Abstract
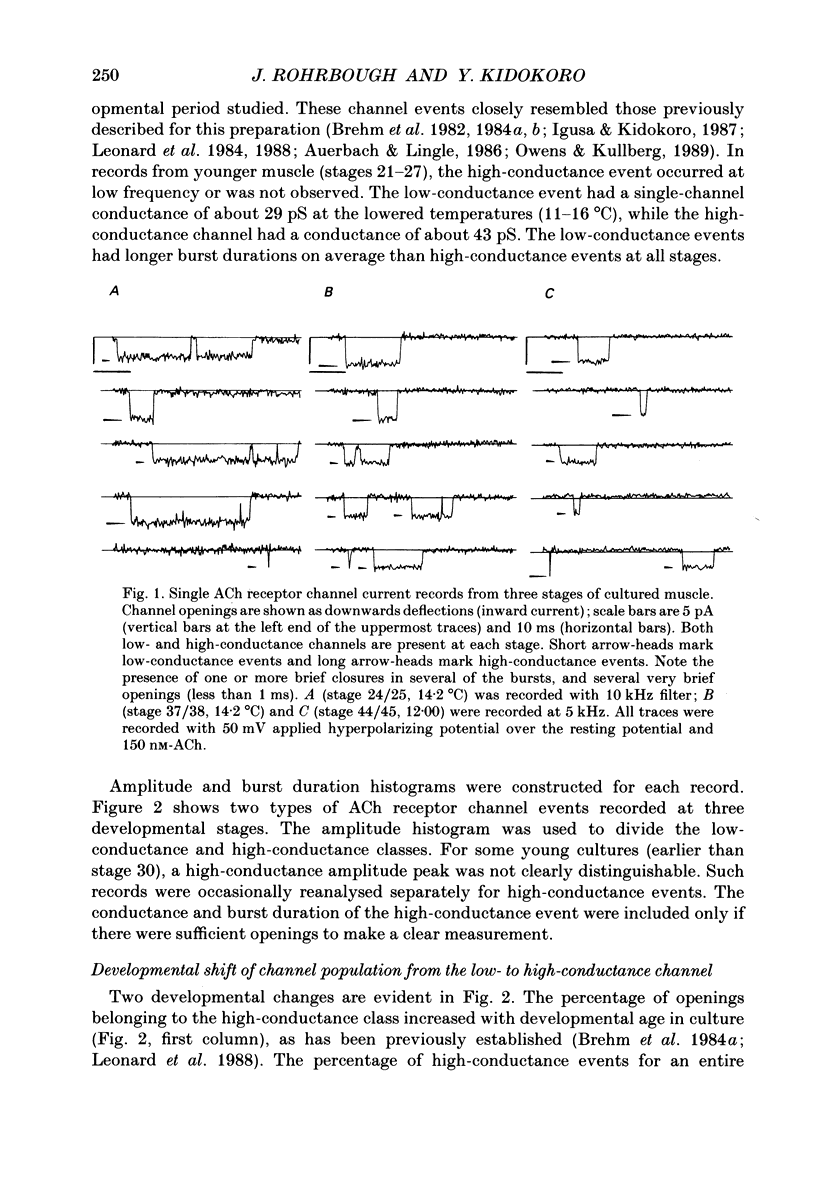
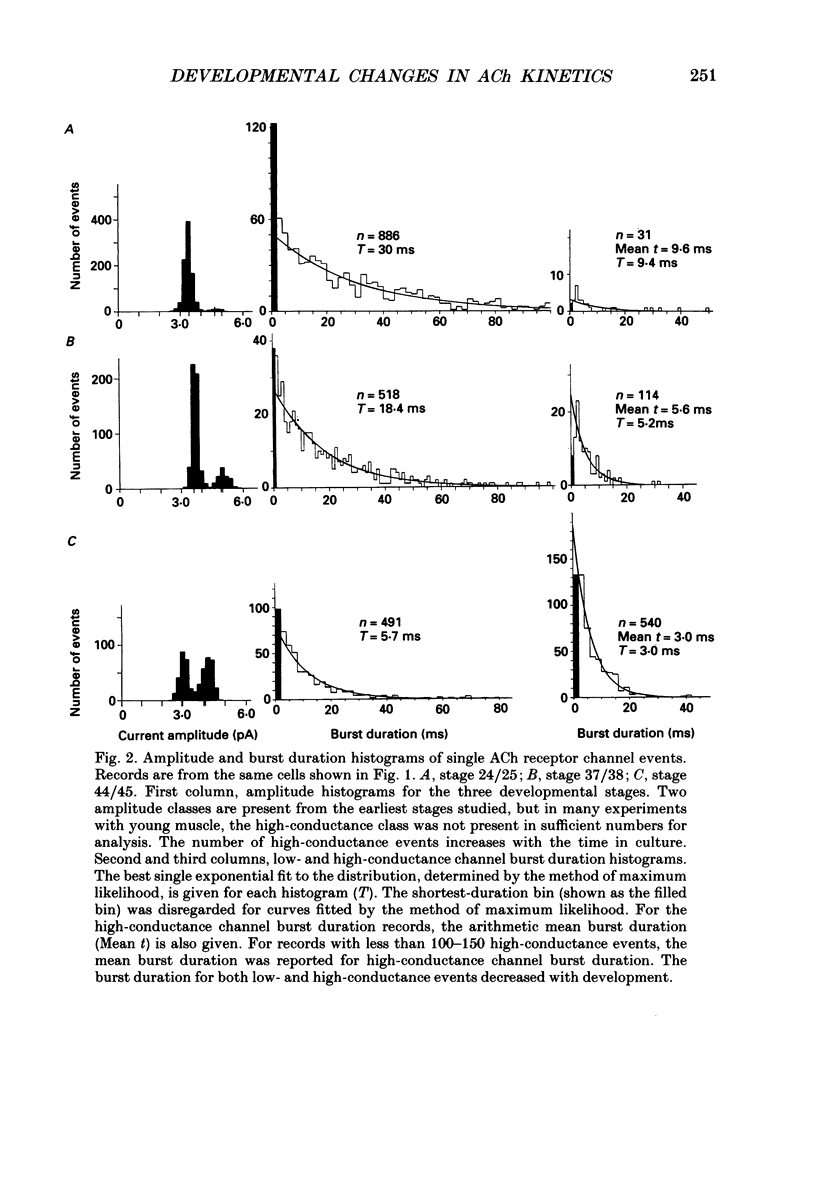
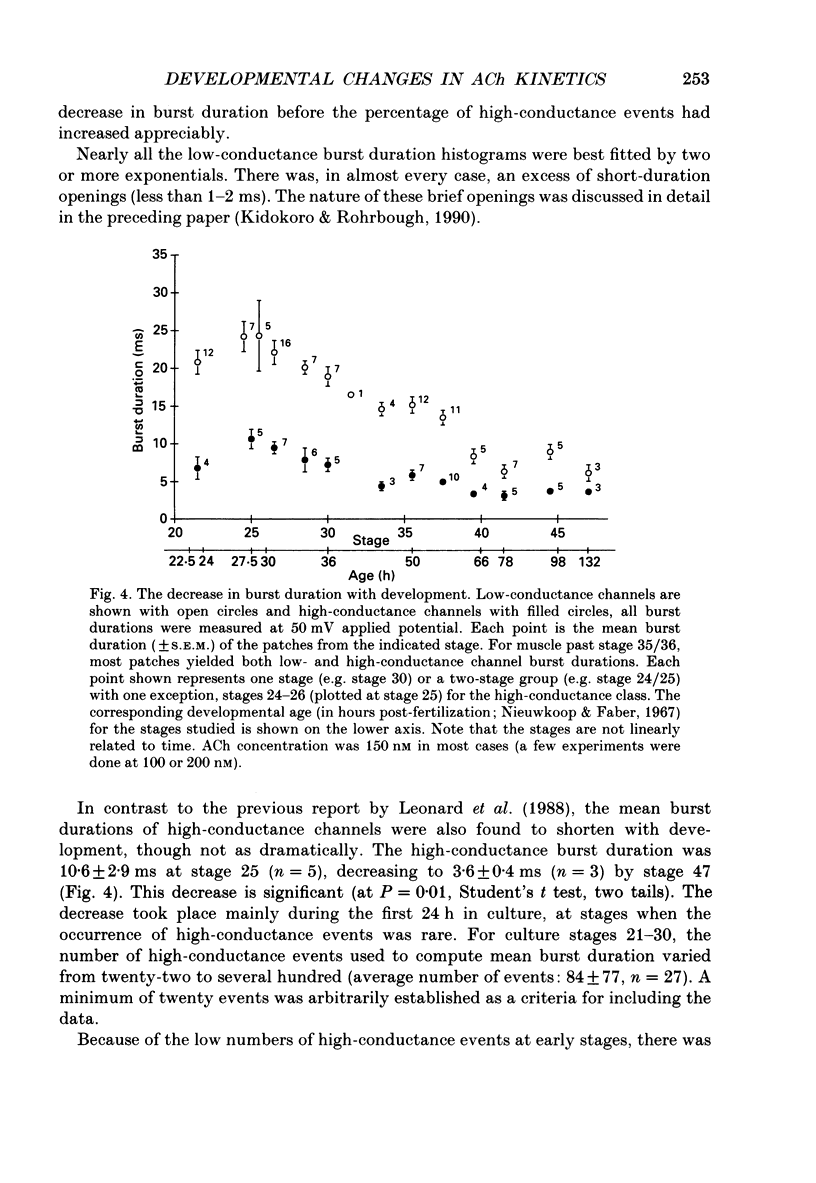
1. Developmental changes in single acetylcholine (ACh) receptor channel properties were analysed in cultured Xenopus myocytes at low temperatures (11-16 degrees C) using the cell-attached patch clamp technique. Single-channel recordings were done at fourteen stages of development ranging from several hours (stage 21) to 5 days (stage 47) after ACh receptors first appear in the muscle membrane. 2. Two types of channels, low conductance and high conductance, which have been described previously, were observed at all stages. At low concentrations of ACh, channel events often occurred as bursts of openings separated by closures briefer than 1 ms. Such bursts were treated as one event. Many brief, isolated channel openings, which were described in the preceding paper, were also observed. Developmental changes in burst duration, brief openings and brief closures were assessed for the period studied in culture. 3. Throughout development, most of the burst duration histograms for the low-conductance channel were not well fitted by a single exponential, having an excess of brief openings. The brief component could be largely accounted for by singly liganded openings, as described