Abstract
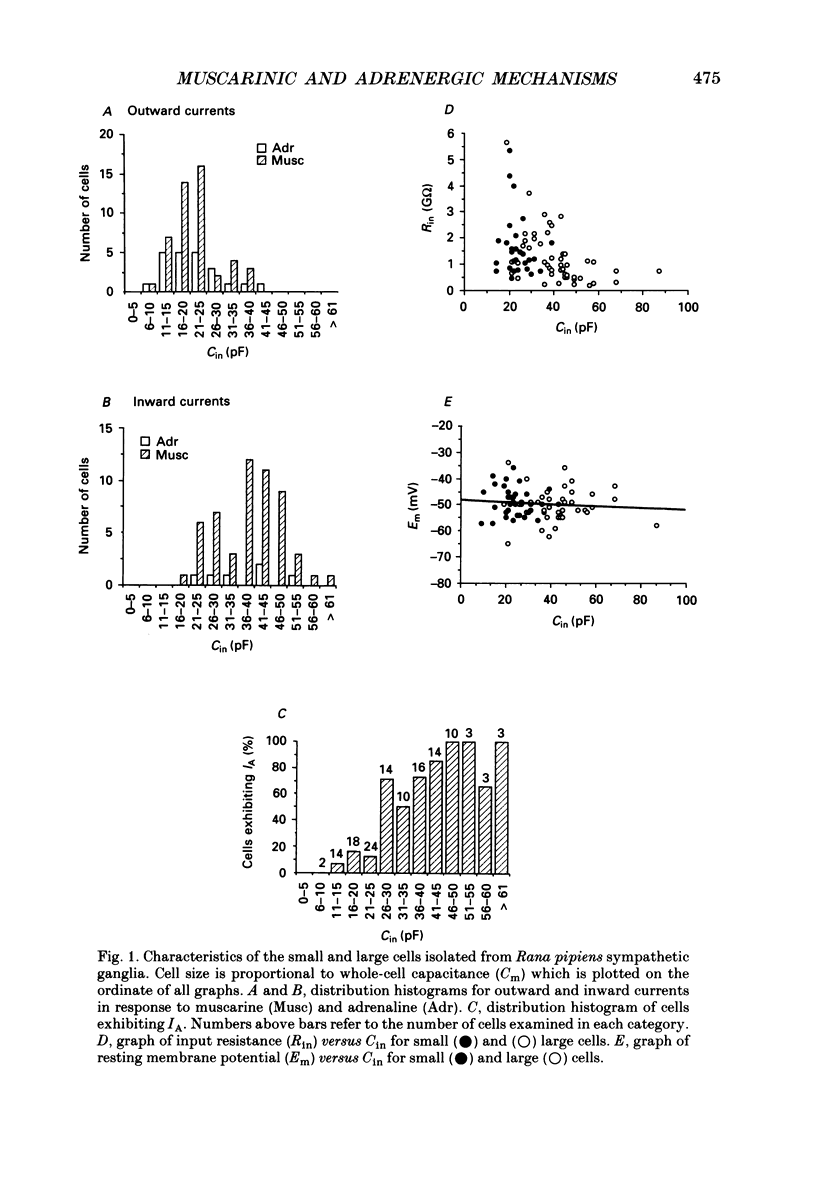
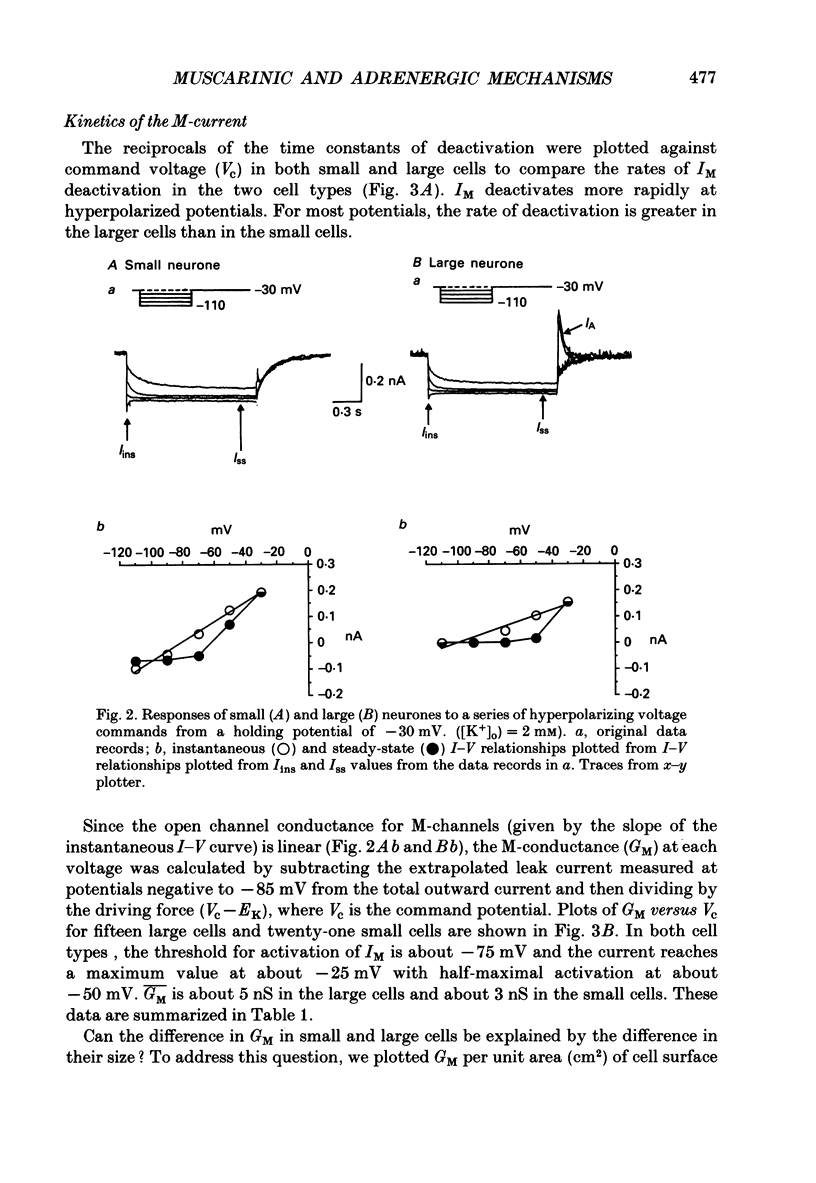
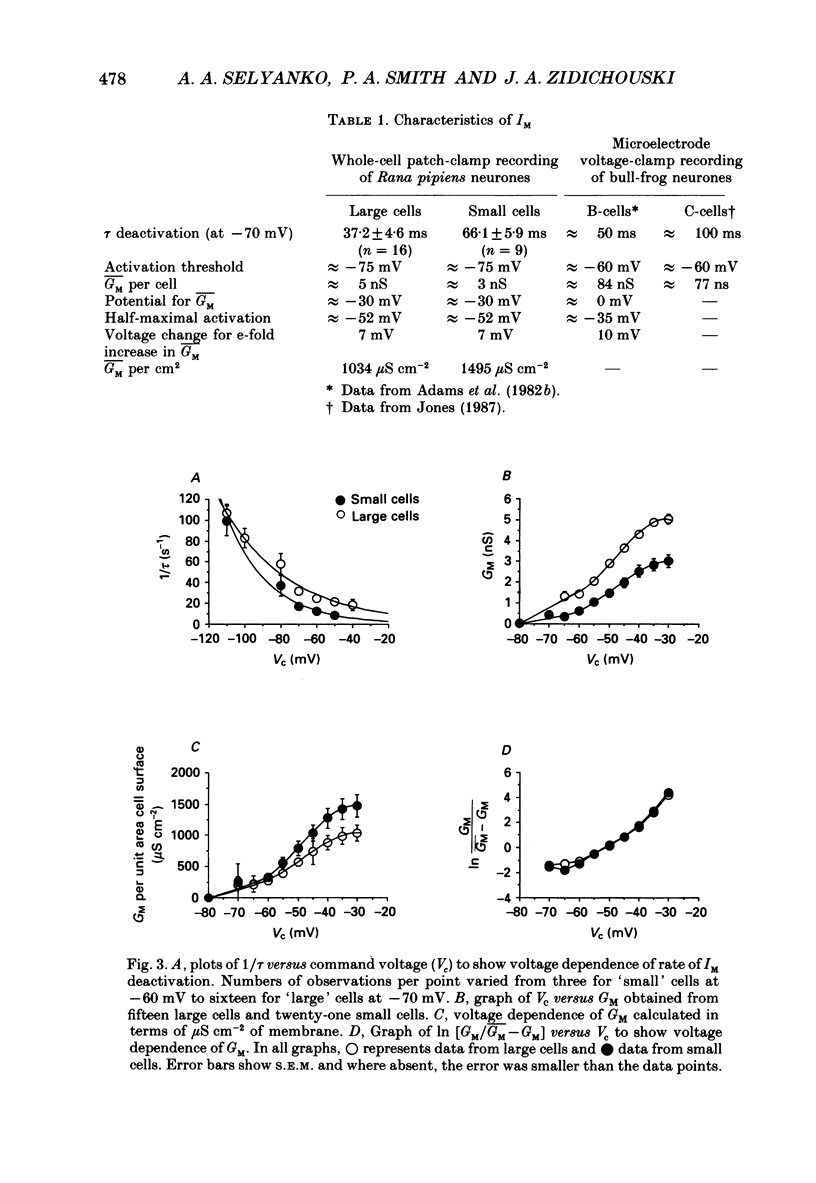
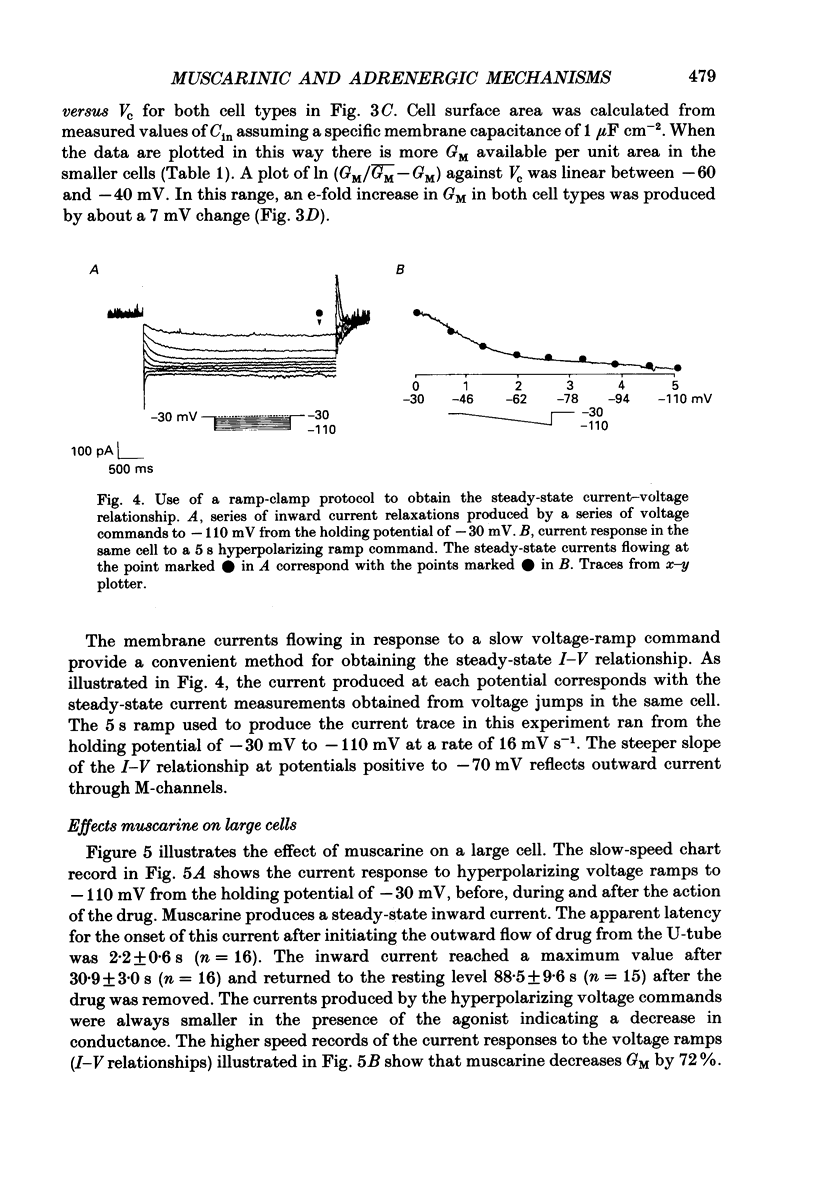
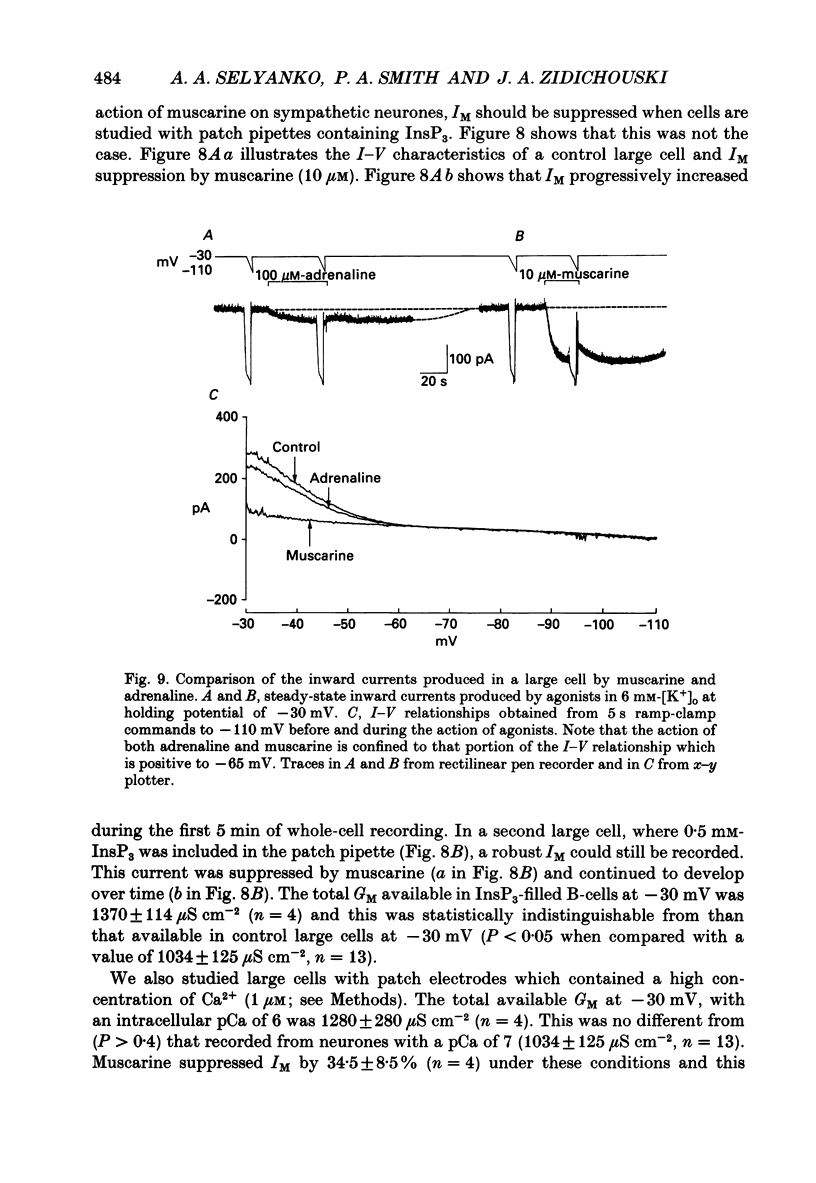
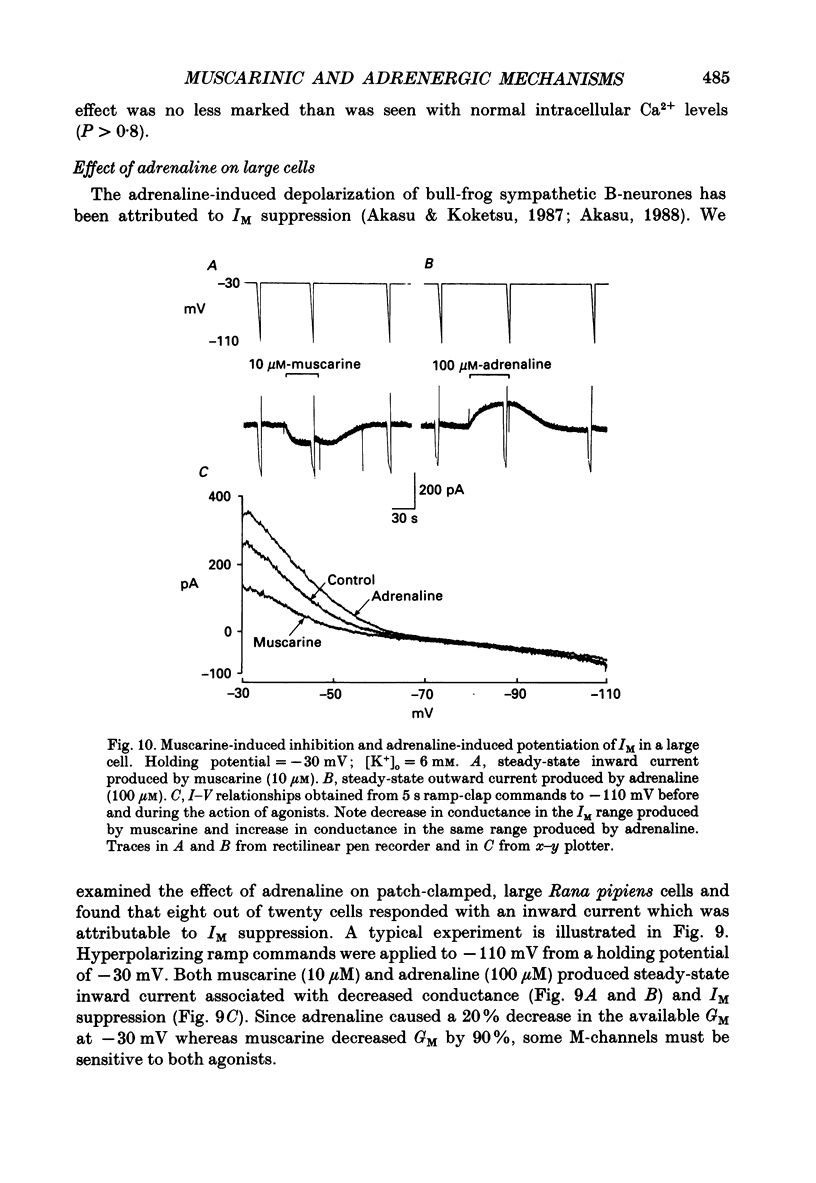
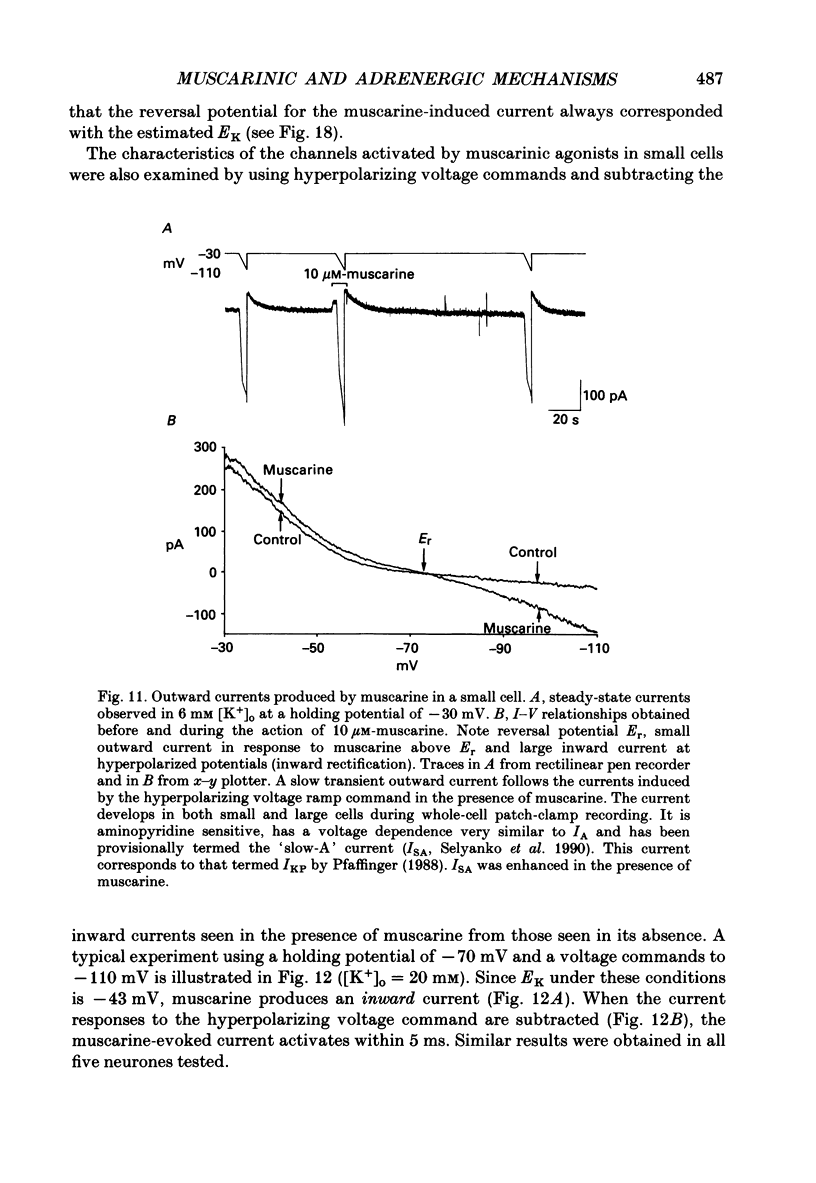
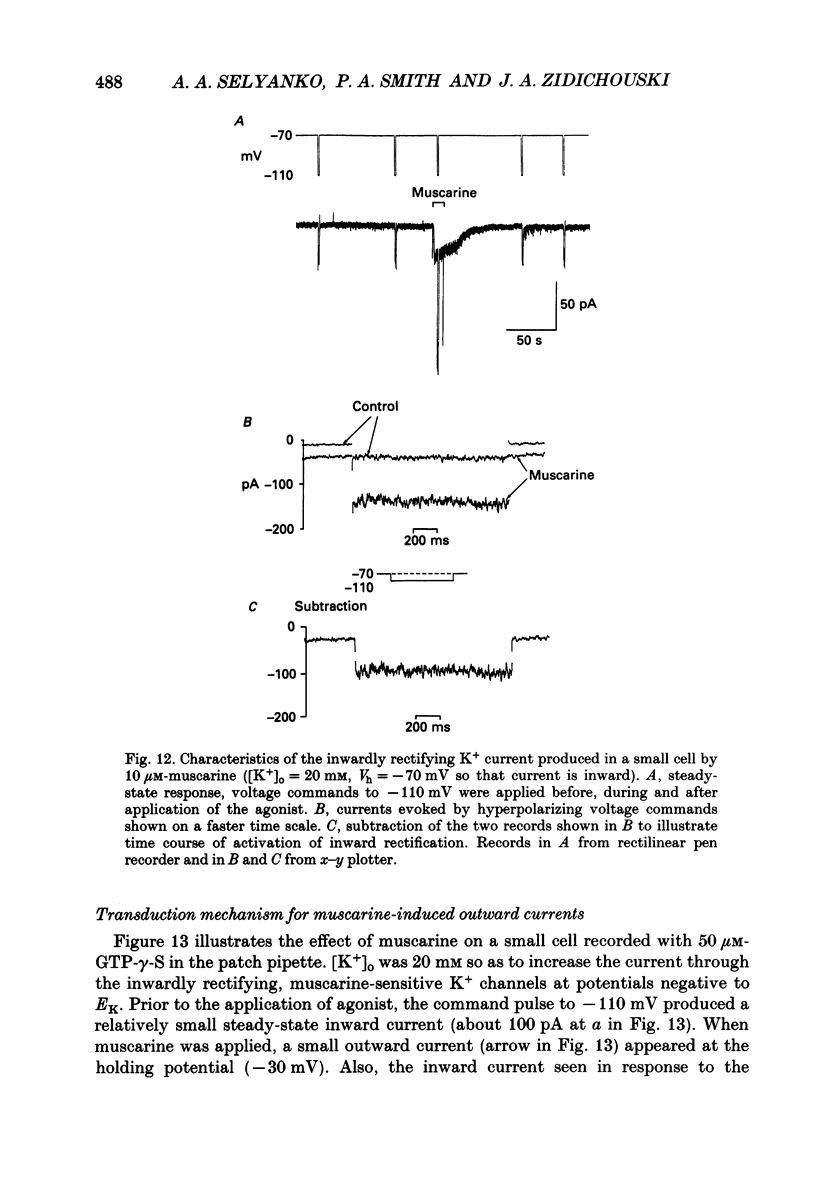
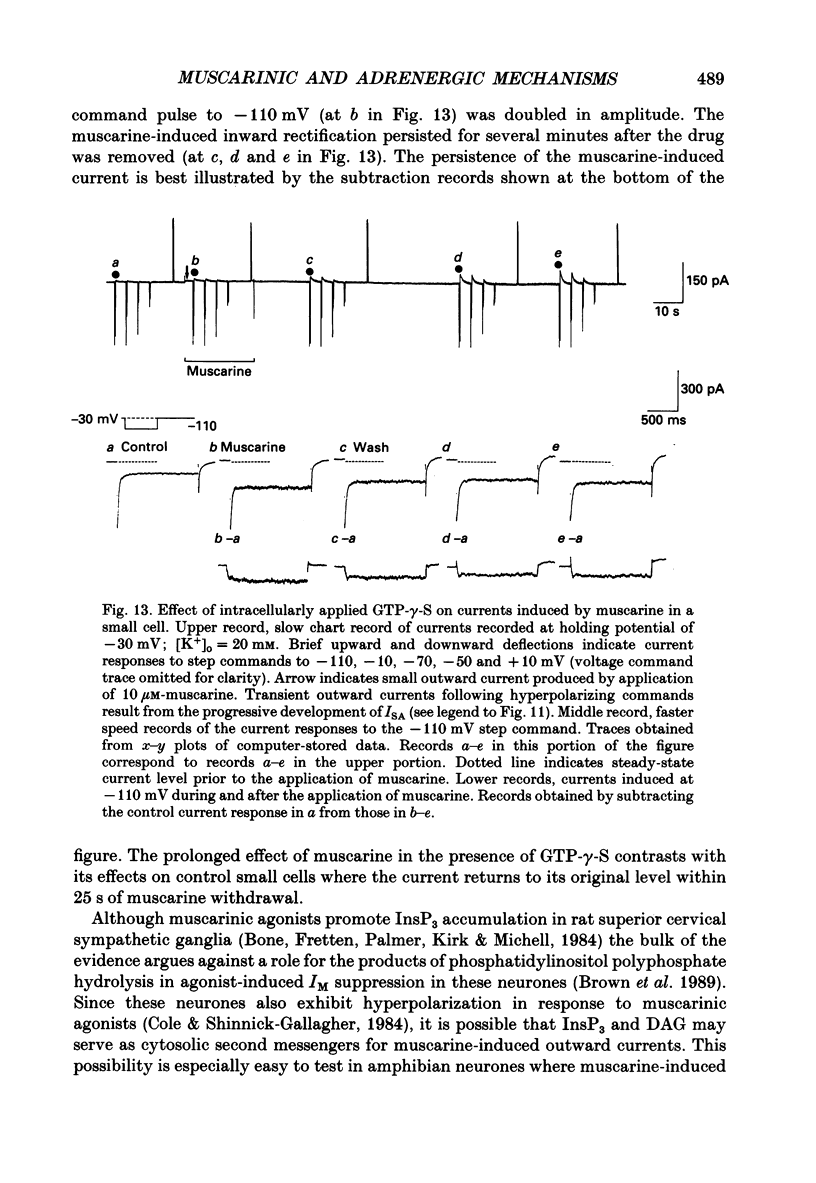
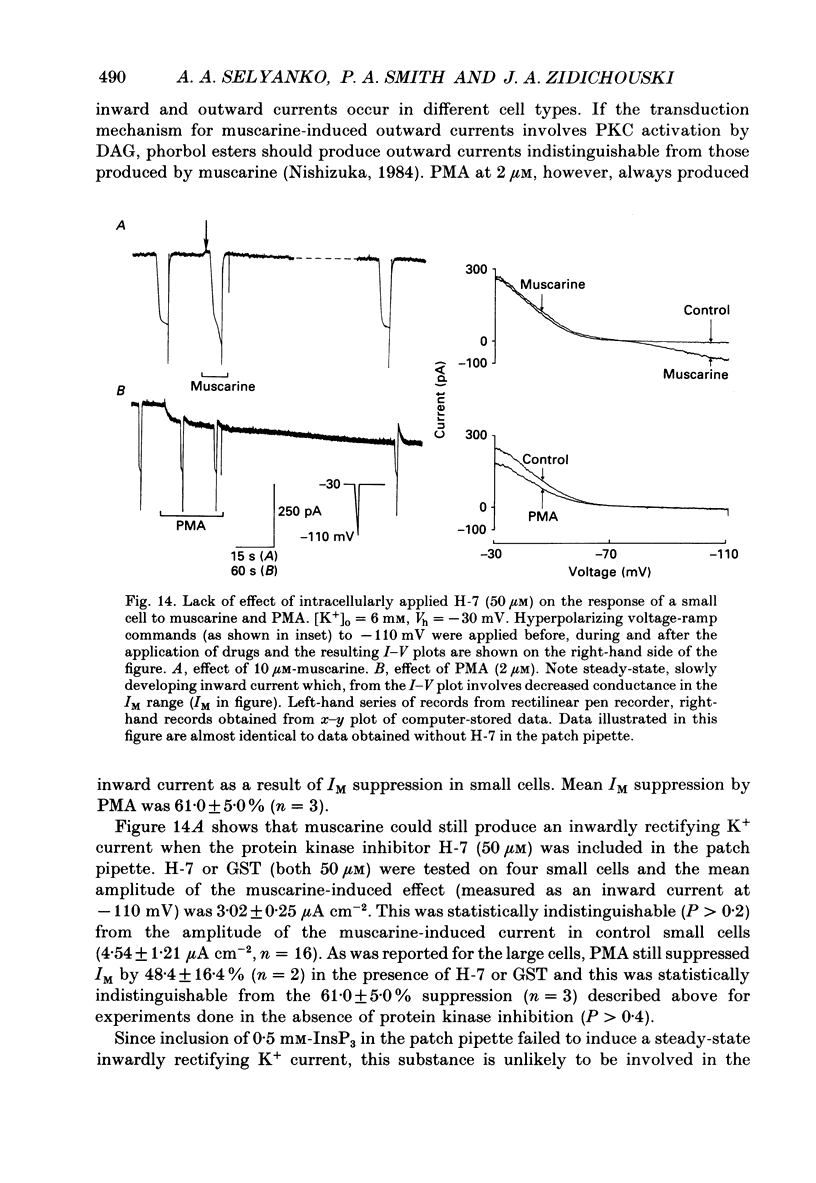
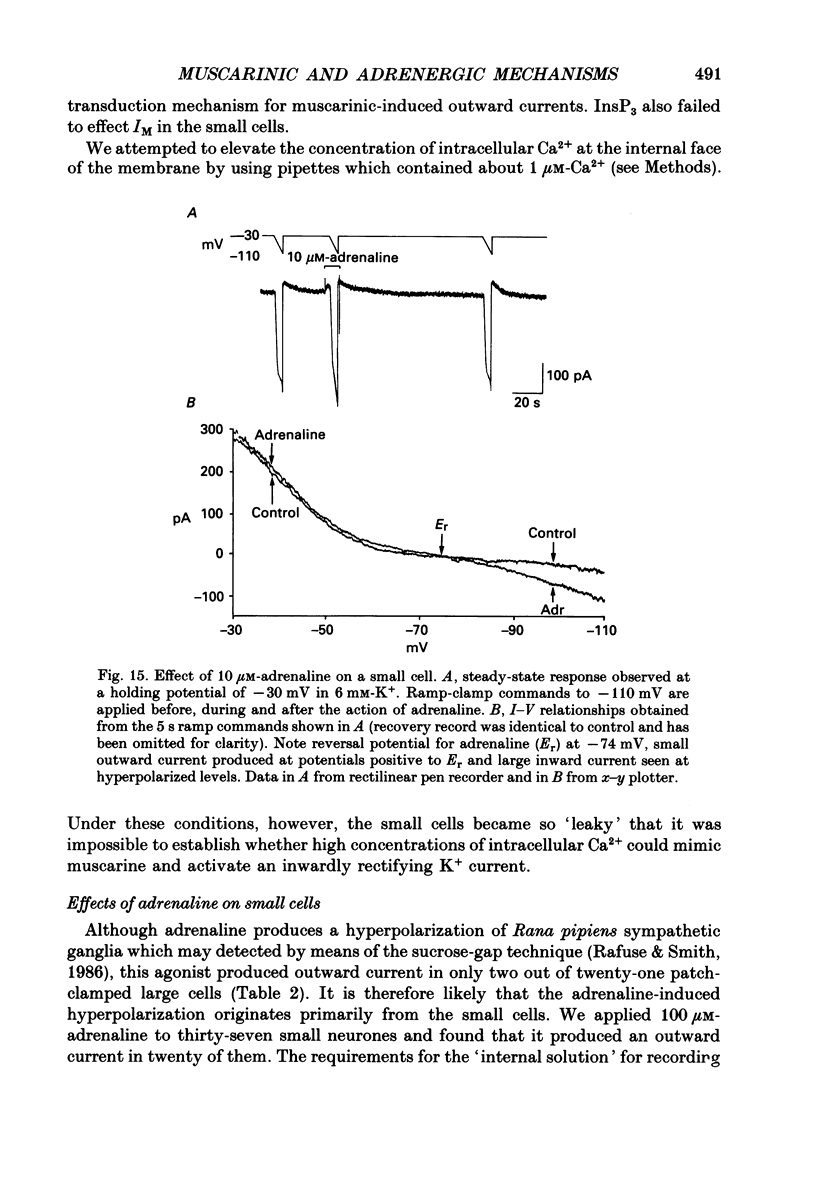
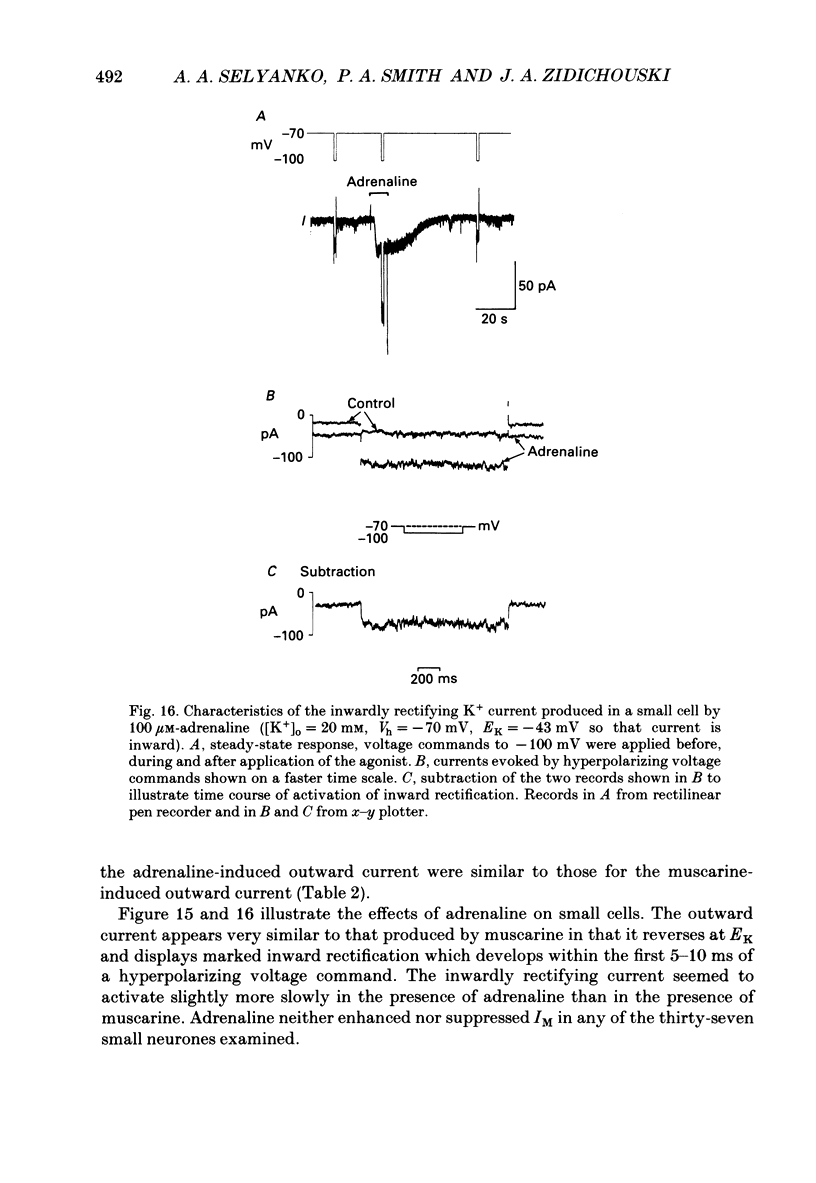
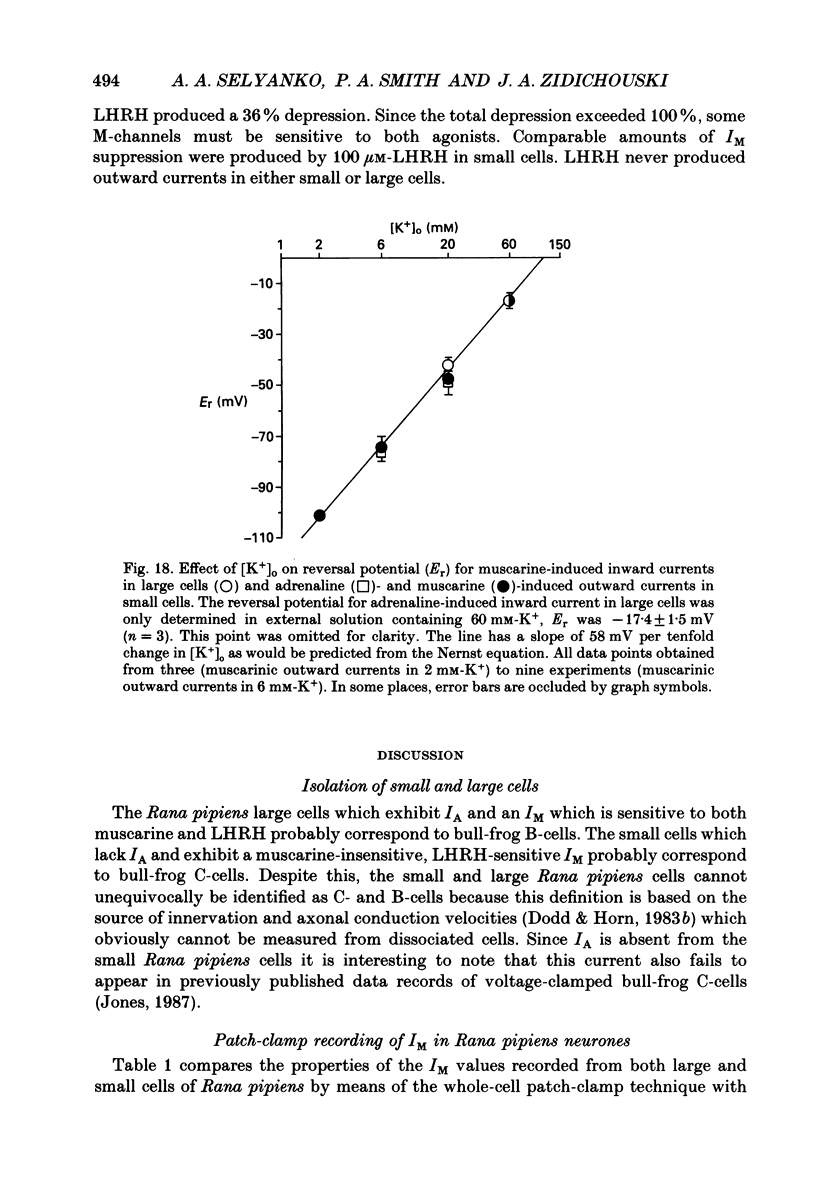
1. Neurones dissociated from Rana pipiens paravertebral sympathetic ganglia were studied by means of the whole-cell patch-clamp technique. Responses to agonists were best recorded when cyclic AMP was included in the patch pipette. 2. Two populations of cells were identified on the basis of size (input capacitance, Cin) and the presence or absence of a fast, transient outward current (A-current, IA). This current was usually present in the 'large' cells (Cin = 40.5 +/- 1.5 pF, n = 66) but absent from 'small' cells (Cin = 21.0 +/- 0.8 pF, n = 70). 3. Both cell types exhibited a slowly activating, non-inactivating K+ current (M-current, IM) which was suppressed by luteinizing hormone-releasing hormone (LHRH, 10-100 microM). Threshold for activation of IM was about -75 mV, half-maximal activation was at -50 mV and the M-conductance GM increased e-fold for at 7 mV change in membrane potential. The maximum value for IM studied in large cells by patch-clamp procedures was less than 0.2 nA. More M-channels were available per unit membrane area in the small cells (GM = 1495 microS cm-2) than in the large cells (GM = 1034 microS cm-2). Time constants for IM deactivation at -70 mV were faster in the large cells (37.2 +/- 4.6 ms, n = 16) than in the small cells (66.1 +/- 5.9 ms, n = 9). 