Abstract
1. Slowly adapting type I (SAI) and type II (SAII) mechanoreceptors in the skin were studied in anaesthetized cats and rats employing mechanical stimuli every 30 s. Individual stimuli rose within 200 ms to a plateau force which was kept constant through a feedback control unit for 2000 ms. 2. In cats, close arterial infusion of neomycin (2.5 mg/min) as sulphate was given through a side branch into the femoral blood stream for 5, 10 or 20 min at a rate of 0.025 ml/min. At other times saline was infused at the same rate. 3. After 20 min of neomycin infusion (total 50 mg) nervous discharge of cat SAI receptors was suppressed to about 30% of the control responses before neomycin infusion. Nervous responses were reduced more profoundly during the plateau phase of stimulation than during the dynamic phase. The interspike interval histogram was severely distorted. 4. In contrast, cat SAII receptors maintained about 70% of their control response after 20 min of neomycin infusion. The interspike interval histogram showed an orderly shift towards longer intervals maintaining its normal shape. 5. In rats, intradermal microinfusion of neomycin (30 micrograms/min) through a glass micropipette into the immediate vicinity of the receptor under investigation resulted in severe transient suppression of SAI receptor responses to about 10% of the control level. Receptor responses recovered almost completely about 1 h after the end of neomycin application. 6. It is concluded that the observed differences between the two types of slowly adapting mechanoreceptors are consistent with the hypothesis that the SAI receptor functions as a secondary sensory receptor, with a synaptic link between the Merkel cell and the primary afferent neurone.
Full text
PDF


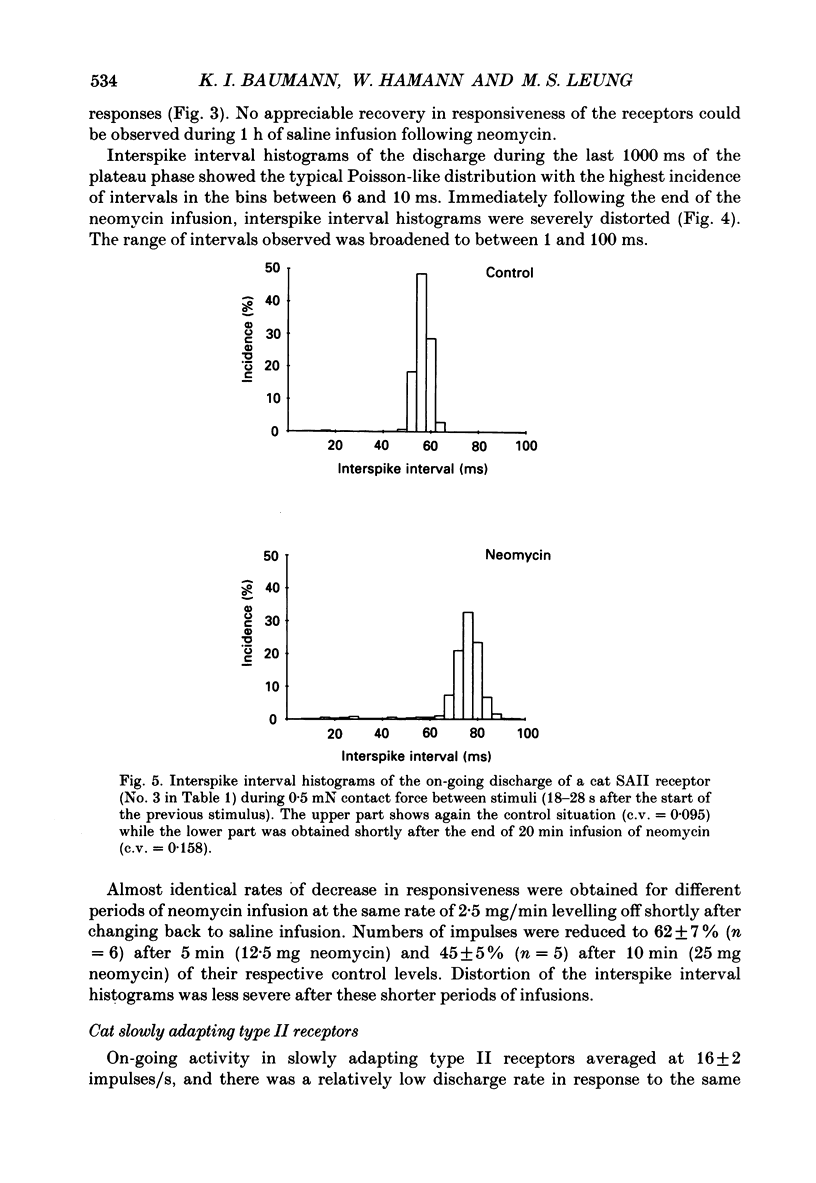
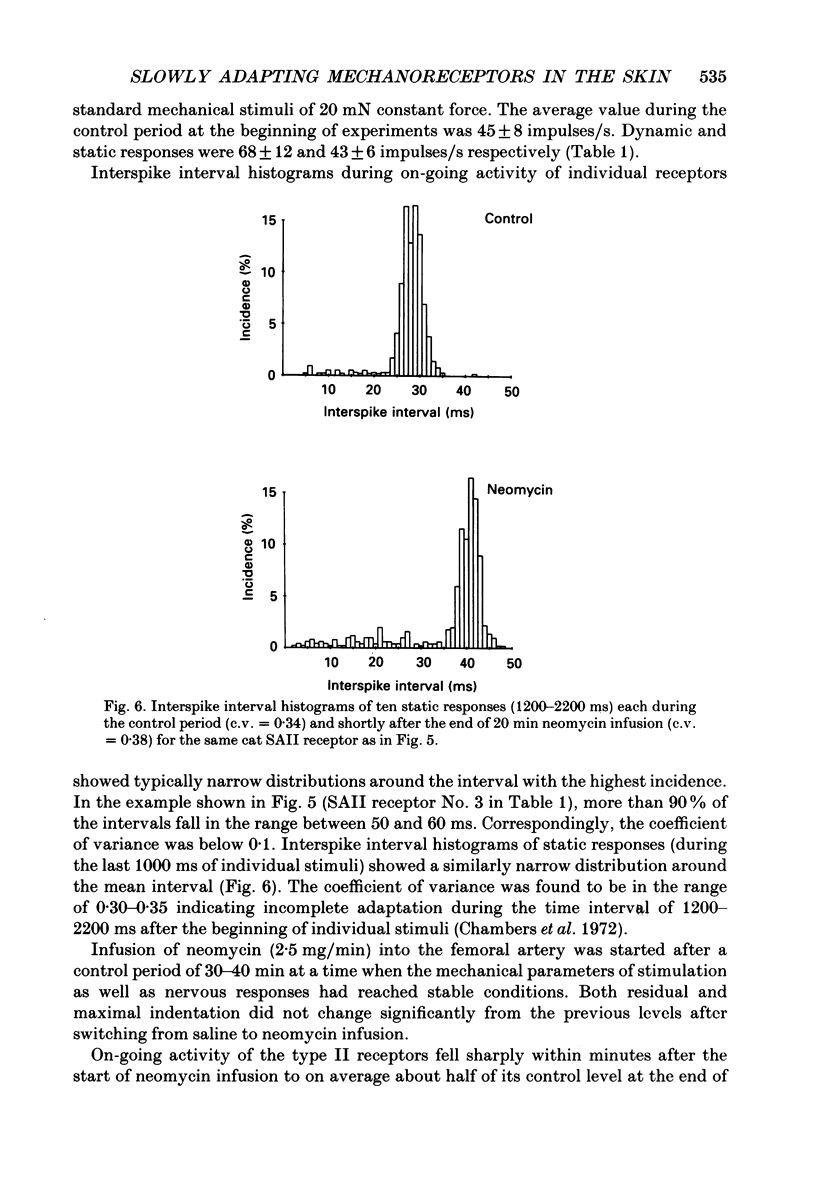
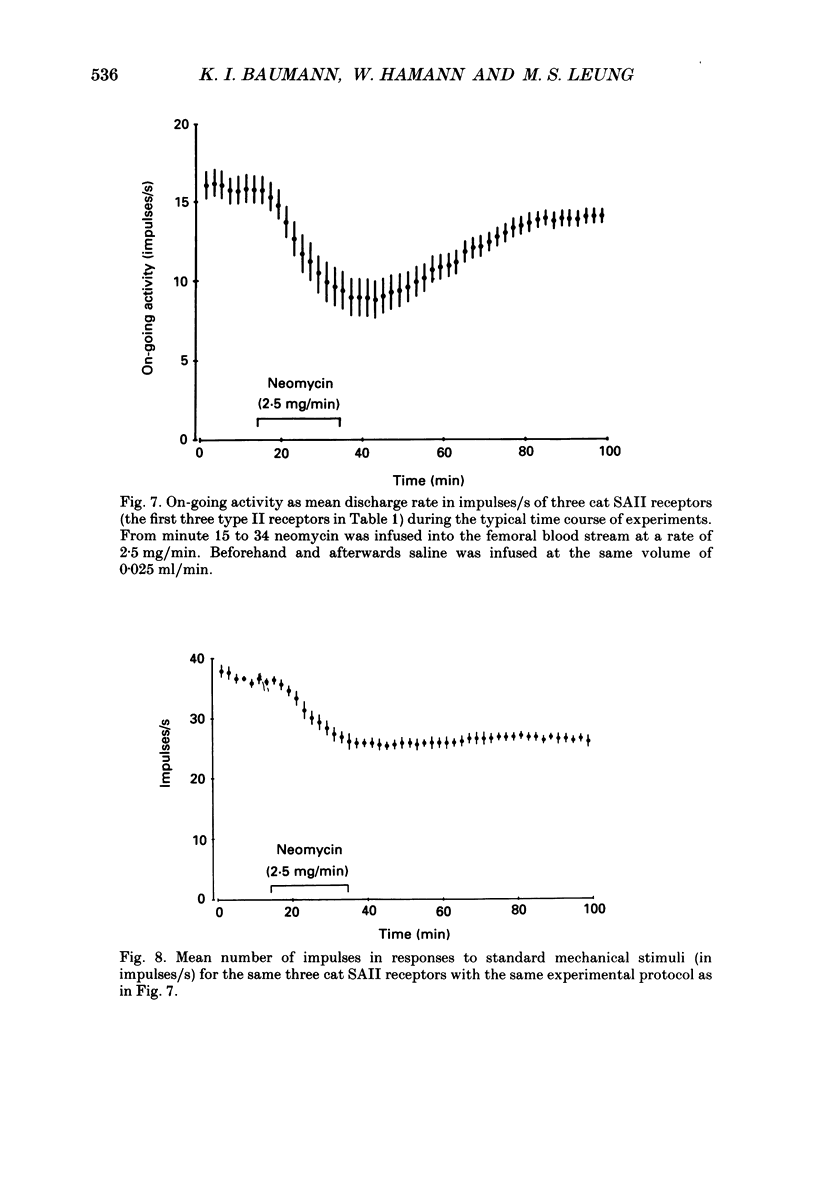
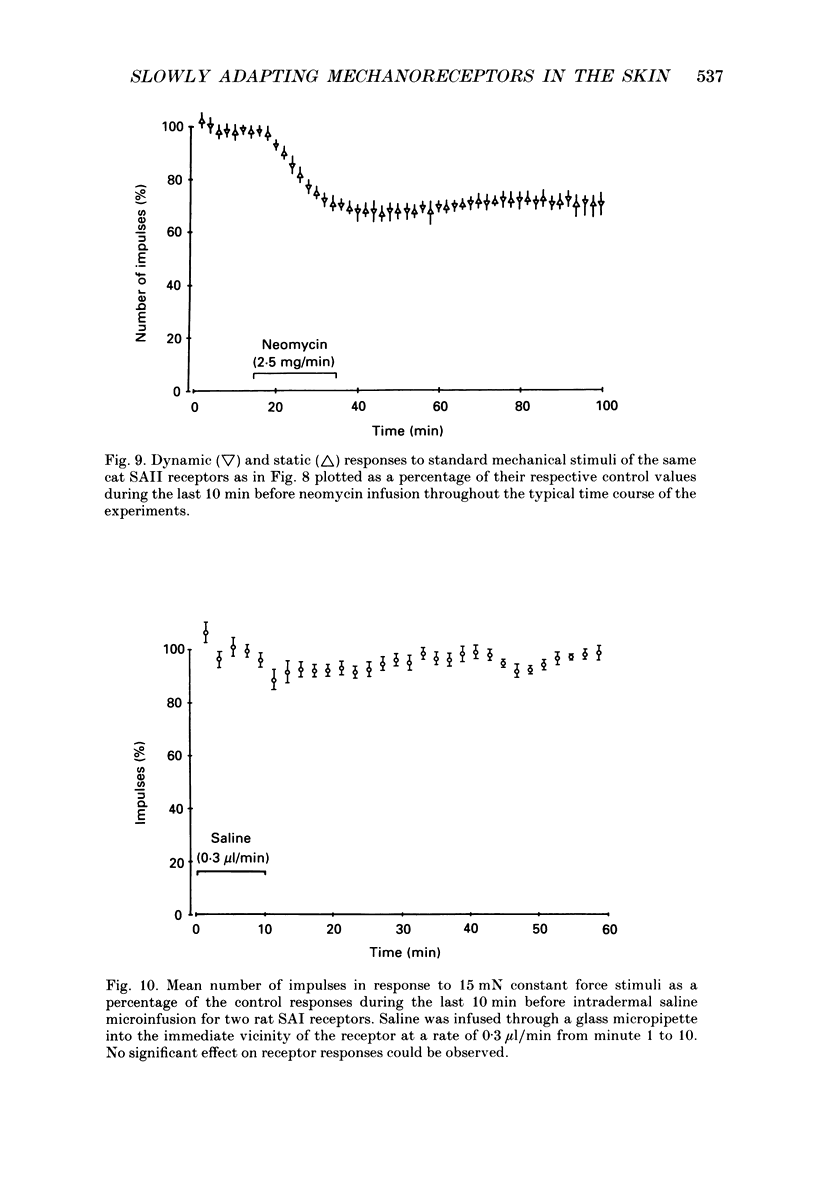
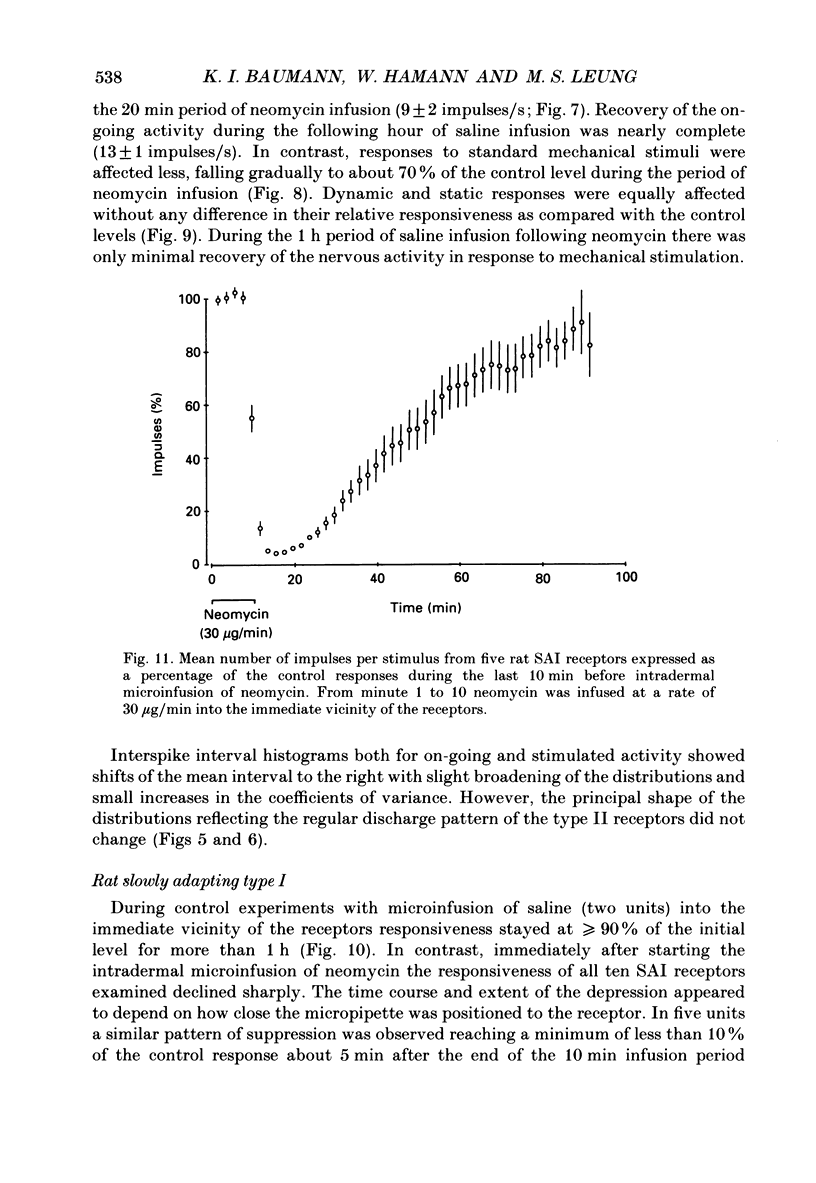
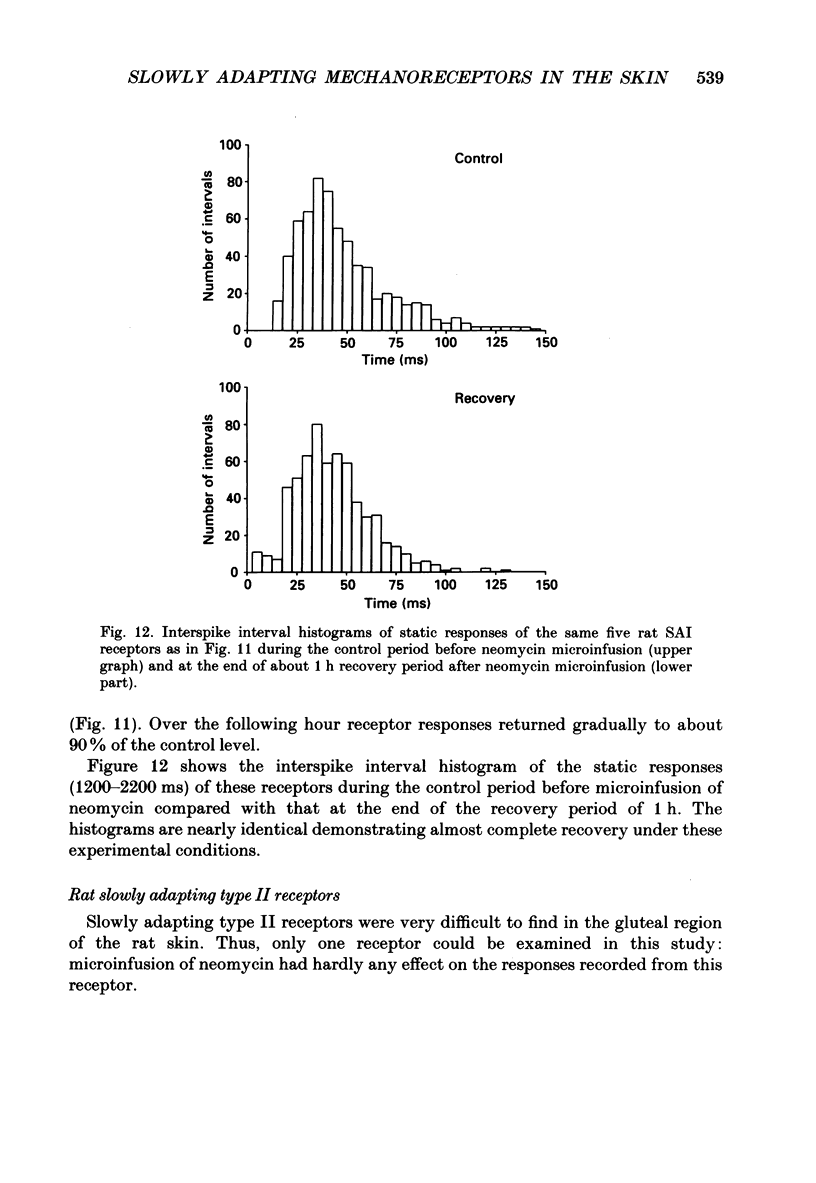





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baumann K. I., Hamann W., Leung M. S. Reduced responsiveness of touch (SA I) receptors in the cat following close arterial infusion of neomycin. Brain Res. 1986 Jul 2;377(1):160–162. doi: 10.1016/0006-8993(86)91201-1. [DOI] [PubMed] [Google Scholar]
