Abstract
1. The voltage dependence and stability of the gating kinetics of the fast Cl-channel in excised patches of membrane from cultured rat skeletal muscle were studied with the patch clamp technique. Up to 10(6) open and shut intervals were analysed from each of five different patches containing a single fast Cl-channel. 2. To test for kinetic stability, plots of the mean durations of consecutive groups of 5-500 open and shut intervals were examined at each voltage. After excluding infrequent entries into both an apparent subconductance state and a long-lived (inactive) shut state, there were no abrupt and sustained changes in the moving means, indicating the absence of obvious shifts to other kinetic modes. The moving means did, however, fluctuate about the overall mean. 3. A comparison of experimental and simulated data indicated that most, but not all, of the fluctuation in the moving means was due to the stochastic variation inherent in the gating process. The fluctuation not accounted for by stochastic variation was mainly expressed as a slow, low-amplitude, component of drift about the mean. This slow component was unlikely to have arisen from measurement errors. 4. To examine whether the slow drift reflected detectable changes in kinetic modes, the data were divided into consecutive groups of 50,000 intervals. The exponential components describing the distributions were remarkably similar among the different groups, with stochastic variation accounting for most of the observed differences. This finding implies a single kinetic mode throughout the experiment. Thus, any changes in channel activity associated with the slow drift would have little effect on the analysis of gating kinetics presented here. 5. Depolarization increased channel open probability, Popen, for all five channels. This increase had a voltage sensitivity of 17 +/- 4 mV per e-fold change (effective