in the preceding paper. The burst durations reported here represent the main component of the distribution. At 150 nM-ACh, high-conductance channel burst duration histograms were well fitted by single exponentials. 4. There was a developmental increase in the percentage of single-channel events belonging to the high-conductance class. The percentage of high-conductance events remained low (less than 10%) for the first day after ACh receptors appeared (stages 20-34), and increased to 60% at stage 47. 5. In addition to a shift in channel population, there was a decrease in both low- and high-conductance channel burst durations during early stages of development (mostly within the first day in culture). The effect was more dramatic for the low-conductance channel: overall, low-conductance channel burst duration at 50 mV hyperpolarization over the resting potential decreased from 24 ms at stage 24/25 to 6 ms at stage 47, a 4-fold decrease. During the same period, high-conductance burst duration decreased from 10.6 to 4 ms. a 2.5-fold decrease. In contrast to the burst duration, the mean open time of 60 microseconds for the brief isolated class of opening in the low-conductance channel did not change with development. 6. Neither the single-channel conductance nor the voltage dependence of burst duration changed with development.(ABSTRACT TRUNCATED AT 400 WORDS)
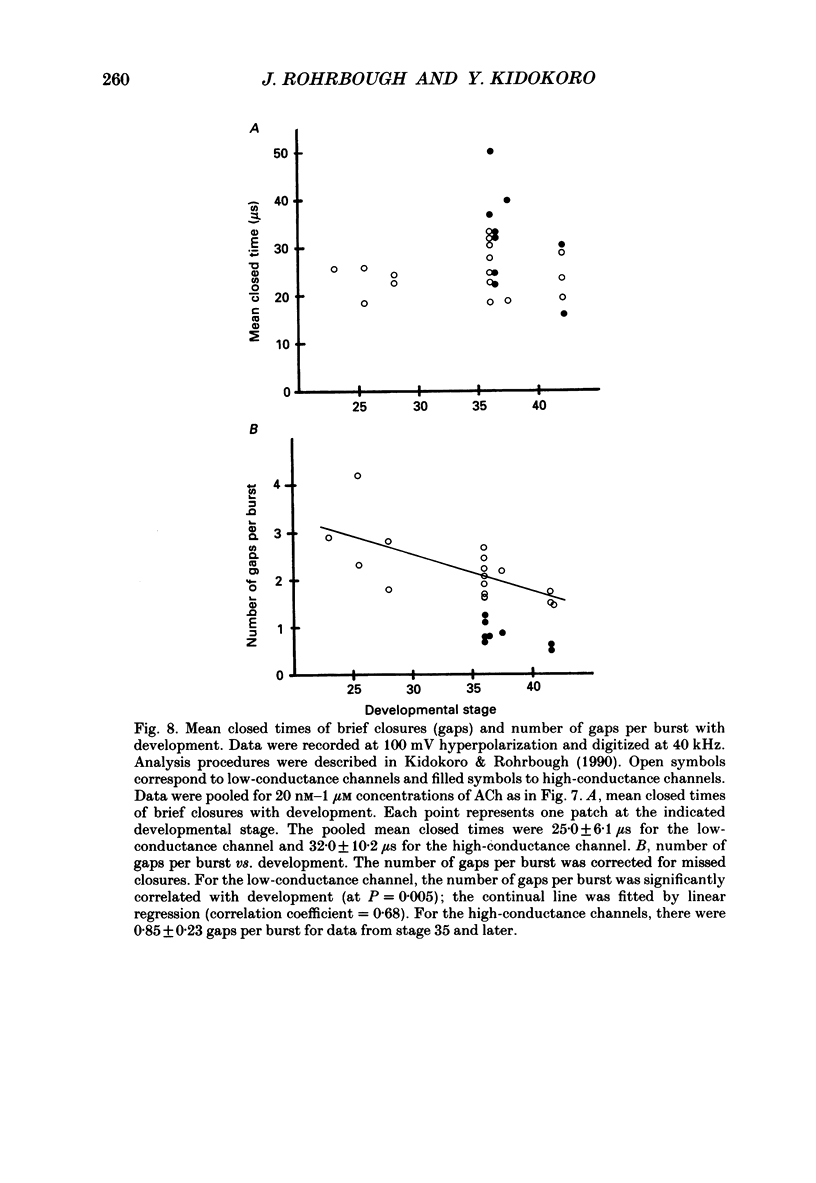
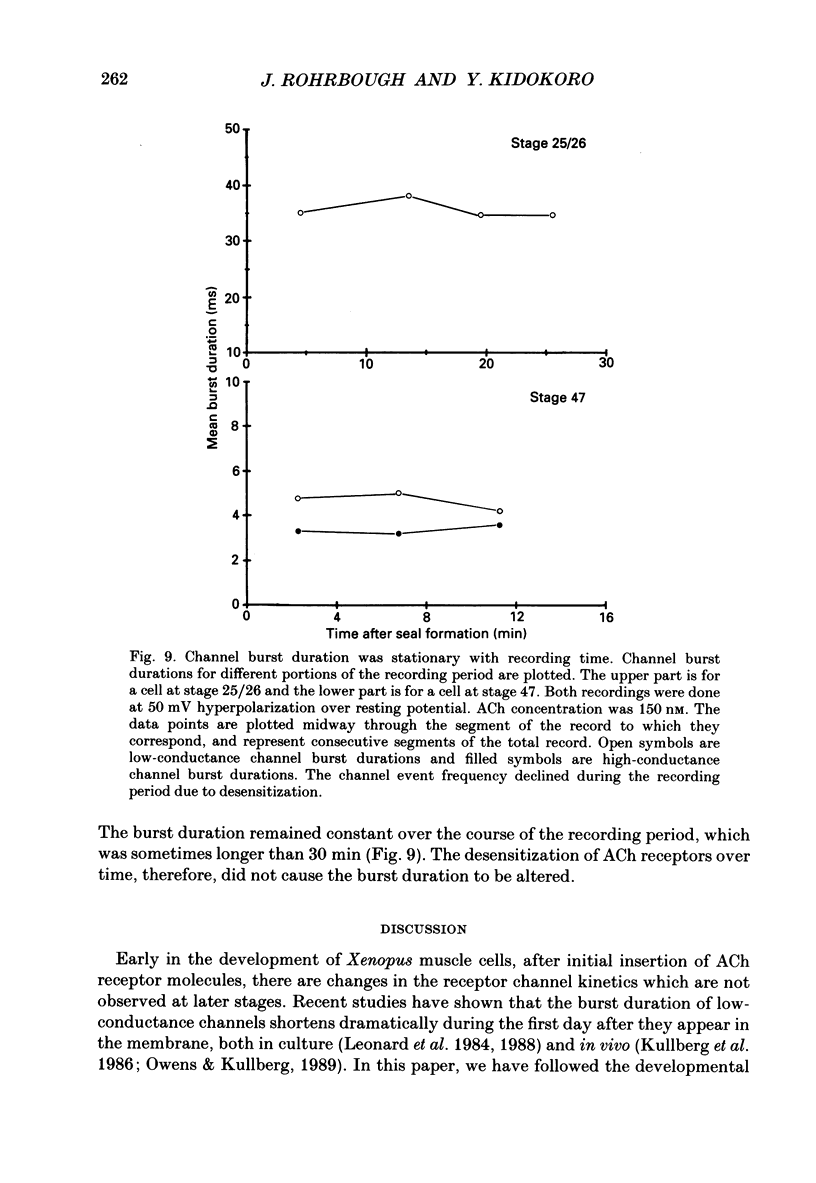
Full text
PDF




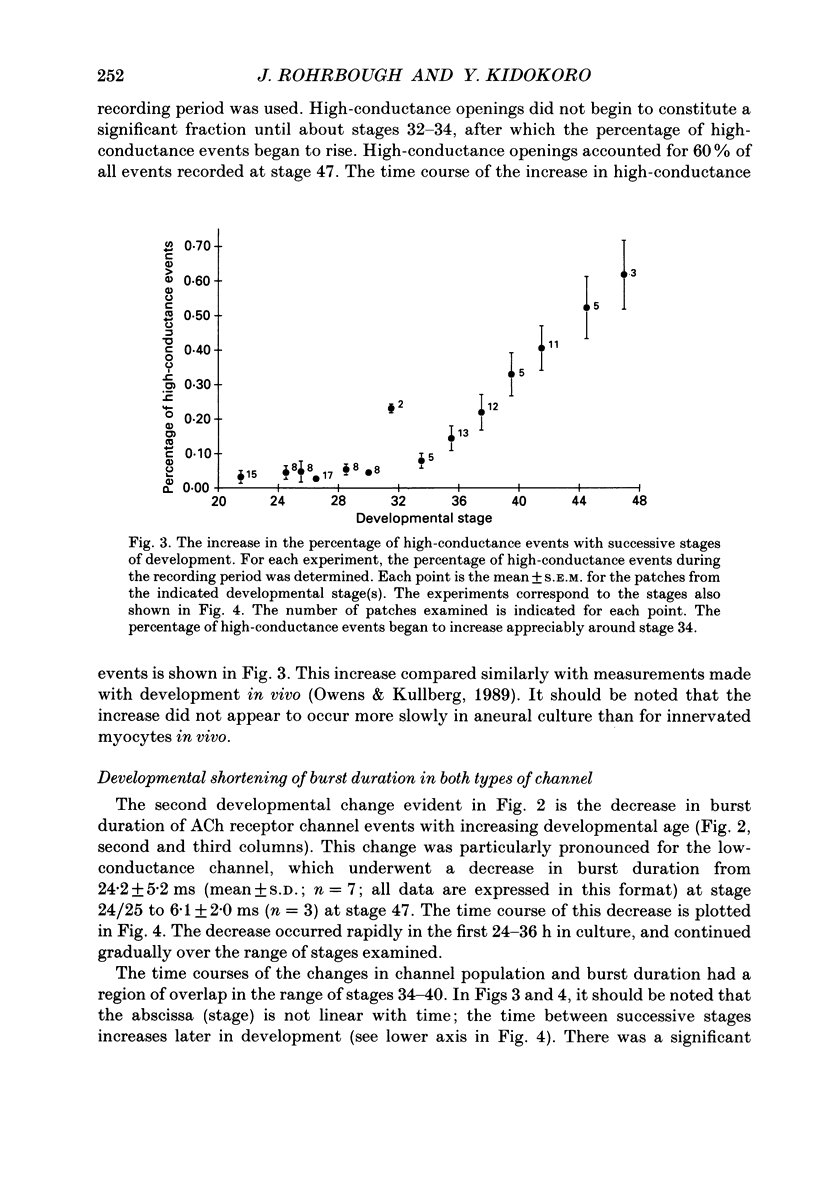












Selected References
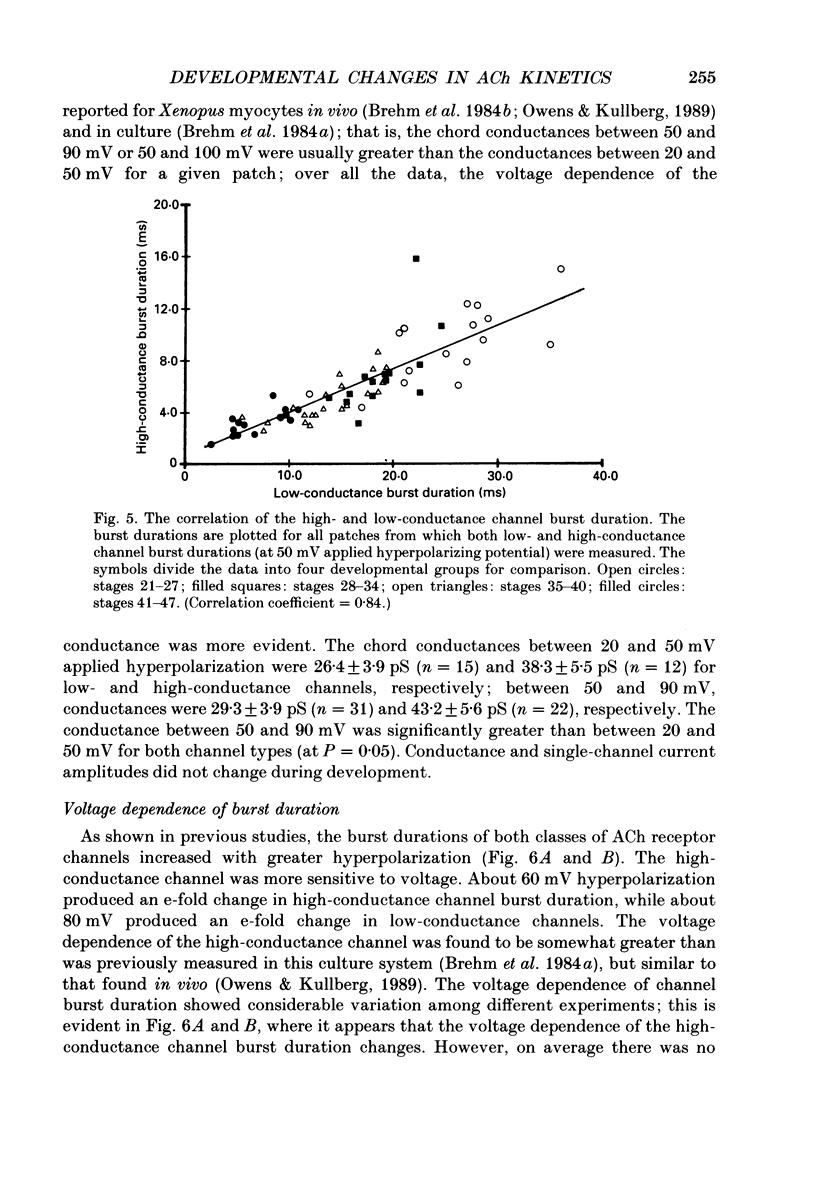
These references are in PubMed. This may not be the complete list of references from this article.
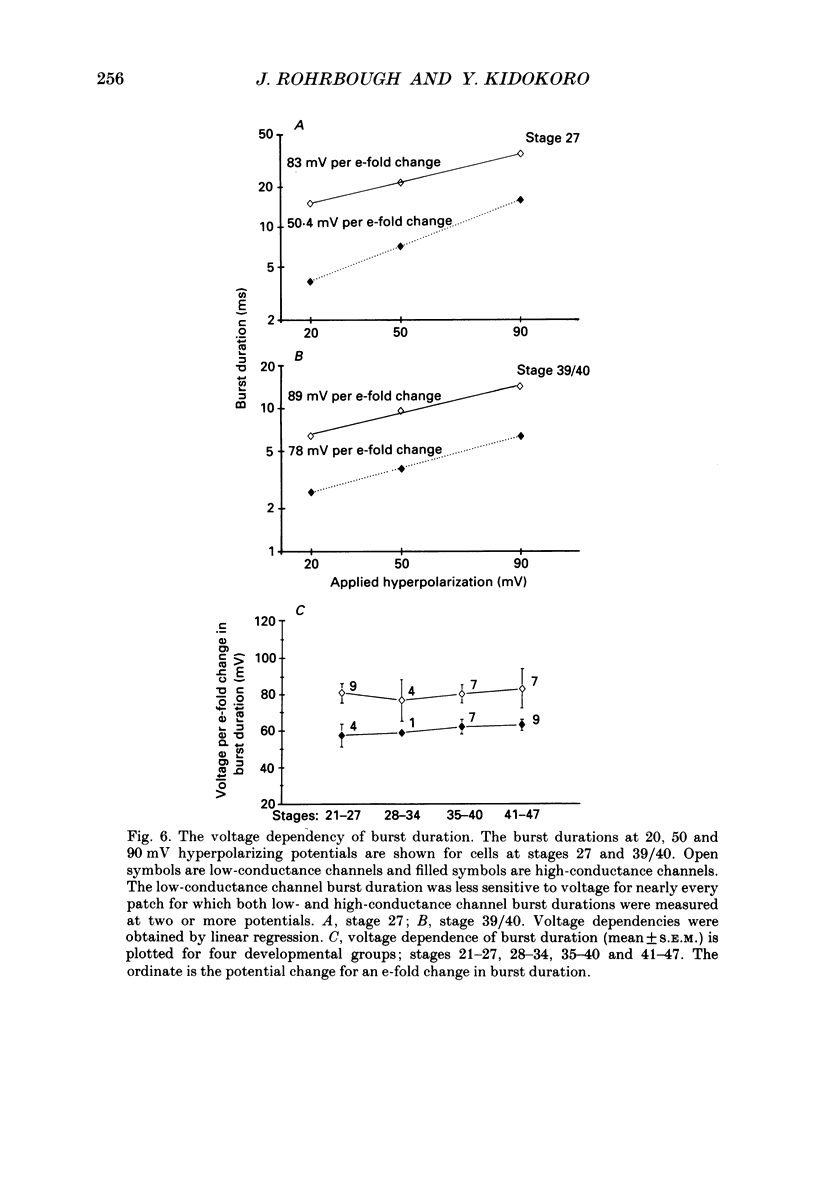
- Albuquerque E. X., Deshpande S. S., Aracava Y., Alkondon M., Daly J. W. A possible involvement of cyclic AMP in the expression of desensitization of the nicotinic acetylcholine receptor. A study with forskolin and its analogs. FEBS Lett. 1986 Apr 7;199(1):113–120. doi: 10.1016/0014-5793(86)81235-2. [DOI] [PubMed] [Google Scholar]
- Auerbach A., Lingle C. J. Heterogeneous kinetic properties of acetylcholine receptor channels in Xenopus myocytes. J Physiol. 1986 Sep;378:119–140. doi: 10.1113/jphysiol.1986.sp016211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackshaw S., Warner A. Onset of acetylcholine sensitivity and endplate activity in developing myotome muscles of Xenopus. Nature. 1976 Jul 15;262(5565):217–218. doi: 10.1038/262217a0. [DOI] [PubMed] [Google Scholar]
- Brehm P., Henderson L. Regulation of acetylcholine receptor channel function during development of skeletal muscle. Dev Biol. 1988 Sep;129(1):1–11. doi: 10.1016/0012-1606(88)90156-x. [DOI] [PubMed] [Google Scholar]
