Abstract
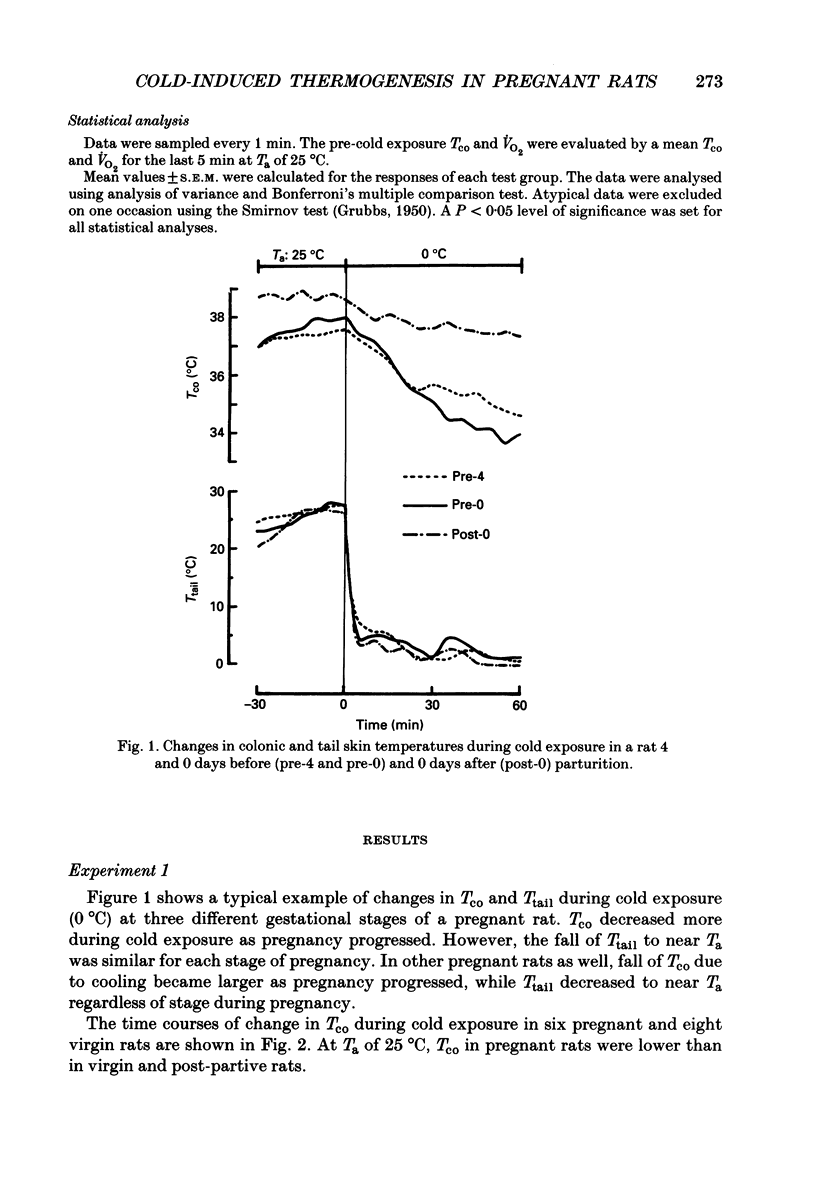
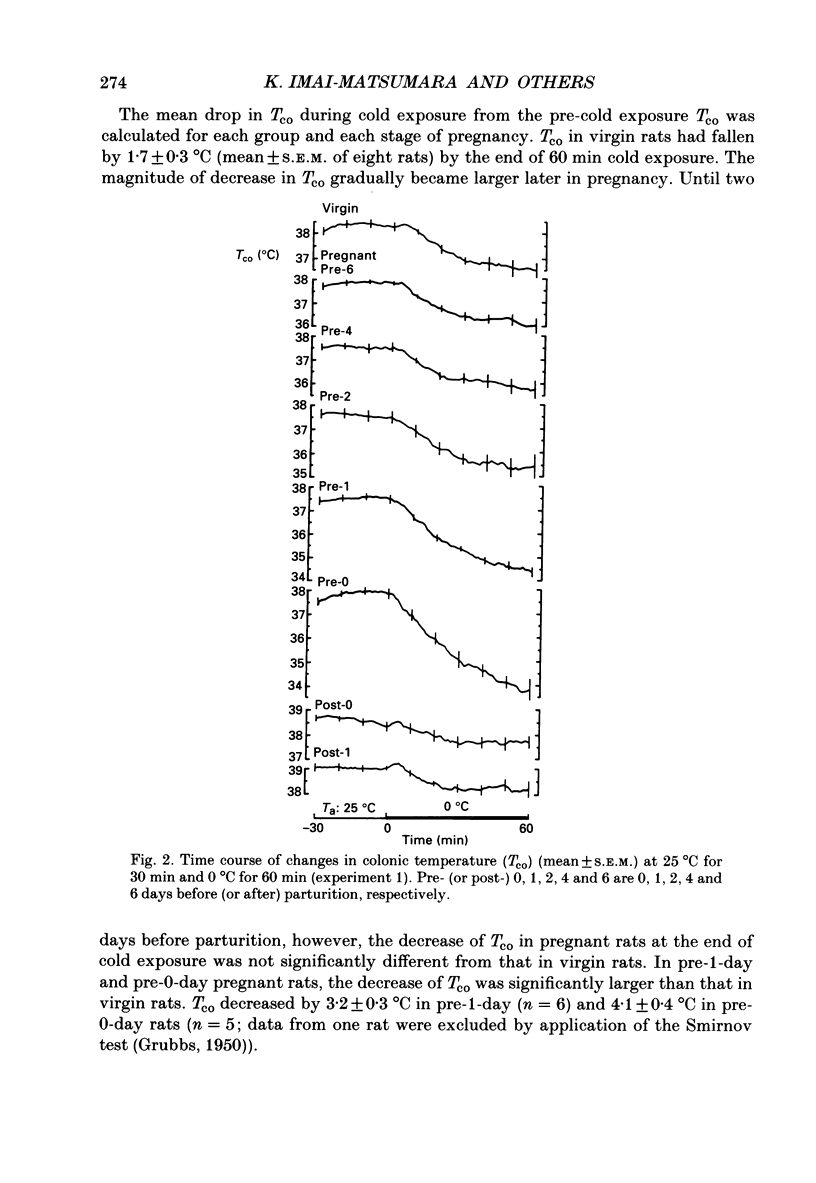
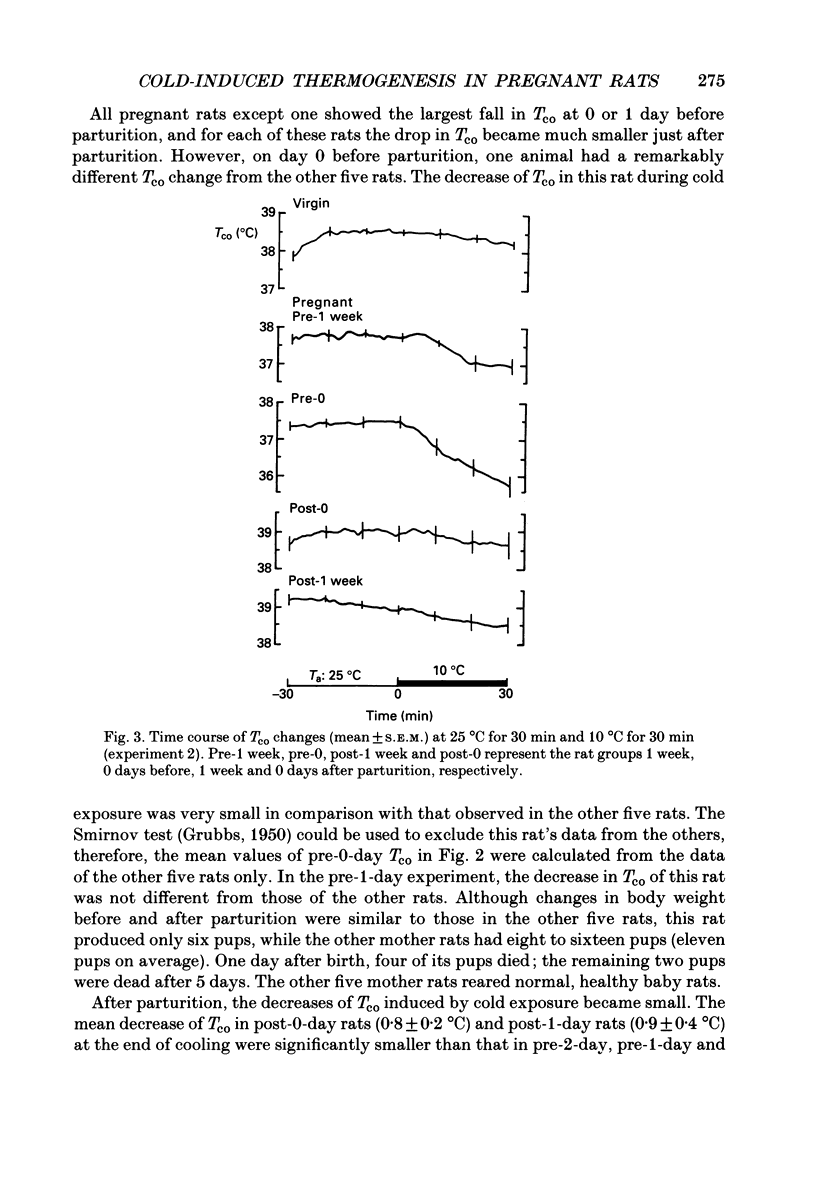
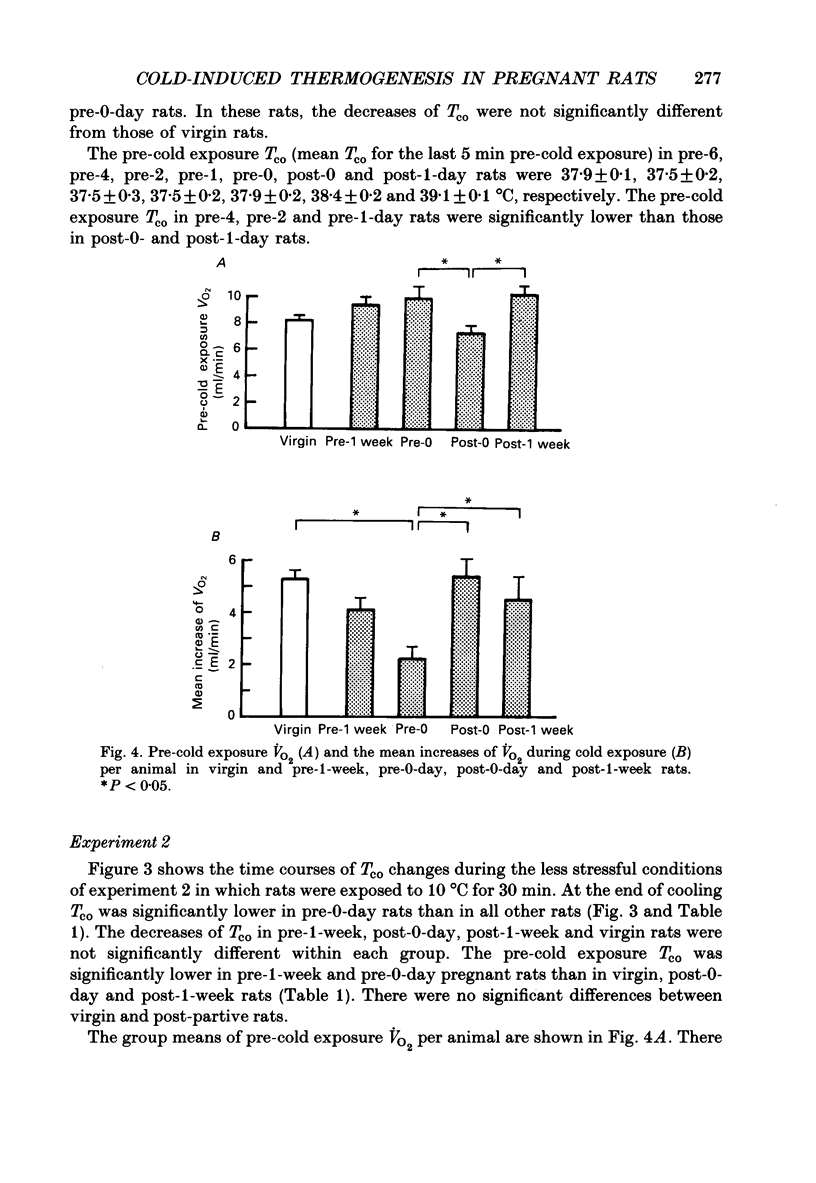
1. Thermoregulation against cold exposure was studied in rats during pregnancy and early lactation, and compared with that of virgin rats. 2. When exposed to 0 degrees C for 60 min, rats which were within 24-48 h of parturition (pre-1-day rats) and those within 24 h of parturition (pre-0-day rats) showed significantly larger falls of colonic temperature (Tco) than virgin rats. The temperature decrease was greatest in the pre-0-day rats, being 4.1 +/- 0.4 degrees C (mean +/- S.E.M.) at the end of cold exposure, compared with a decrease of 1.7 +/- 0.3 degrees C in the virgin rats. The tail skin temperature fell to 0 degrees C during cooling in all virgin rats and in pregnant rats at each gestational stage. 3. During cold exposure at 10 degrees C for 30 min, pre-0-day rats also showed significantly larger falls in Tco (1.8 +/- 0.6 degrees C) than virgin (0.4 +/- 0.2 degrees C), pre-1-week (0.8 +/- 0.3 degrees C), post-0-day (0.3 +/- 0.3 degrees C) or post-1-week rats (0.4 +/- 0.3 degrees C). Although body weights in pre-0-day rats were far larger than those in virgin rats, the increase in oxygen consumption per animal during cold exposure was 50% lower in pre-0-day rats (2.2 +/- 0.5 ml/min) than in virgin rats (5.3 +/- 0.3 ml/min). There was no difference in basal oxygen consumption per animal between the late pregnant and virgin rats. 4. Within 24 h after parturition, both the decrease of Tco and the increase of oxygen consumption during cold exposure returned to the values observed in virgin rats. 5. The present results demonstrate clearly that cold-induced thermogenesis is significantly suppressed in rats at a late stage of pregnancy.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADOLPH E. F. Some differences in responses to low temperatures between warm-blooded and cold-blooded vertebrates. Am J Physiol. 1951 Jul;166(1):92–103. doi: 10.1152/ajplegacy.1951.166.1.92. [DOI] [PubMed] [Google Scholar]
- Abrams R., Caton D., Curet L. B., Crenshaw C., Mann L., Barron D. H. Fetal brain-maternal aorta temperature differences in sheep. Am J Physiol. 1969 Dec;217(6):1619–1622. doi: 10.1152/ajplegacy.1969.217.6.1619. [DOI] [PubMed] [Google Scholar]
- Ahokas R. A., Anderson G. D., Lipshitz J. Cardiac output and uteroplacental blood flow in diet-restricted and diet-repleted pregnant rats. Am J Obstet Gynecol. 1983 May 1;146(1):6–13. doi: 10.1016/0002-9378(83)90918-3. [DOI] [PubMed] [Google Scholar]
- Alexander G. Body temperature control in mammalian young. Br Med Bull. 1975 Jan;31(1):62–68. doi: 10.1093/oxfordjournals.bmb.a071243. [DOI] [PubMed] [Google Scholar]
- Conklin P., Heggeness F. W. Maturation of tempeature homeostasis in the rat. Am J Physiol. 1971 Feb;220(2):333–336. doi: 10.1152/ajplegacy.1971.220.2.333. [DOI] [PubMed] [Google Scholar]
- Edwards M. J., Penny R. H., Zevnik I. A brain cell deficit in newborn guinea-pigs following prenatal hyperthermia. Brain Res. 1971 May 7;28(2):341–345. doi: 10.1016/0006-8993(71)90666-4. [DOI] [PubMed] [Google Scholar]
- Foster D. O., Frydman M. L. Tissue distribution of cold-induced thermogenesis in conscious warm- or cold-acclimated rats reevaluated from changes in tissue blood flow: the dominant role of brown adipose tissue in the replacement of shivering by nonshivering thermogenesis. Can J Physiol Pharmacol. 1979 Mar;57(3):257–270. doi: 10.1139/y79-039. [DOI] [PubMed] [Google Scholar]