gating charge of 1.6 +/- 0.32 electronic charges at 23 degrees C). Popen was 0.5 at -31 +/- 4 mV. 6. The depolarization-induced increase in Popen typically arose from a decrease in the mean shut time (19 +/- 4 mV per e-fold change; effective gating charge of 1.3 +/- 0.3 at 23 degrees C) and an increase in the mean open time (109 +/- 61 mV per e-fold change; effective gating charge of -0.24 +/- 0.13). 7. Neither plots of Popen versus voltage nor plots of the mean open and mean shut time versus voltage were completely described by a single Boltzmann distribution, suggesting multiple voltage-sensitive steps in channel gating.(ABSTRACT TRUNCATED AT 400 WORDS)
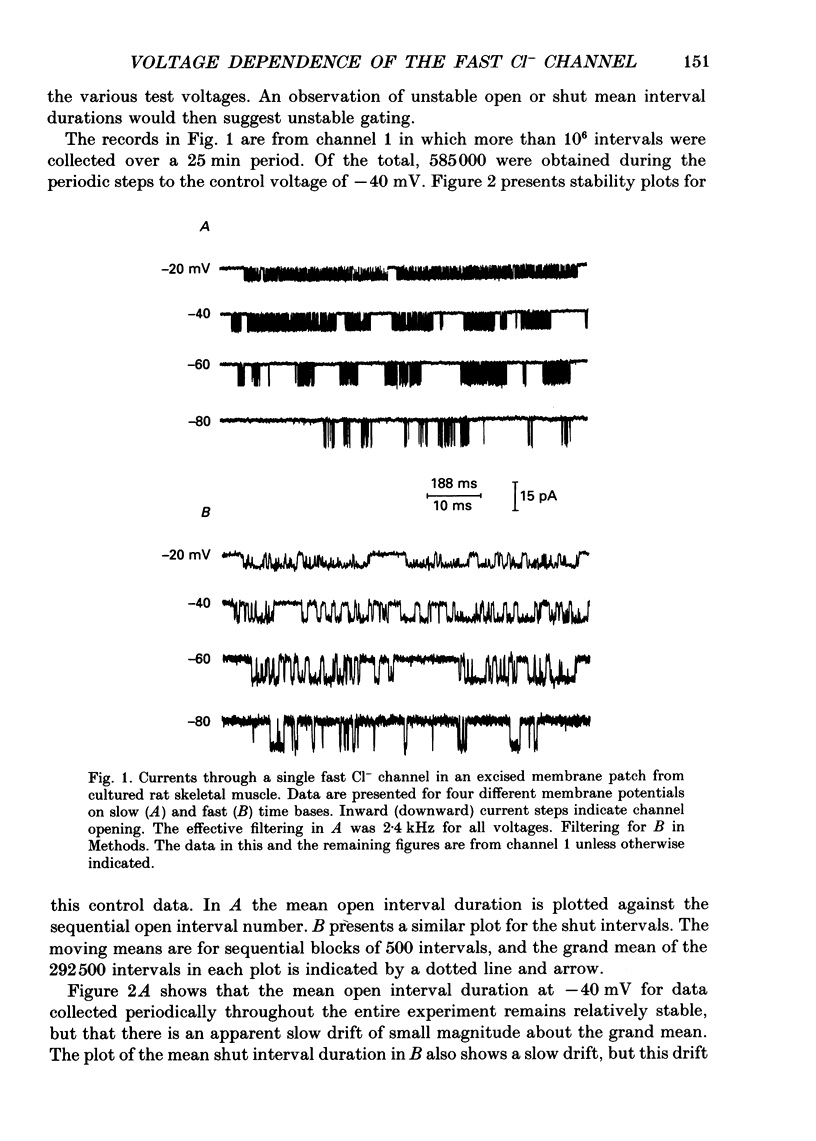
Full text
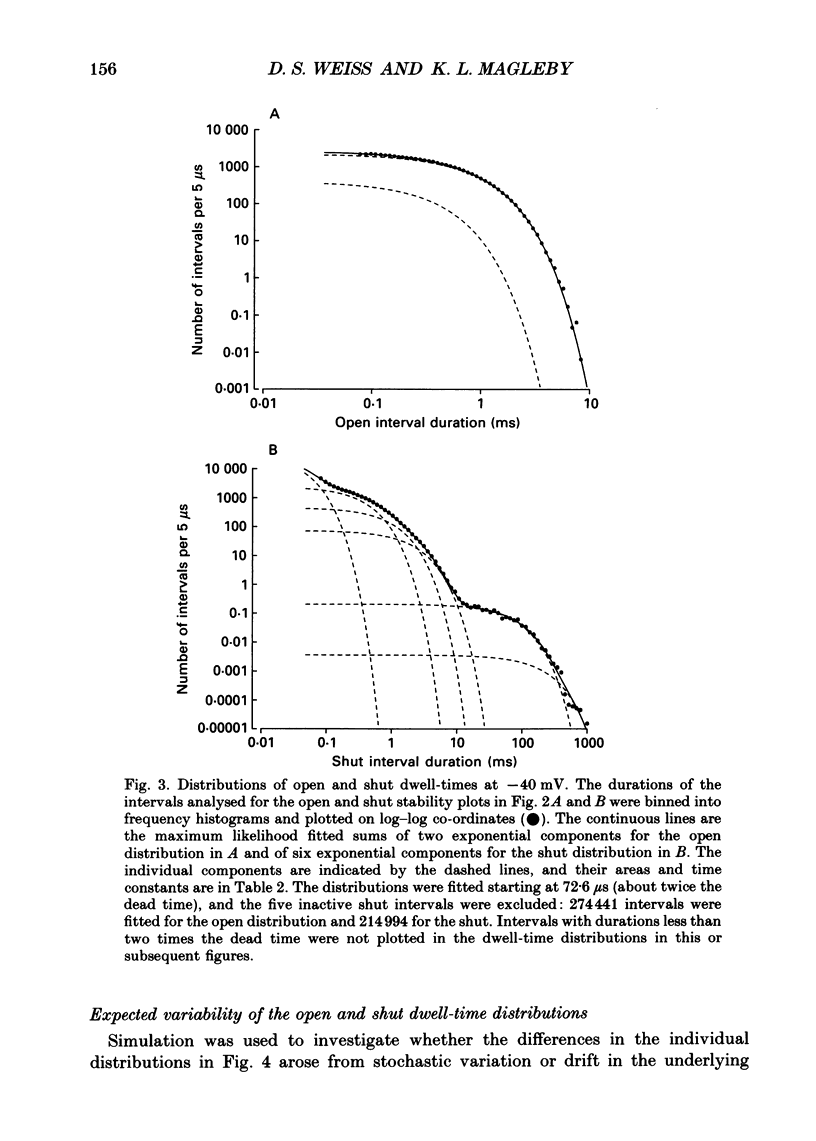
PDF





