Abstract
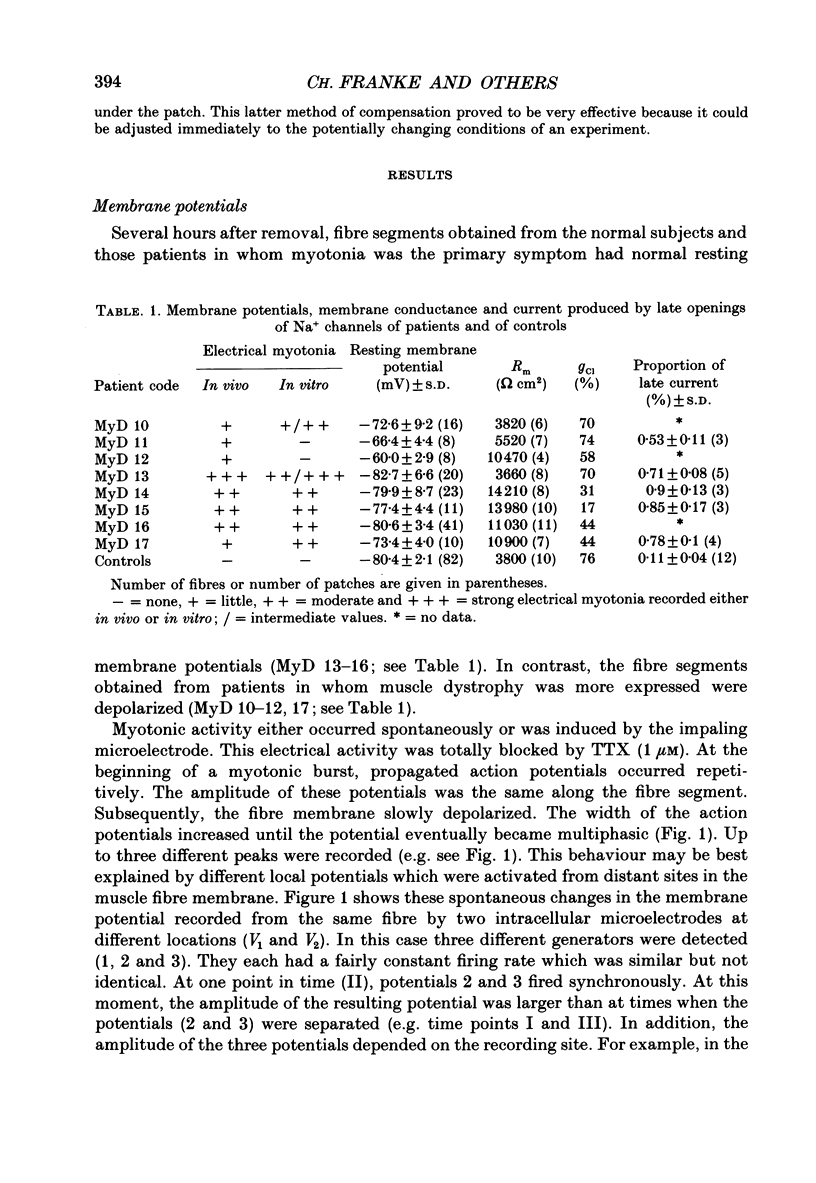
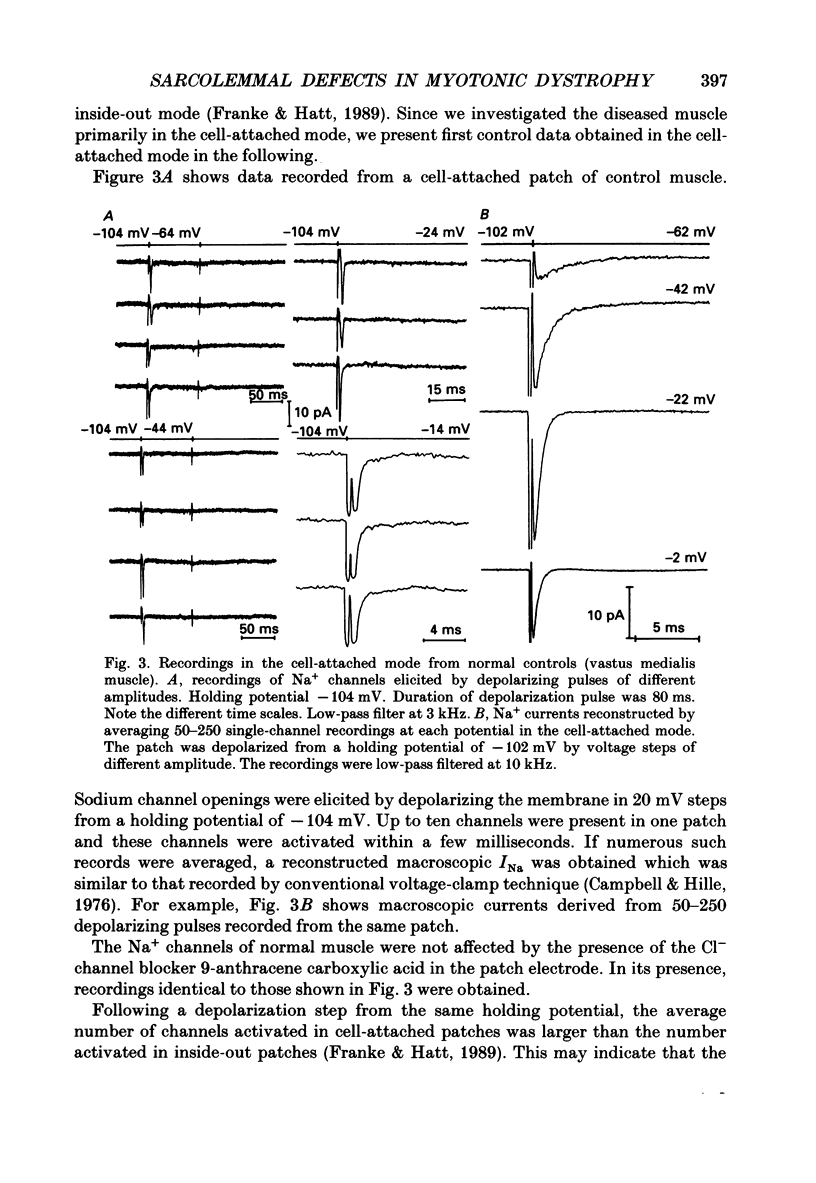
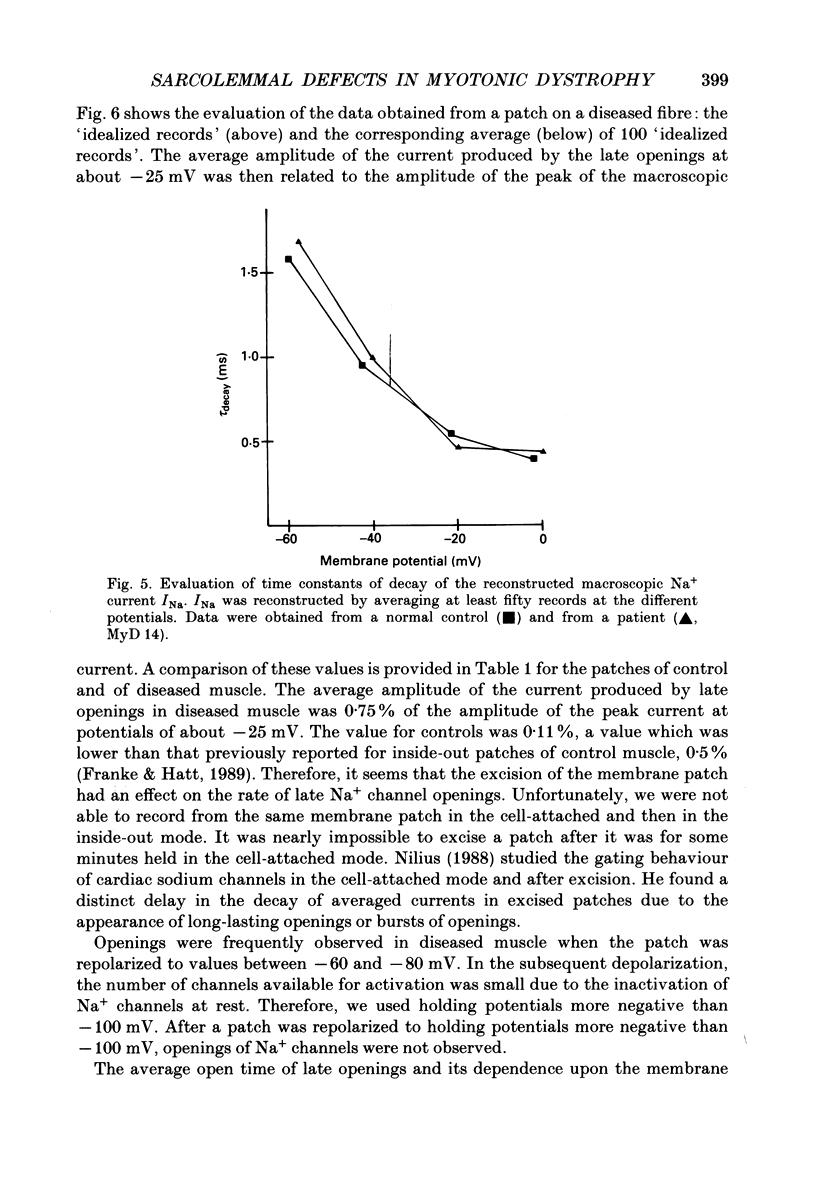
1. Electrical and contractile properties of resealed fibre segments were investigated by a variety of in vitro techniques. The preparations were removed from skeletal muscles of normal subjects and of eight patients with myotonic dystrophy. 2. Several hours after removal, fibre segments from normal subjects and those patients in whom myotonia was the primary symptom had resting membrane potentials of approximately -80 mV. In contrast, fibre segments obtained from patients in whom muscle dystrophy was more expressed were depolarized (-60 to -70 mV). 3. Contractions induced in fibre segments of myotonic muscle which had normal potentials were characterized by slowed relaxation which was due to electrical after-activity. 4. After single stimuli, long-lasting (3-100) runs of action potentials were recorded intracellularly from the myotonic muscle. In some of these fibre segments complex repetitive discharges were observed: multiple sites of locally gated currents were identified. 5. The three-electrode voltage clamp was used to determine the total membrane conductance, gm, and the ion component conductances. All fibres of a particular patient had similar conductances. However, the Cl- conductance varied from patient to patient from normal (74% of gm) to low values (30% of gm). The K+ conductance was normal in all fibres of all patients. 6. The patch-clamp technique was used to record currents through single Na+ channels of the sarcolemma. After treatment of the fibre segments with collagenase gigaohm seals were routinely obtained. The rate of success was greater when using the cell-attached mode than the inside-out mode. 7. Sodium channel currents were elicited by depolarizing voltage steps which produced an initial burst of Na+ channel openings. Up to ten channels were activated simultaneously when the patch was depolarized to potentials more positive than -30 mV. The Na+ channels re-opened very rarely in controls. The macroscopic sodium current, INa, was reconstructed by averaging depolarizing pulses. The time constant of rapid decay of INa reflecting macroscopic inactivation, the onset of INa and the amplitude of INa were voltage dependent. The mean amplitude of the current produced by re-openings was on average only 0.11 +/- 0.04% of the amplitude of the peak current. 