- Baumann K. I., Hamann W., Leung M. S. Responsiveness of slowly adapting cutaneous mechanoreceptors after close arterial infusion of neomycin in cats. Prog Brain Res. 1988;74:43–49. doi: 10.1016/s0079-6123(08)62996-9. [DOI] [PubMed] [Google Scholar]
- Chambers M. R., Andres K. H., von Duering M., Iggo A. The structure and function of the slowly adapting type II mechanoreceptor in hairy skin. Q J Exp Physiol Cogn Med Sci. 1972 Oct;57(4):417–445. doi: 10.1113/expphysiol.1972.sp002177. [DOI] [PubMed] [Google Scholar]
- Diamond J., Mills L. R., Mearow K. M. Evidence that the Merkel cell is not the transducer in the mechanosensory Merkel cell-neurite complex. Prog Brain Res. 1988;74:51–56. doi: 10.1016/s0079-6123(08)62997-0. [DOI] [PubMed] [Google Scholar]
- FJALLBRANT N., IGGO A. The effect of histamine, 5-hydroxytryptamine and acetylcholine on cutaneous afferent fibres. J Physiol. 1961 May;156:578–590. doi: 10.1113/jphysiol.1961.sp006694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiekers J. F. Effects of the aminoglycoside antibiotics, streptomycin and neomycin, on neuromuscular transmission. I. Presynaptic considerations. J Pharmacol Exp Ther. 1983 Jun;225(3):487–495. [PubMed] [Google Scholar]