4. Muscarine (10 microM) produced inward current in the large cells as a result of IM suppression. In 40% of the large cells, some of the M-channels were also sensitive to adrenaline (10-100 microM). In a few large cells (less than 10%) adrenaline produced outward current by increasing IM. 5. Muscarine failed to effect IM in the small cells and instead produced an inwardly rectifying K+ current which activated within 5 ms at -110 mV. The outward current produced in twenty out of thirty-seven small cells by adrenaline was occluded by that produced by muscarine, suggesting that both agonists affect the same K+ channels. 6. Inclusion of the protein kinase inhibitors, 1-(5-isoquinolinyl-sulphonyl)-2-methyl piperazine (H-7, 50 microM) or gold sodium thiomalate (GST, 50 microM) in the pipette solution failed to antagonize either muscarine-induced current. Both currents were prolonged when the 'internal solution' contained GTP-gamma-S (50 microM). 7. Phorbol-12-myristate-13-acetate (PMA, 2-5 microM) produced an inward current as a result of IM suppression in both small and large cells.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF




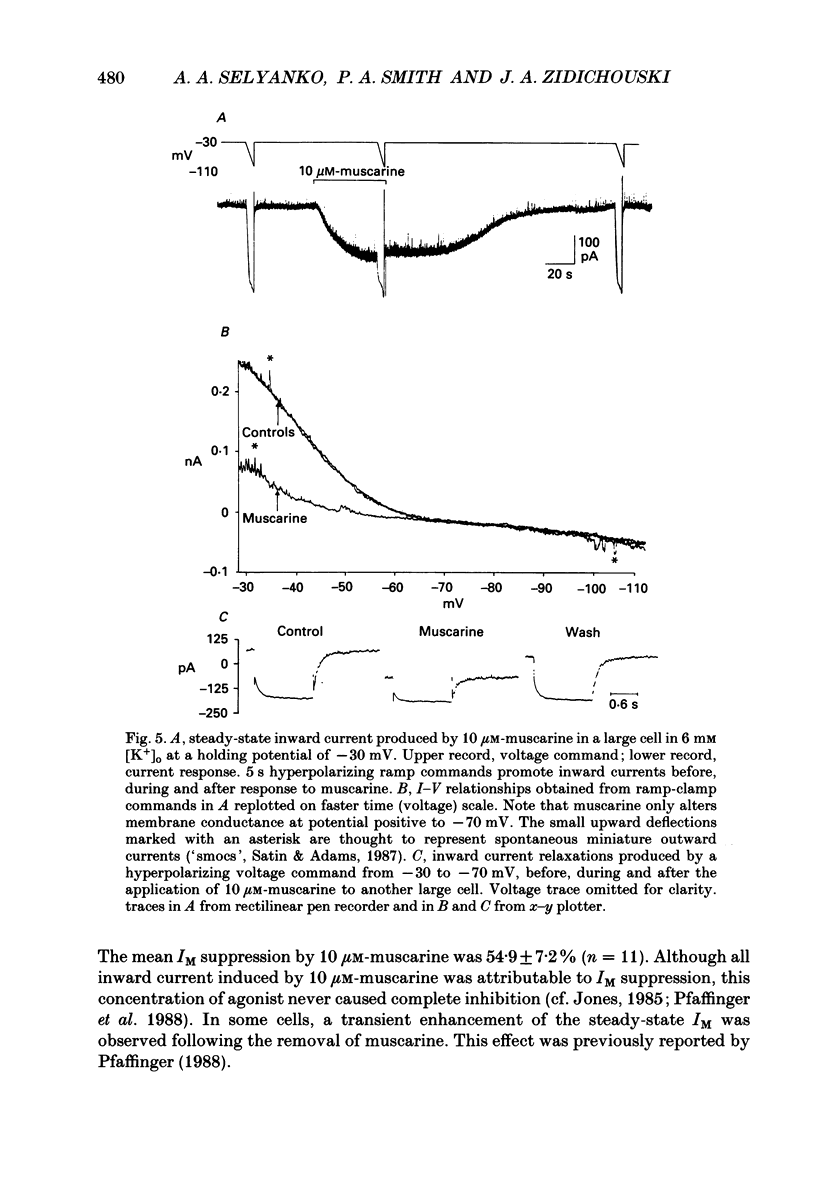
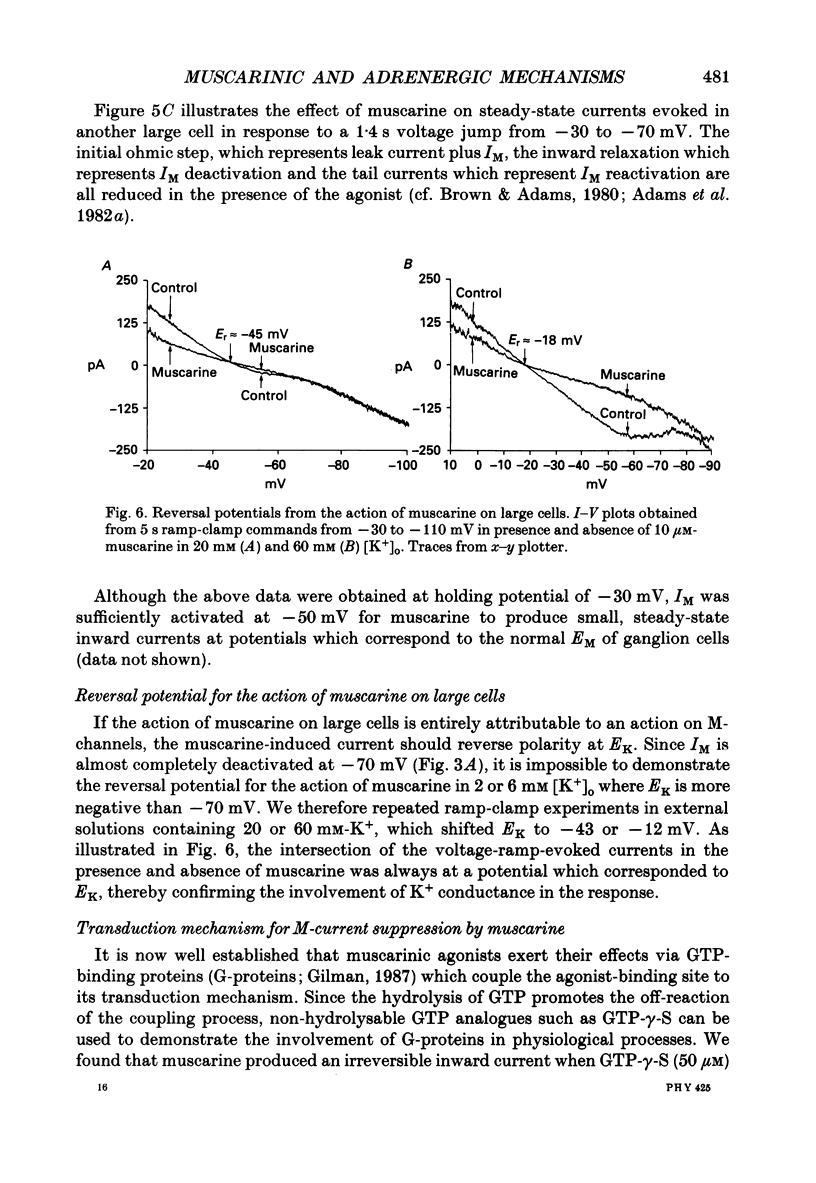
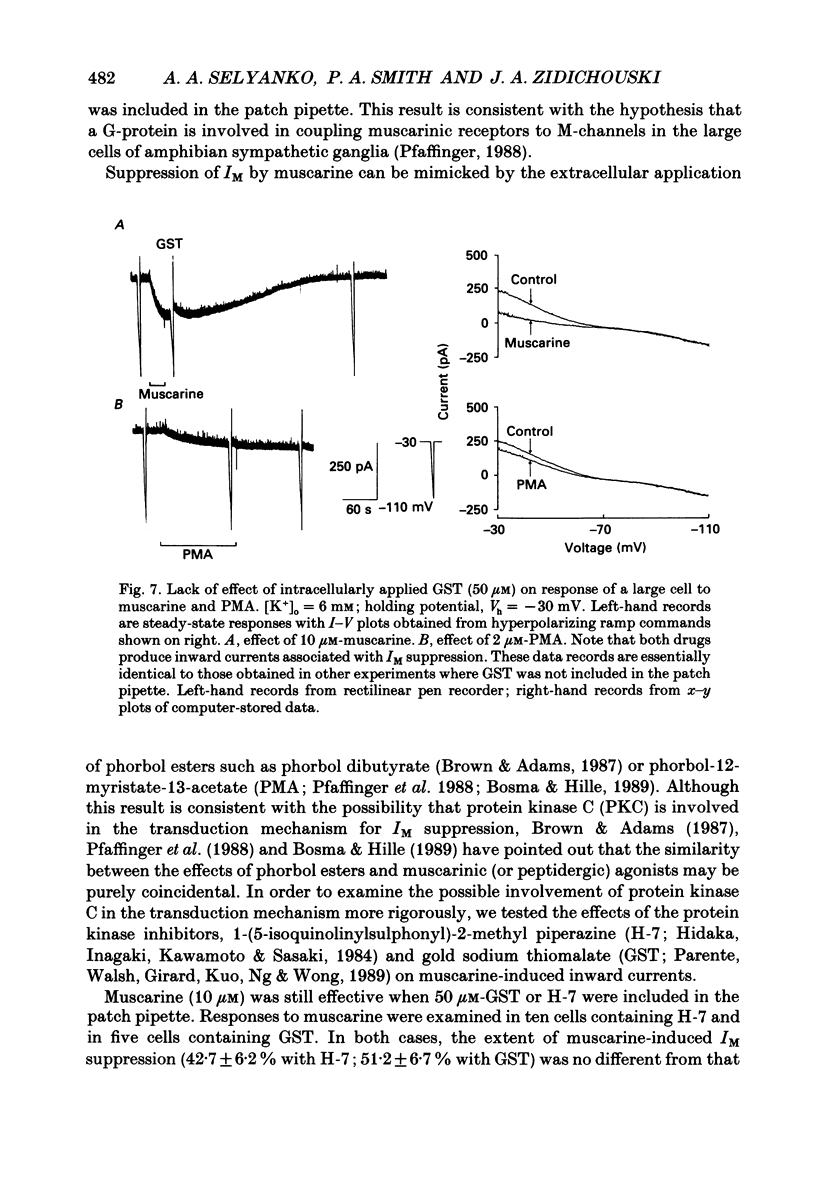
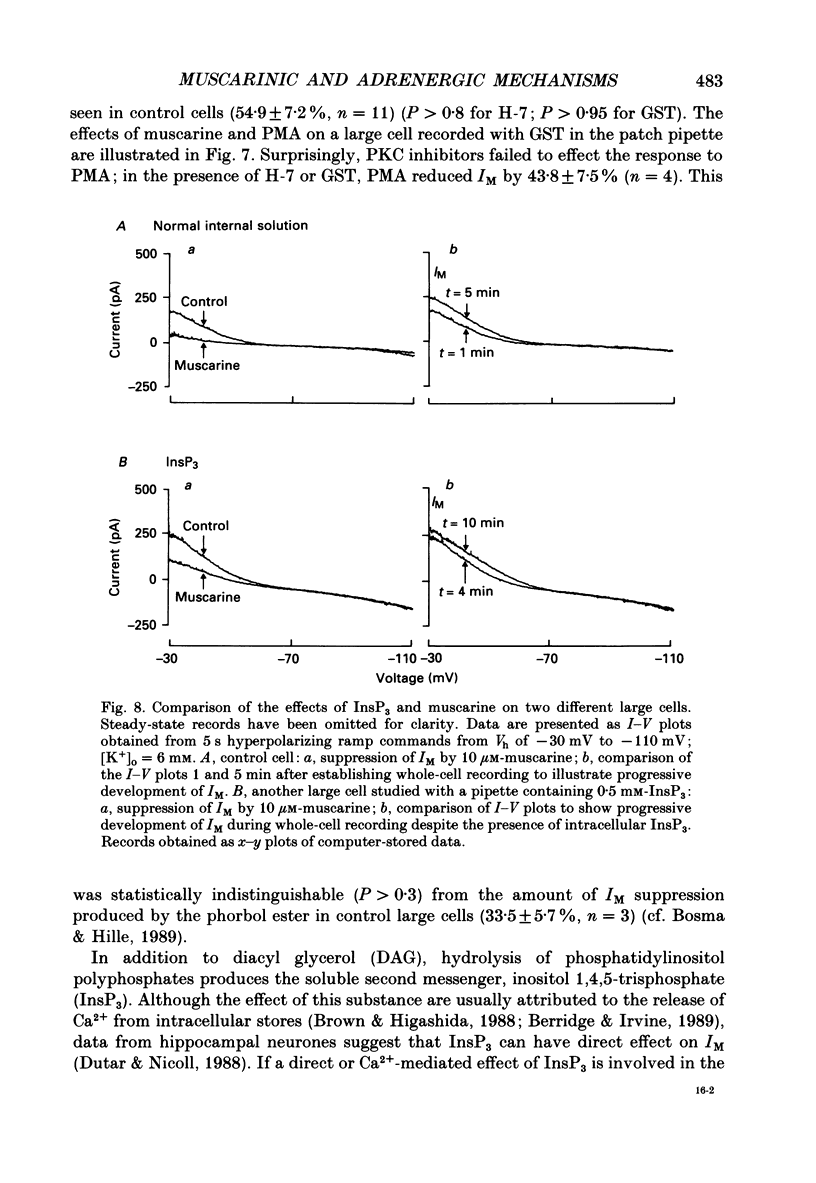