8. Late openings of the Na+ channels were frequent in patches on the myotonic fibre segments. The amplitude of the current produced by re-openings was as high as about 0.75 +/- 0.11% of the amplitude of the peak current.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almers W., Roberts W. M., Ruff R. L. Voltage clamp of rat and human skeletal muscle: measurements with an improved loose-patch technique. J Physiol. 1984 Feb;347:751–768. doi: 10.1113/jphysiol.1984.sp015094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldin S., Jäger U., Ruppersberg J. P., Pentz S., Rüdel R. Cultivation, morphology, and electrophysiology of contractile rat myoballs. Pflugers Arch. 1987 Aug;409(4-5):462–467. doi: 10.1007/BF00583802. [DOI] [PubMed] [Google Scholar]
- Bryant S. H., Morales-Aguilera A. Chloride conductance in normal and myotonic muscle fibres and the action of monocarboxylic aromatic acids. J Physiol. 1971 Dec;219(2):367–383. doi: 10.1113/jphysiol.1971.sp009667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton F., Dörstelmann U., Hutter O. F. Single-channel activity in sarcolemmal vesicles from human and other mammalian muscles. Muscle Nerve. 1988 Oct;11(10):1029–1038. doi: 10.1002/mus.880111004. [DOI] [PubMed] [Google Scholar]
- Campbell D. T., Hille B. Kinetic and pharmacological properties of the sodium channel of frog skeletal muscle. J Gen Physiol. 1976 Mar;67(3):309–323. doi: 10.1085/jgp.67.3.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coërs C., Telerman-Toppet N., Gerard J. M. Terminal innervation ratio in neuromuscular disease. II. Disorders of lower motor neuron, peripheral nerve, and muscle. Arch Neurol. 1973 Oct;29(4):215–222. doi: 10.1001/archneur.1973.00490280027003. [DOI] [PubMed] [Google Scholar]