- Brehm P., Kidokoro Y., Moody-Corbett F. Acetylcholine receptor channel properties during development of Xenopus muscle cells in culture. J Physiol. 1984 Dec;357:203–217. doi: 10.1113/jphysiol.1984.sp015497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brehm P., Kream R. M., Moody-Corbett F. Transcriptional and translational requirements for developmental alterations in acetylcholine receptor channel function in Xenopus myotomal muscle. Dev Biol. 1987 Sep;123(1):222–230. doi: 10.1016/0012-1606(87)90444-1. [DOI] [PubMed] [Google Scholar]
- Brehm P., Kullberg R., Moody-Corbett F. Properties of non-junctional acetylcholine receptor channels on innervated muscle of Xenopus laevis. J Physiol. 1984 May;350:631–648. doi: 10.1113/jphysiol.1984.sp015222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brehm P., Steinbach J. H., Kidokoro Y. Channel open time of acetylcholine receptors on Xenopus muscle cells in dissociated cell culture. Dev Biol. 1982 May;91(1):93–102. doi: 10.1016/0012-1606(82)90012-4. [DOI] [PubMed] [Google Scholar]
- Brehm P., Yeh E., Patrick J., Kidokoro Y. Metabolism of acetylcholine receptors on embryonic amphibian muscle. J Neurosci. 1983 Jan;3(1):101–107. doi: 10.1523/JNEUROSCI.03-01-00101.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devreotes P. N., Fambrough D. M. Acetylcholine receptor turnover in membranes of developing muscle fibers. J Cell Biol. 1975 May;65(2):335–358. doi: 10.1083/jcb.65.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischbach G. D., Schuetze S. M. A post-natal decrease in acetylcholine channel open time at rat end-plates. J Physiol. 1980 Jun;303:125–137. doi: 10.1113/jphysiol.1980.sp013275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg A. S., Nakajima S., Nakajima Y. Functional properties of newly inserted acetylcholine receptors in embryonic Xenopus muscle cells. Brain Res. 1985 Apr;351(2):289–296. doi: 10.1016/0165-3806(85)90200-7. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Sakmann B. Multiple conductance states of single acetylcholine receptor channels in embryonic muscle cells. Nature. 1981 Dec 3;294(5840):462–464. doi: 10.1038/294462a0. [DOI] [PubMed] [Google Scholar]
- Huganir R. L., Miles K., Greengard P. Phosphorylation of the nicotinic acetylcholine receptor by an endogenous tyrosine-specific protein kinase. Proc Natl Acad Sci U S A. 1984 Nov;81(22):6968–6972. doi: 10.1073/pnas.81.22.6968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igusa Y., Kidokoro Y. Two types of acetylcholine receptor channels in developing Xenopus muscle cells in culture: further kinetic analyses. J Physiol. 