- Findlater G. S., Cooksey E. J., Anand A., Paintal A. S., Iggo A. The effects of hypoxia on slowly adapting type I (SAI) cutaneous mechanoreceptors in the cat and rat. Somatosens Res. 1987;5(1):1–17. doi: 10.3109/07367228709144615. [DOI] [PubMed] [Google Scholar]
- Horch K. W., Whitehorn D., Burgess P. R. Impulse generation in type I cutaneous mechanoreceptors. J Neurophysiol. 1974 Mar;37(2):267–281. doi: 10.1152/jn.1974.37.2.267. [DOI] [PubMed] [Google Scholar]
- Hudspeth A. J. Extracellular current flow and the site of transduction by vertebrate hair cells. J Neurosci. 1982 Jan;2(1):1–10. doi: 10.1523/JNEUROSCI.02-01-00001.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iggo A., Andres K. H. Morphology of cutaneous receptors. Annu Rev Neurosci. 1982;5:1–31. doi: 10.1146/annurev.ne.05.030182.000245. [DOI] [PubMed] [Google Scholar]
- Iggo A., Muir A. R. The structure and function of a slowly adapting touch corpuscle in hairy skin. J Physiol. 1969 Feb;200(3):763–796. doi: 10.1113/jphysiol.1969.sp008721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynn B., Carpenter S. E. Primary afferent units from the hairy skin of the rat hind limb. Brain Res. 1982 Apr 22;238(1):29–43. doi: 10.1016/0006-8993(82)90768-5. [DOI] [PubMed] [Google Scholar]
- Mason J. M., Ledsome J. R. Effects of obstruction of the mitral orifice or distention of the pulmonary vein--atrial junctions on renal and hind-limb vascular resistance in the dog. Circ Res. 1974 Jul;35(1):24–32. doi: 10.1161/01.res.35.1.24. [DOI] [PubMed] [Google Scholar]
- Munger B. L., Ide C. The structure and function of cutaneous sensory receptors. Arch Histol Cytol. 1988 Mar;51(1):1–34. doi: 10.1679/aohc.51.1. [DOI] [PubMed] [Google Scholar]
- Ohmori H. Mechano-electrical transduction currents in isolated vestibular hair cells of the chick. J Physiol. 1985 Feb;359:189–217. doi: 10.1113/jphysiol.1985.sp015581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmori H. Studies of ionic currents in the isolated vestibular hair cell of the chick. J Physiol. 1984 May;350:561–581. doi: 10.1113/jphysiol.1984.sp015218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacitti E. G., Findlater G. S. Calcium channel blockers and Merkel cells. Prog Brain Res. 1988;74:37–42. doi: 10.1016/s0079-6123(08)62995-7. [DOI] [PubMed] [Google Scholar]
- Prado W. A., Corrado A. P., Marseillan R. F. Competitive antagonism between calcium and antibiotics at the neuromuscular junction. Arch Int Pharmacodyn Ther. 1978 Feb;231(2):297–307. [PubMed] [Google Scholar]
- Smith K. R., Jr, Creech B. J. Effects of pharmacological agents on the physiological responses of hair discs. Exp Neurol. 1967 Dec;19(4):477–482. doi: 10.1016/0014-4886(67)90167-7. [DOI] [PubMed] [Google Scholar]
- Tolliver J. M., Warnick J. E. Aminoglycoside-induced blockade of reflex activity in the isolated spinal cord from the neonatal rat. Neurotoxicology. 1987 Summer;8(2):255–267. [PubMed] [Google Scholar]
- Tsai M. C. Effect of neomycin on post-tetanic twitch tension of the mouse diaphragm preparation. Br J Pharmacol. 1987 Apr;90(4):625–633. doi: 10.1111/j.1476-5381.1987.tb11214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright J. M., Collier B. The effects of neomycin upon transmitter release and action. J Pharmacol Exp Ther. 1977 Mar;200(3):576–587. [PubMed] [Google Scholar]