- Franke C., Dudel J. Single glutamate-gated synaptic channels at the crayfish neuromuscular junction. I. The effect of enzyme treatment. Pflugers Arch. 1987 Mar;408(3):300–306. doi: 10.1007/BF02181473. [DOI] [PubMed] [Google Scholar]
- Franke C., Hatt H. Characteristics of single Na+ channels of adult human skeletal muscle. Pflugers Arch. 1990 Jan;415(4):399–406. doi: 10.1007/BF00373616. [DOI] [PubMed] [Google Scholar]
- Furman R. E., Barchi R. L. The pathophysiology of myotonia produced by aromatic carboxylic acids. Ann Neurol. 1978 Oct;4(4):357–365. doi: 10.1002/ana.410040411. [DOI] [PubMed] [Google Scholar]
- Gruener R., Stern L. Z., Markovitz D., Gerdes C. Electrophysiologic properties of intercostal muscle fibers in human neuromuscular diseases. Muscle Nerve. 1979 May-Jun;2(3):165–172. doi: 10.1002/mus.880020303. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hofmann W. W., Rowe G. Electrophysiological study of myotonia. Nature. 1966 Nov 26;212(5065):954–954. [PubMed] [Google Scholar]
- Kirsch G. E., Anderson M. F. Sodium channel kinetics in normal and denervated rabbit muscle membrane. Muscle Nerve. 1986 Oct;9(8):738–747. doi: 10.1002/mus.880090810. [DOI] [PubMed] [Google Scholar]
- Kohlhardt M., Fröbe U., Herzig J. W. Properties of normal and non-inactivating single cardiac Na+ channels. Proc R Soc Lond B Biol Sci. 1987 Oct 22;232(1266):71–93. doi: 10.1098/rspb.1987.0062. [DOI] [PubMed] [Google Scholar]
- Kwieciński H., Lehmann-Horn F., Rüdel R. Drug-induced myotonia in human intercostal muscle. Muscle Nerve. 1988 Jun;11(6):576–581. doi: 10.1002/mus.880110609. [DOI] [PubMed] [Google Scholar]
- Lehmann-Horn F., Küther G., Ricker K., Grafe P., Ballanyi K., Rüdel R. Adynamia episodica hereditaria with myotonia: a non-inactivating sodium current and the effect of extracellular pH. Muscle Nerve. 1987 May;10(4):363–374. doi: 10.1002/mus.880100414. [DOI] [PubMed] [Google Scholar]
- Lehmann-Horn F., Rüdel R., Dengler R., Lorković H., Haass A., Ricker K. Membrane defects in paramyotonia congenita with and without myotonia in a warm environment. Muscle Nerve. 1981 Sep-Oct;4(5):396–406. doi: 10.1002/mus.880040508. [DOI] [PubMed] [Google Scholar]