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. R., Brown D. A., Constanti A. M-currents and other potassium currents in bullfrog sympathetic neurones. J Physiol. 1982 Sep;330:537–572. doi: 10.1113/jphysiol.1982.sp014357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams P. R., Brown D. A., Constanti A. Pharmacological inhibition of the M-current. J Physiol. 1982 Nov;332:223–262. doi: 10.1113/jphysiol.1982.sp014411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams P. R., Brown D. A. Synaptic inhibition of the M-current: slow excitatory post-synaptic potential mechanism in bullfrog sympathetic neurones. J Physiol. 1982 Nov;332:263–272. doi: 10.1113/jphysiol.1982.sp014412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akasu T. Adrenaline depolarization in paravertebral sympathetic neurones of bullfrogs. Pflugers Arch. 1988 Jan;411(1):80–87. doi: 10.1007/BF00581650. [DOI] [PubMed] [Google Scholar]
- Akasu T., Gallagher J. P., Koketsu K., Shinnick-Gallagher P. Slow excitatory post-synaptic currents in bull-frog sympathetic neurones. J Physiol. 1984 Jun;351:583–593. doi: 10.1113/jphysiol.1984.sp015264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akasu T., Koketsu K. Evidence for epinephrine-induced depolarization in neurons of bullfrog sympathetic ganglia. Brain Res. 1987 Mar 10;405(2):375–379. doi: 10.1016/0006-8993(87)90309-x. [DOI] [PubMed] [Google Scholar]
- Andrade R., Aghajanian G. K. Opiate- and alpha 2-adrenoceptor-induced hyperpolarizations of locus ceruleus neurons in brain slices: reversal by cyclic adenosine 3':5'-monophosphate analogues. J Neurosci. 1985 Sep;5(9):2359–2364. doi: 10.1523/JNEUROSCI.05-09-02359.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belles B., Malécot C. O., Hescheler J., Trautwein W. "Run-down" of the Ca current during long whole-cell recordings in guinea pig heart cells: role of phosphorylation and intracellular calcium. Pflugers Arch. 1988 Apr;411(4):353–360. doi: 10.1007/BF00587713. