y., Irisawa H. Resting K conductances in pacemaker and non-pacemaker heart cells of the rabbit. Jpn J Physiol. 1984;34(2):245–254. doi: 10.2170/jjphysiol.34.245. [DOI] [PubMed] [Google Scholar]
- Priola D. V., Fellows C., Moorehouse J., Sanchez R. Mechanical activity of canine mitral valve in situ. Am J Physiol. 1970 Dec;219(6):1647–1651. doi: 10.1152/ajplegacy.1970.219.6.1647. [DOI] [PubMed] [Google Scholar]
- Rozanski G. J. Electrophysiological properties of automatic fibers in rabbit atrioventricular valves. Am J Physiol. 1987 Oct;253(4 Pt 2):H720–H727. doi: 10.1152/ajpheart.1987.253.4.H720. [DOI] [PubMed] [Google Scholar]
- Rozanski G. J., Jalife J. Automaticity in atrioventricular valve leaflets of rabbit heart. Am J Physiol. 1986 Mar;250(3 Pt 2):H397–H406. doi: 10.1152/ajpheart.1986.250.3.H397. [DOI] [PubMed] [Google Scholar]
- Rozanski G. J., Lipsius S. L., Randall W. C. Functional characteristics of sinoatrial and subsidiary pacemaker activity in the canine right atrium. Circulation. 1983 Jun;67(6):1378–1387. doi: 10.1161/01.cir.67.6.1378. [DOI] [PubMed] [Google Scholar]
- Sakmann B., Trube G. Voltage-dependent inactivation of inward-rectifying single-channel currents in the guinea-pig heart cell membrane. J Physiol. 1984 Feb;347:659–683. doi: 10.1113/jphysiol.1984.sp015089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah A. K., Cohen I. S., Datyner N. B. Background K+ current in isolated canine cardiac Purkinje myocytes. Biophys J. 1987 Oct;52(4):519–525. doi: 10.1016/S0006-3495(87)83241-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibasaki T. Conductance and kinetics of delayed rectifier potassium channels in nodal cells of the rabbit heart. J Physiol. 1987 Jun;387:227–250. doi: 10.1113/jphysiol.1987.sp016571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata E. F., Giles W. R. Ionic currents that generate the spontaneous diastolic depolarization in individual cardiac pacemaker cells. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7796–7800. doi: 10.1073/pnas.82.22.7796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenblick E. H., Napolitano L. M., Daggett W. M., Cooper T. An intrinsic neuromuscular basis for mitral valve motion in the dog. Circ Res. 1967 Jul;21(1):9–15. doi: 10.1161/01.res.21.1.9. [DOI] [PubMed] [Google Scholar]
- TRAUTWEIN W., KASSEBAUM D. G. On the mechanism of spontaneous impulse generation in the pacemaker of the heart. J Gen Physiol. 1961 Nov;45:317–330. doi: 10.1085/jgp.45.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tourneur Y., Mitra R., Morad M., Rougier O. Activation properties of the inward-rectifying potassium channel on mammalian heart cells. J Membr Biol. 1987;97(2):127–135. doi: 10.1007/BF01869419. [DOI] [PubMed] [Google Scholar]
- Wit A. L., Cranefield P. F. Triggered activity in cardiac muscle fibers of the simian mitral valve. Circ Res. 1976 Feb;38(2):85–98. doi: 10.1161/01.res.38.2.85. [DOI] [PubMed] [Google Scholar]
- Wit A. L., Fenoglio J. J., Jr, Hordof A. J., Reemtsma K. Ultrastructure and transmembrane potentials of cardiac muscle in the human anterior mitral valve leaflet. Circulation. 1979 Jun;59(6):1284–1292. doi: 10.1161/01.cir.59.6.1284. [DOI] [PubMed] [Google Scholar]
- Wit A. L., Fenoglio J. J., Jr, Wagner B. M., Bassett A. L. Electrophysiological properties of cardiac muscle in the anterior mitral valve leaflet and the adjacent atrium in the dog. Possible implications for the genesis of atrial dysrhythmias. Circ Res. 1973 Jun;32(6):731–745. doi: 10.1161/01.res.32.6.731. [DOI] [PubMed] [Google Scholar]
- Yanagihara K., Irisawa H. Potassium current during the pacemaker depolarization in rabbit sinoatrial node cell. Pflugers Arch. 1980 Dec;388(3):255–260. doi: 10.1007/BF00658491. [DOI] [PubMed] [Google Scholar]