- Lehmann-Horn F., Rüdel R., Ricker K. Membrane defects in paramyotonia congenita (Eulenburg). Muscle Nerve. 1987 Sep;10(7):633–641. doi: 10.1002/mus.880100709. [DOI] [PubMed] [Google Scholar]
- MACDERMOT V. The histology of the neuromuscular junction in dystrophia myotonica. Brain. 1961 Mar;84:75–84. doi: 10.1093/brain/84.1.75. [DOI] [PubMed] [Google Scholar]
- Merickel M., Gray R., Chauvin P., Appel S. Cultured muscle from myotonic muscular dystrophy patients: altered membrane electrical properties. Proc Natl Acad Sci U S A. 1981 Jan;78(1):648–652. doi: 10.1073/pnas.78.1.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilius B. Modal gating behavior of cardiac sodium channels in cell-free membrane patches. Biophys J. 1988 Jun;53(6):857–862. doi: 10.1016/S0006-3495(88)83166-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patlak J. B., Ortiz M. Two modes of gating during late Na+ channel currents in frog sartorius muscle. J Gen Physiol. 1986 Feb;87(2):305–326. doi: 10.1085/jgp.87.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricker K., Rüdel R., Lehmann-Horn F., Küther G. Muscle stiffness and electrical activity in paramyotonia congenita. Muscle Nerve. 1986 May;9(4):299–305. doi: 10.1002/mus.880090403. [DOI] [PubMed] [Google Scholar]
- Rüdel R., Lehmann-Horn F. Membrane changes in cells from myotonia patients. Physiol Rev. 1985 Apr;65(2):310–356. doi: 10.1152/physrev.1985.65.2.310. [DOI] [PubMed] [Google Scholar]
- Rüdel R., Ruppersberg J. P., Spittelmeister W. Abnormalities of the fast sodium current in myotonic dystrophy, recessive generalized myotonia, and adynamia episodica. Muscle Nerve. 1989 Apr;12(4):281–287. doi: 10.1002/mus.880120405. [DOI] [PubMed] [Google Scholar]
- Tahmoush A. J., Askanas V., Nelson P. G., Engel W. K. Electrophysiologic properties of aneurally cultured muscle from patients with myotonic muscular atrophy. Neurology. 1983 Mar;33(3):311–316. doi: 10.1212/wnl.33.3.311. [DOI] [PubMed] [Google Scholar]
- Trautmann A., Delaporte C., Marty A. Voltage-dependent channels of human muscle cultures. Pflugers Arch. 1986 Feb;406(2):163–172. doi: 10.1007/BF00586678. [DOI] [PubMed] [Google Scholar]