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol phosphates and cell signalling. Nature. 1989 Sep 21;341(6239):197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- Bone E. A., Fretten P., Palmer S., Kirk C. J., Michell R. H. Rapid accumulation of inositol phosphates in isolated rat superior cervical sympathetic ganglia exposed to V1-vasopressin and muscarinic cholinergic stimuli. Biochem J. 1984 Aug 1;221(3):803–811. doi: 10.1042/bj2210803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosma M. M., Hille B. Protein kinase C is not necessary for peptide-induced suppression of M current or for desensitization of the peptide receptors. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2943–2947. doi: 10.1073/pnas.86.8.2943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Adams P. R. Effects of phorbol dibutyrate on M currents and M current inhibition in bullfrog sympathetic neurons. Cell Mol Neurobiol. 1987 Sep;7(3):255–269. doi: 10.1007/BF00711303. [DOI] [PubMed] [Google Scholar]
- Brown D. A., Adams P. R. Muscarinic suppression of a novel voltage-sensitive K+ current in a vertebrate neurone. Nature. 1980 Feb 14;283(5748):673–676. doi: 10.1038/283673a0. [DOI] [PubMed] [Google Scholar]
- Brown D. A., Higashida H. Inositol 1,4,5-trisphosphate and diacylglycerol mimic bradykinin effects on mouse neuroblastoma x rat glioma hybrid cells. J Physiol. 1988 Mar;397:185–207. doi: 10.1113/jphysiol.1988.sp016995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Marrion N. V., Smart T. G. On the transduction mechanism for muscarine-induced inhibition of M-current in cultured rat sympathetic neurones. J Physiol. 