1987 Aug;389:271–300. doi: 10.1113/jphysiol.1987.sp016657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidokoro Y., Rohrbough J. Acetylcholine receptor channels in Xenopus myocyte culture; brief openings, brief closures and slow desensitization. J Physiol. 1990 Jun;425:227–244. doi: 10.1113/jphysiol.1990.sp018100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullberg R. W., Brehm P., Steinbach J. H. Nonjunctional acetylcholine receptor channel open time decreases during development of Xenopus muscle. Nature. 1981 Jan 29;289(5796):411–413. doi: 10.1038/289411a0. [DOI] [PubMed] [Google Scholar]
- Kullberg R. W., Lentz T. L., Cohen M. W. Development of the myotomal neuromuscular junction in Xenopus laevis: an electrophysiological and fine-structural study. Dev Biol. 1977 Oct 1;60(1):101–129. doi: 10.1016/0012-1606(77)90113-0. [DOI] [PubMed] [Google Scholar]
- Kullberg R., Kasprzak H. Gating kinetics of nonjunctional acetylcholine receptor channels in developing Xenopus muscle. J Neurosci. 1985 Apr;5(4):970–976. doi: 10.1523/JNEUROSCI.05-04-00970.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullberg R., Owens J. L. Comparative development of end-plate currents in two muscles of Xenopus laevis. J Physiol. 