- Giralt M., Villarroya F., Mampel T., Iglesias R. Impaired basal and noradrenaline-induced iodothyronine 5'-deiodinase activity in brown adipose tissue from pregnant and lactating rats. Biochem Biophys Res Commun. 1986 Aug 14;138(3):1315–1321. doi: 10.1016/s0006-291x(86)80426-0. [DOI] [PubMed] [Google Scholar]
- Gunn T. R., Gluckman P. D. Development of temperature regulation in the fetal sheep. J Dev Physiol. 1983 Jun;5(3):167–179. [PubMed] [Google Scholar]
- Leduc B. The effect of hyperventilation on maternal placental blood flow in pregnant rabbits. J Physiol. 1972 Sep;225(2):339–348. doi: 10.1113/jphysiol.1972.sp009943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACFARLANE W. V., PENNYCUIK P. R., THRIFT E. Resorption and loss of foetuses in rats living at 35degree C. J Physiol. 1957 Mar 11;135(3):451–459. doi: 10.1113/jphysiol.1957.sp005723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morishige W. K., Pepe G. J., Rothchild I. Serum luteinizing hormone, prolactin and progesterone levels during pregnancy in the rat. Endocrinology. 1973 May;92(5):1527–1530. doi: 10.1210/endo-92-5-1527. [DOI] [PubMed] [Google Scholar]