1989 Jun;413:469–488. doi: 10.1113/jphysiol.1989.sp017664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busis N. A., Weight F. F., Smith P. A. Synaptic potentials in sympathetic ganglia: are they mediated by cyclic nucleotides? Science. 1978 Jun 2;200(4345):1079–1081. doi: 10.1126/science.206964. [DOI] [PubMed] [Google Scholar]
- Cole A. E., Shinnick-Gallagher P. Muscarinic inhibitory transmission in mammalian sympathetic ganglia mediated by increased potassium conductance. Nature. 1984 Jan 19;307(5948):270–271. doi: 10.1038/307270a0. [DOI] [PubMed] [Google Scholar]
- Dodd J., Horn J. P. A reclassification of B and C neurones in the ninth and tenth paravertebral sympathetic ganglia of the bullfrog. J Physiol. 1983 Jan;334:255–269. doi: 10.1113/jphysiol.1983.sp014493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd J., Horn J. P. Muscarinic inhibition of sympathetic C neurones in the bullfrog. J Physiol. 1983 Jan;334:271–291. doi: 10.1113/jphysiol.1983.sp014494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutar P., Nicoll R. A. Stimulation of phosphatidylinositol (PI) turnover may mediate the muscarinic suppression of the M-current in hippocampal pyramidal cells. Neurosci Lett. 1988 Feb 15;85(1):89–94. doi: 10.1016/0304-3940(88)90434-x. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Hidaka H., Inagaki M., Kawamoto S., Sasaki Y. Isoquinolinesulfonamides, novel and potent inhibitors of cyclic nucleotide dependent protein kinase and protein kinase C. Biochemistry. 1984 Oct 9;23(21):5036–5041. doi: 10.1021/bi00316a032. [DOI] [PubMed] [Google Scholar]
- Jones S. W. A muscarine-resistant M-current in C cells of bullfrog sympathetic ganglia. Neurosci Lett. 1987 Mar 9;74(3):309–314. doi: 10.1016/0304-3940(87)90315-6. [DOI] [PubMed] [Google Scholar]