1986 May;374:413–427. doi: 10.1113/jphysiol.1986.sp016088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard R. J., Nakajima S., Nakajima Y., Carlson C. G. Early development of two types of nicotinic acetylcholine receptors. J Neurosci. 1988 Nov;8(11):4038–4048. doi: 10.1523/JNEUROSCI.08-11-04038.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard R. J., Nakajima S., Nakajima Y., Takahashi T. Differential development of two classes of acetylcholine receptors in Xenopus muscle in culture. Science. 1984 Oct 5;226(4670):55–57. doi: 10.1126/science.6474189. [DOI] [PubMed] [Google Scholar]
- Middleton P., Jaramillo F., Schuetze S. M. Forskolin increases the rate of acetylcholine receptor desensitization at rat soleus endplates. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4967–4971. doi: 10.1073/pnas.83.13.4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton P., Rubin L. L., Schuetze S. M. Desensitization of acetylcholine receptors in rat myotubes is enhanced by agents that elevate intracellular cAMP. J Neurosci. 1988 Sep;8(9):3405–3412. doi: 10.1523/JNEUROSCI.08-09-03405.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles K., Anthony D. T., Rubin L. L., Greengard P., Huganir R. L. Regulation of nicotinic acetylcholine receptor phosphorylation in rat myotubes by forskolin and cAMP. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6591–6595. doi: 10.1073/pnas.84.18.6591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishina M., Takai T., Imoto K., Noda M., Takahashi T., Numa S., Methfessel C., Sakmann B. Molecular distinction between fetal and adult forms of muscle acetylcholine receptor. Nature. 1986 May 22;321(6068):406–411. doi: 10.1038/321406a0. [DOI] [PubMed] [Google Scholar]
- Owens J. L., Kullberg R. In vivo development of nicotinic acetylcholine receptor channels in Xenopus myotomal muscle. J Neurosci. 1989 Mar;9(3):1018–1028. doi: 10.1523/JNEUROSCI.09-03-01018.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakmann B., Brenner H. R. Change in synaptic channel gating during neuromuscular development. Nature. 1978 Nov 23;276(5686):401–402. doi: 10.1038/276401a0. [DOI] [PubMed] [Google Scholar]
- Siegelbaum S. A., Trautmann A., Koenig J. Single acetylcholine-activated channel currents in developing muscle cells. Dev Biol. 1984 Aug;104(2):366–379. doi: 10.1016/0012-1606(84)90092-7. [DOI] [PubMed] [Google Scholar]
- Vicini S., Schuetze S. M. Gating properties of acetylcholine receptors at developing rat endplates. J Neurosci. 1985 Aug;5(8):2212–2224. doi: 10.1523/JNEUROSCI.05-08-02212.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]