- Perkins M. N., Rothwell N. J., Stock M. J., Stone T. W. Activation of brown adipose tissue thermogenesis by the ventromedial hypothalamus. Nature. 1981 Jan 29;289(5796):401–402. doi: 10.1038/289401a0. [DOI] [PubMed] [Google Scholar]
- SHAH M. K. Reciprocal egg transplantations to study the embryo-uterine relationship in heat-induced failure of pregnancy in rabbits. Nature. 1956 Jun 16;177(4520):1134–1135. doi: 10.1038/1771134a0. [DOI] [PubMed] [Google Scholar]
- Smith R. E., Horwitz B. A. Brown fat and thermogenesis. Physiol Rev. 1969 Apr;49(2):330–425. doi: 10.1152/physrev.1969.49.2.330. [DOI] [PubMed] [Google Scholar]
- Trayhurn P., Douglas J. B., McGuckin M. M. Brown adipose tissue thermogenesis is 'suppressed' during lactation in mice. Nature. 1982 Jul 1;298(5869):59–60. doi: 10.1038/298059a0. [DOI] [PubMed] [Google Scholar]
- Villarroya F., Mampel T. Changes in brown adipose tissue lipoprotein lipase activity and lipogenesis rate during pregnancy and lactation in the rat. Biochem Int. 1986 Sep;13(3):511–519. [PubMed] [Google Scholar]