- Jones S. W., Adams P. R., Brownstein M. J., Rivier J. E. Teleost luteinizing hormone-releasing hormone: action on bullfrog sympathetic ganglia is consistent with role as neurotransmitter. J Neurosci. 1984 Feb;4(2):420–429. doi: 10.1523/JNEUROSCI.04-02-00420.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S. W. Muscarinic and peptidergic excitation of bull-frog sympathetic neurones. J Physiol. 1985 Sep;366:63–87. doi: 10.1113/jphysiol.1985.sp015785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S. W. On the resting potential of isolated frog sympathetic neurons. Neuron. 1989 Aug;3(2):153–161. doi: 10.1016/0896-6273(89)90028-7. [DOI] [PubMed] [Google Scholar]
- Krishtal O. A., Pidoplichko V. I. A receptor for protons in the nerve cell membrane. Neuroscience. 1980;5(12):2325–2327. doi: 10.1016/0306-4522(80)90149-9. [DOI] [PubMed] [Google Scholar]
- Kuffler S. W., Sejnowski T. J. Peptidergic and muscarinic excitation at amphibian sympathetic synapses. J Physiol. 1983 Aug;341:257–278. doi: 10.1113/jphysiol.1983.sp014805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E. The use of the patch clamp technique to study second messenger-mediated cellular events. Neuroscience. 1988 Sep;26(3):727–734. doi: 10.1016/0306-4522(88)90094-2. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- North R. A. Twelfth Gaddum memorial lecture. Drug receptors and the inhibition of nerve cells. Br J Pharmacol. 1989 Sep;98(1):13–28. doi: 10.1111/j.1476-5381.1989.tb16855.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PORTZEHL H., CALDWELL P. C., RUEEGG J. C. THE DEPENDENCE OF CONTRACTION AND RELAXATION OF MUSCLE FIBRES FROM THE CRAB MAIA SQUINADO ON THE INTERNAL CONCENTRATION OF FREE CALCIUM IONS. Biochim Biophys Acta. 1964 May 25;79:581–591. doi: 10.1016/0926-6577(64)90224-4. [DOI] [PubMed] [Google Scholar]
- Parente J. E., Walsh M. P., Girard P. R., Kuo J. F., Ng D. S., Wong K. Effects of gold coordination complexes on neutrophil function are mediated via inhibition of protein kinase C. Mol Pharmacol. 1989 Jan;35(1):26–33. [PubMed] [Google Scholar]
- Pfaffinger P. J., Leibowitz M. D., Subers E. M., Nathanson N. M., Almers W., Hille B. Agonists that suppress M-current elicit phosphoinositide turnover and Ca2+ transients, but these events do not explain M-current suppression. Neuron. 1988 Aug;1(6):477–484. doi: 10.1016/0896-6273(88)90178-x. [DOI] [PubMed] [Google Scholar]
- Pfaffinger P. Muscarine and t-LHRH suppress M-current by activating an IAP-insensitive G-protein. J Neurosci. 1988 Sep;8(9):3343–3353. doi: 10.1523/JNEUROSCI.08-09-03343.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafuse P. E., Smith P. A. Alpha 2-adrenergic hyperpolarization is not involved in slow synaptic inhibition in amphibian sympathetic ganglia. Br J Pharmacol. 1986 Feb;87(2):409–416. doi: 10.1111/j.1476-5381.1986.tb10831.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satin L. S., Adams P. R. Spontaneous miniature outward currents in cultured bullfrog neurons. Brain Res. 1987 Jan 20;401(2):331–339. doi: 10.1016/0006-8993(87)91417-x. [DOI] [PubMed] [Google Scholar]
- Sims S. M., Singer J. J., Walsh J. V., Jr Antagonistic adrenergic-muscarinic regulation of M current in smooth muscle cells. Science. 1988 Jan 8;239(4836):190–193. doi: 10.1126/science.2827305. [DOI] [PubMed] [Google Scholar]
- Smith P. A., Weight F. F. The pathway for the slow inhibitory postsynaptic potential in bullfrog sympathetic ganglia. J Neurophysiol. 1986 Sep;56(3):823–834. doi: 10.1152/jn.1986.56.3.823. [DOI] [PubMed] [Google Scholar]
- Tosaka T., Chichibu S., Libet B. Intracellular analysis of slow inhibitors and excitatory postsynaptic potentials in sympathetic ganglia of the frog. J Neurophysiol. 1968 May;31(3):396–409. doi: 10.1152/jn.1968.31.3.396. [DOI] [PubMed] [Google Scholar]
- Tsuji S., Kuba K. Muscarinic regulation of two ionic currents in the bullfrog sympathetic neurone. Pflugers Arch. 1988 Apr;411(4):361–370. doi: 10.1007/BF00587714. [DOI] [PubMed] [Google Scholar]
- Weight F. F., Padjen A. Acetylcholine and slow synaptic inhibition in frog sympathetic ganglion cells. Brain Res. 1973 May 30;55(1):225–228. doi: 10.1016/0006-8993(73)90506-4. [DOI] [PubMed] [Google Scholar]
- Weight F. F., Votava J. Slow synaptic excitation in sympathetic ganglion cells: evidence for synaptic inactivation of potassium conductance. Science. 1970 Nov 13;170(3959):755–758. doi: 10.1126/science.170.3959.755. [DOI] [PubMed] [Google Scholar]
- Williams J. T., North R. A., Tokimasa T. Inward rectification of resting and opiate-activated potassium currents in rat locus coeruleus neurons. J Neurosci. 1988 Nov;8(11):4299–4306. doi: 10.1523/JNEUROSCI.08-11-04299.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zidichouski J. A., Kehoe M. P., Wong K., Smith P. A. Elevation of intracellular cyclic AMP concentration fails to inhibit adrenaline-induced hyperpolarization in amphibian sympathetic neurons. Br J Pharmacol. 1989 Apr;96(4):779–784. doi: 10.1111/j.1476-5381.1989.tb11883.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