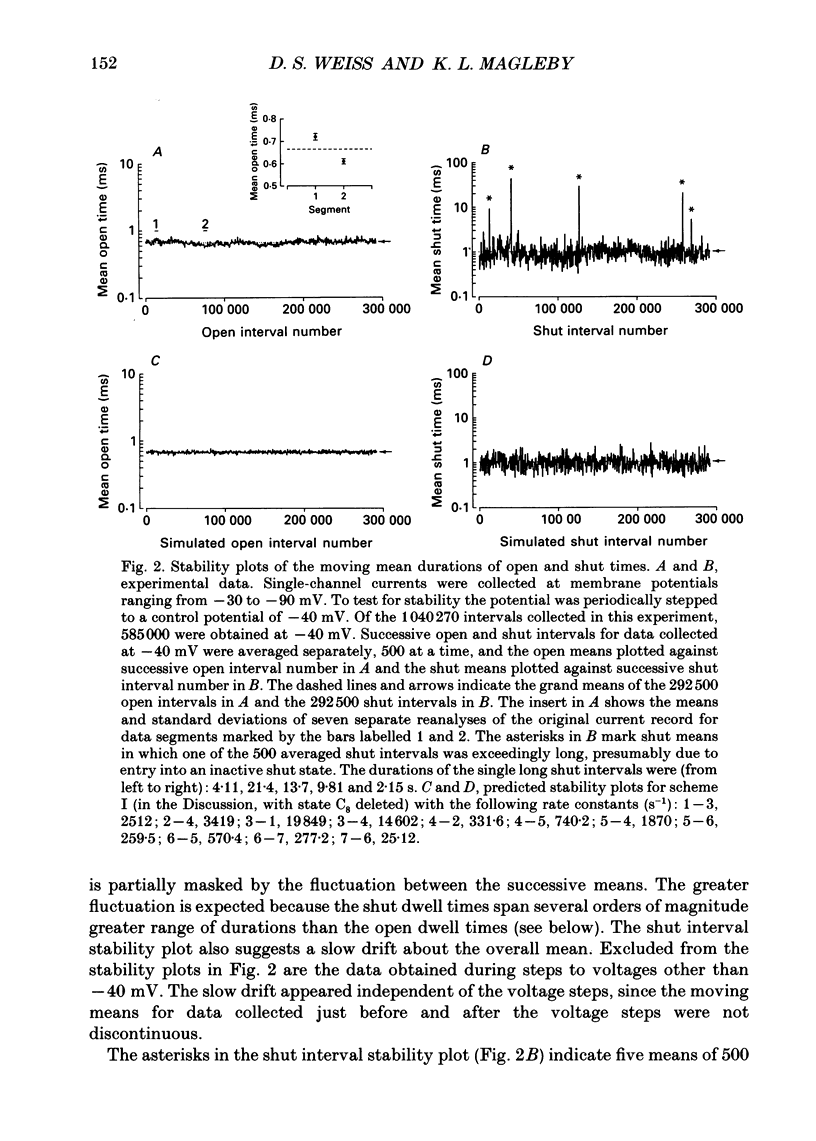




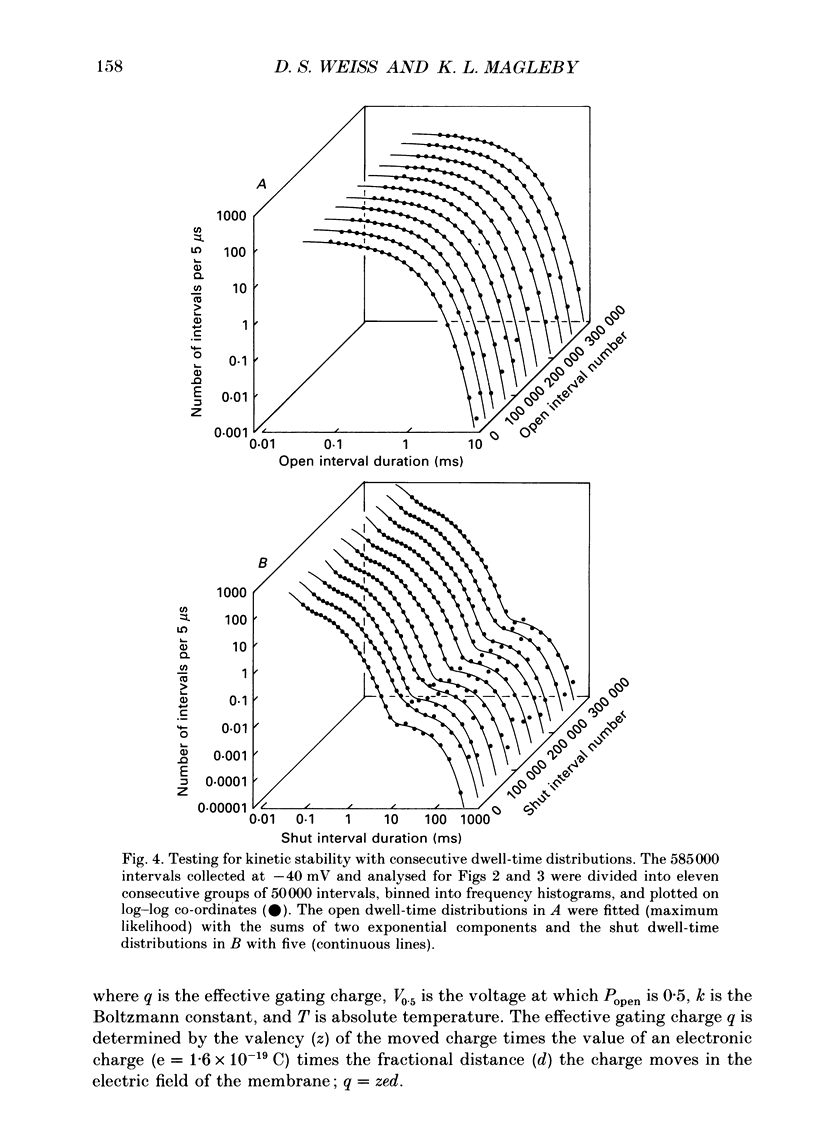
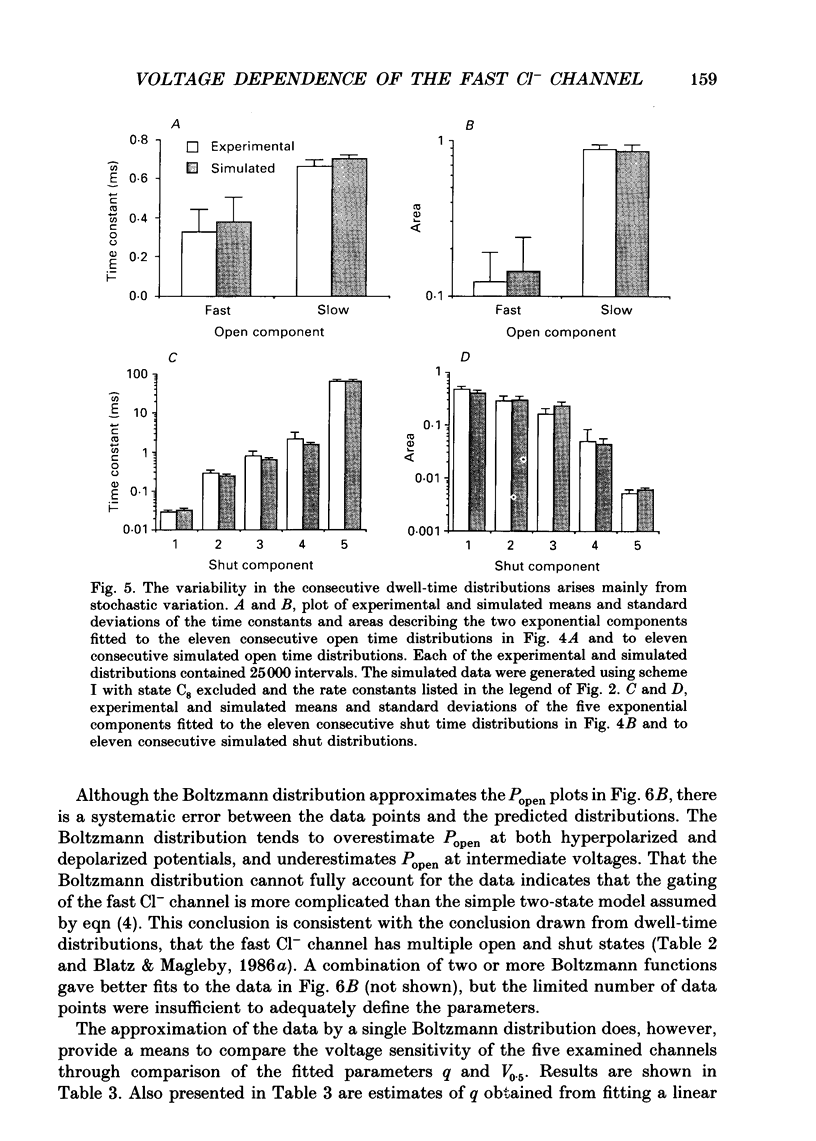
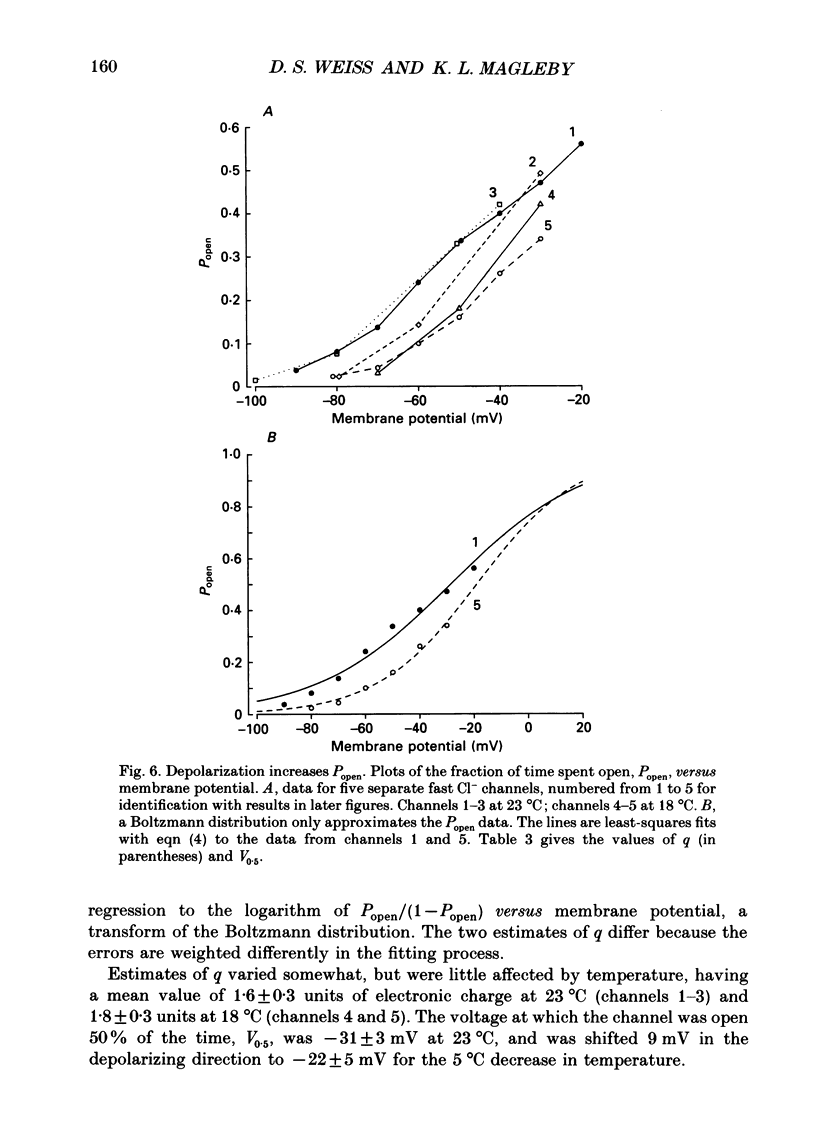

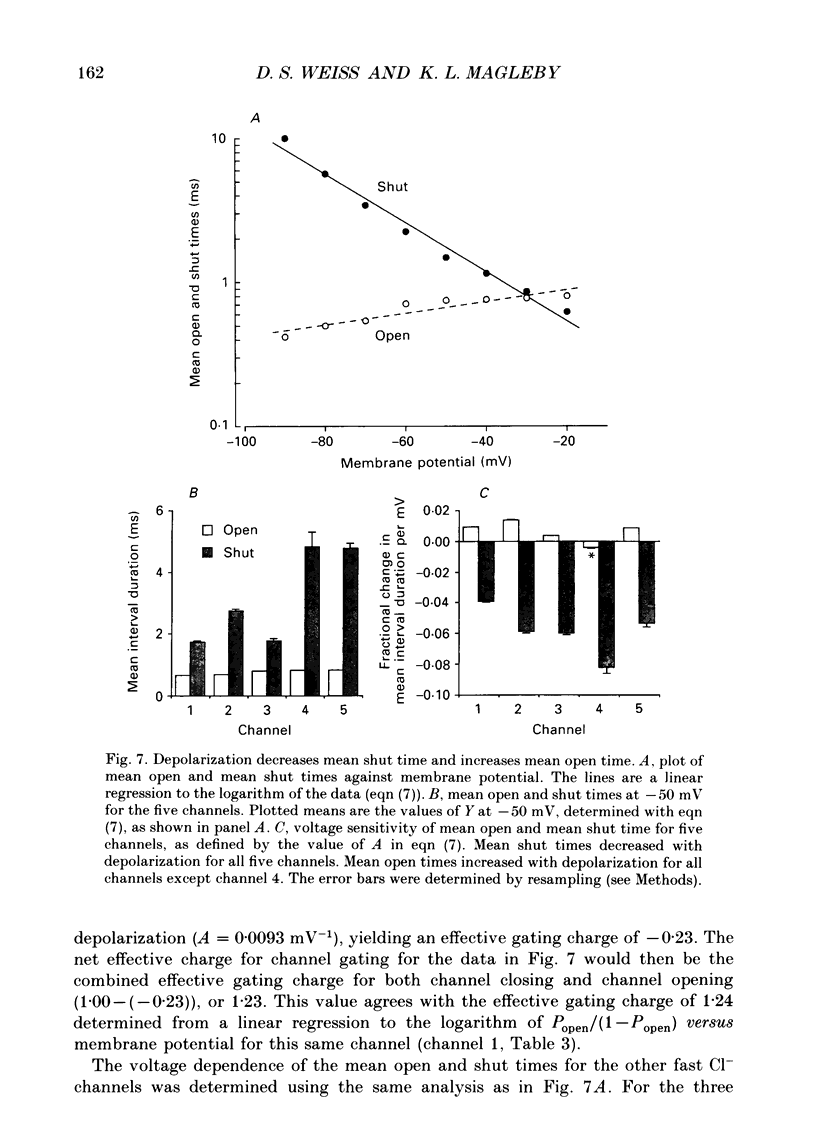



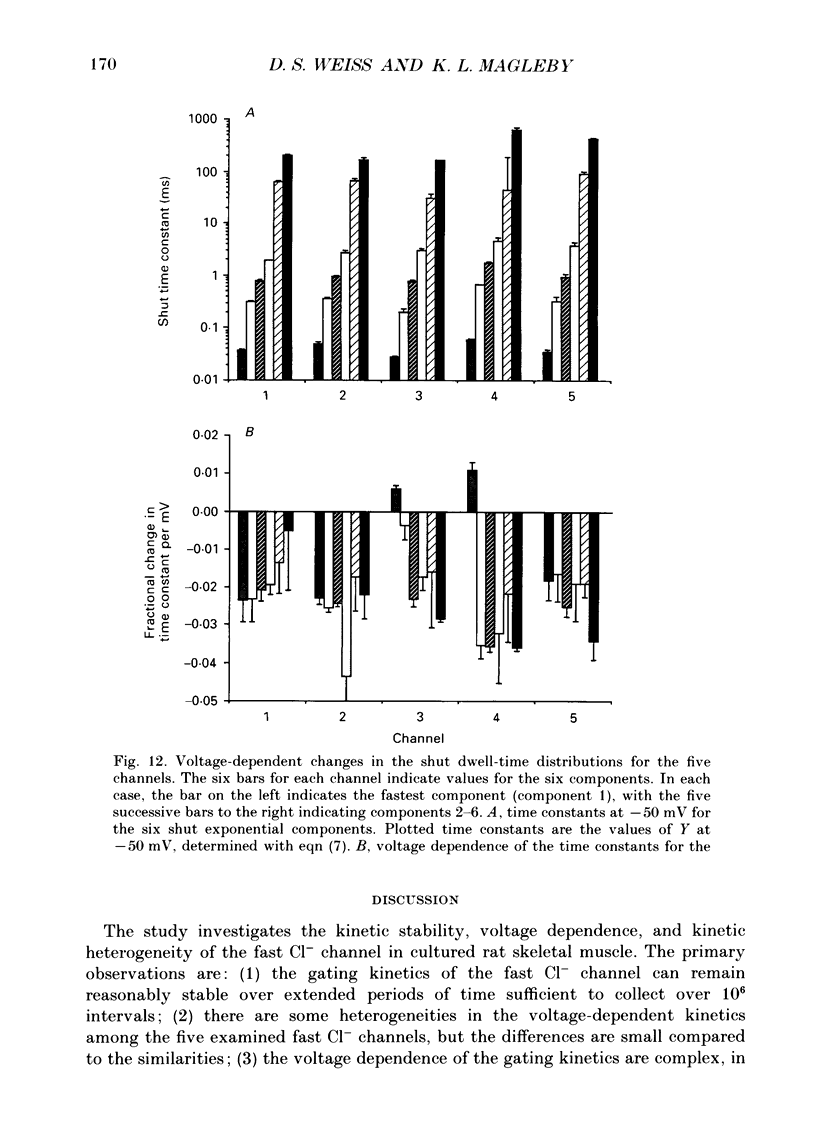
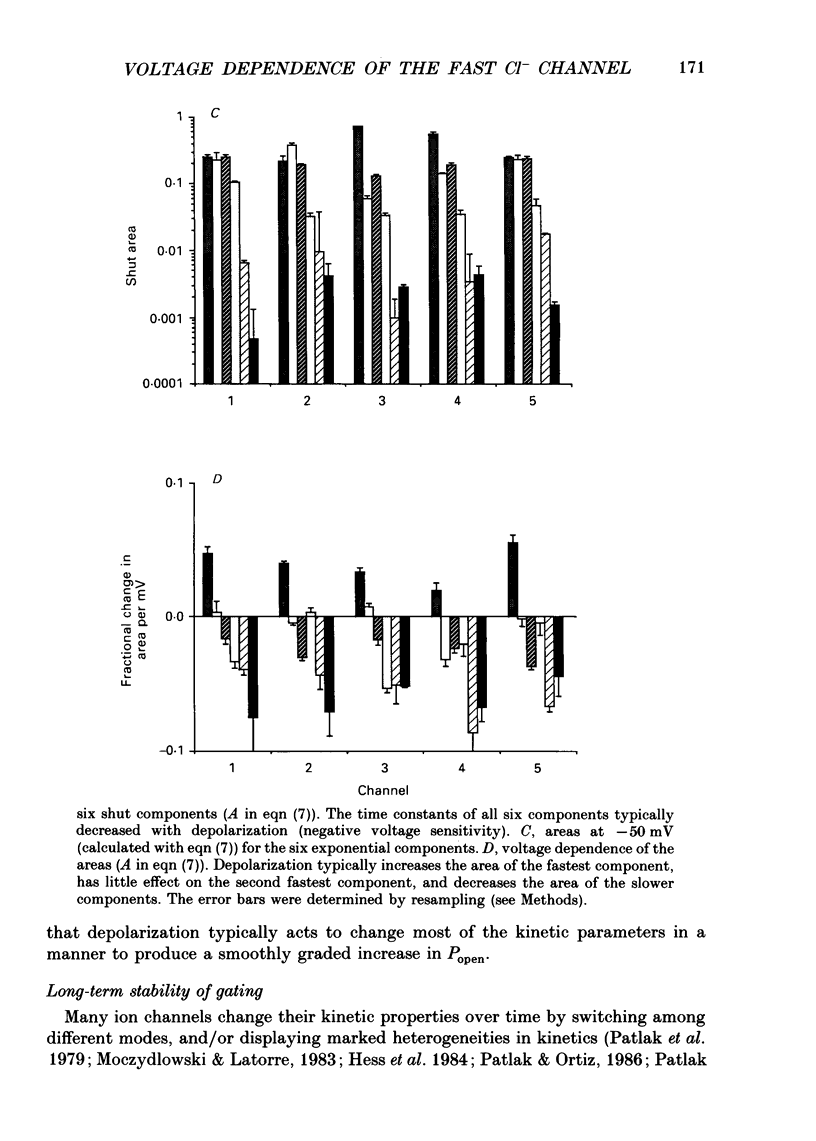





Images in this article
Selected References
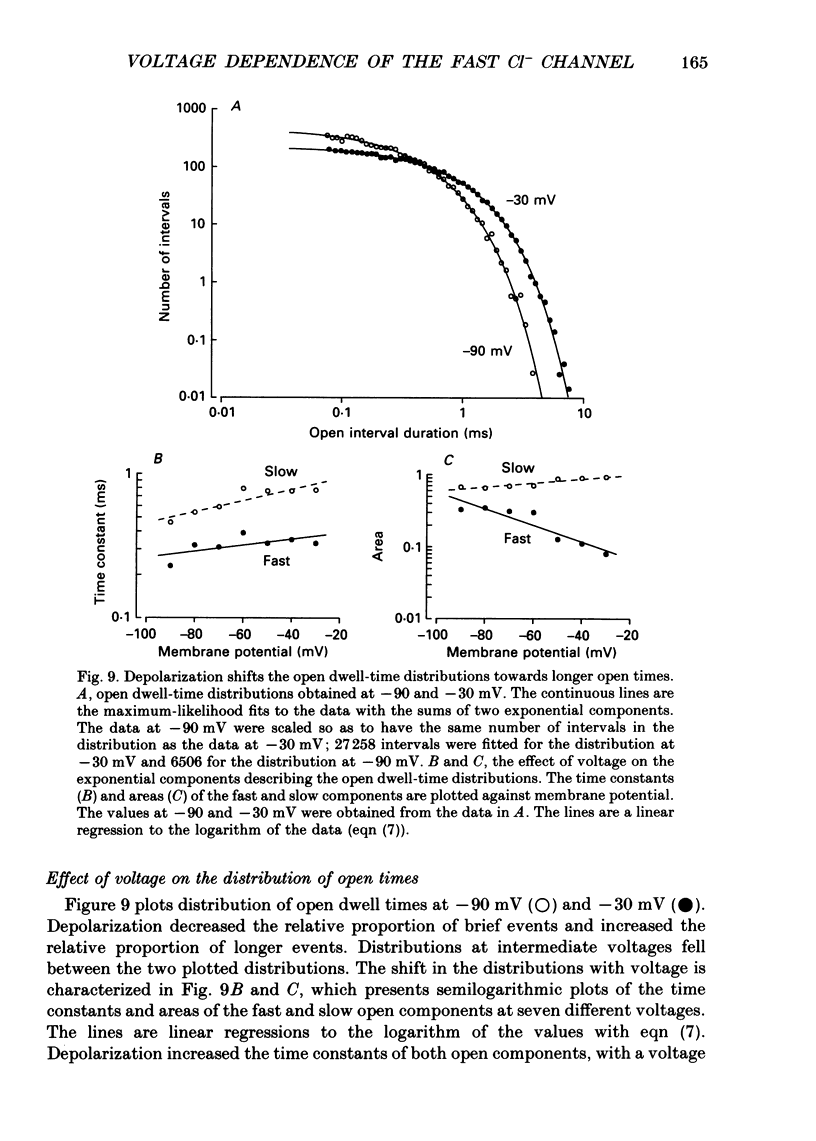
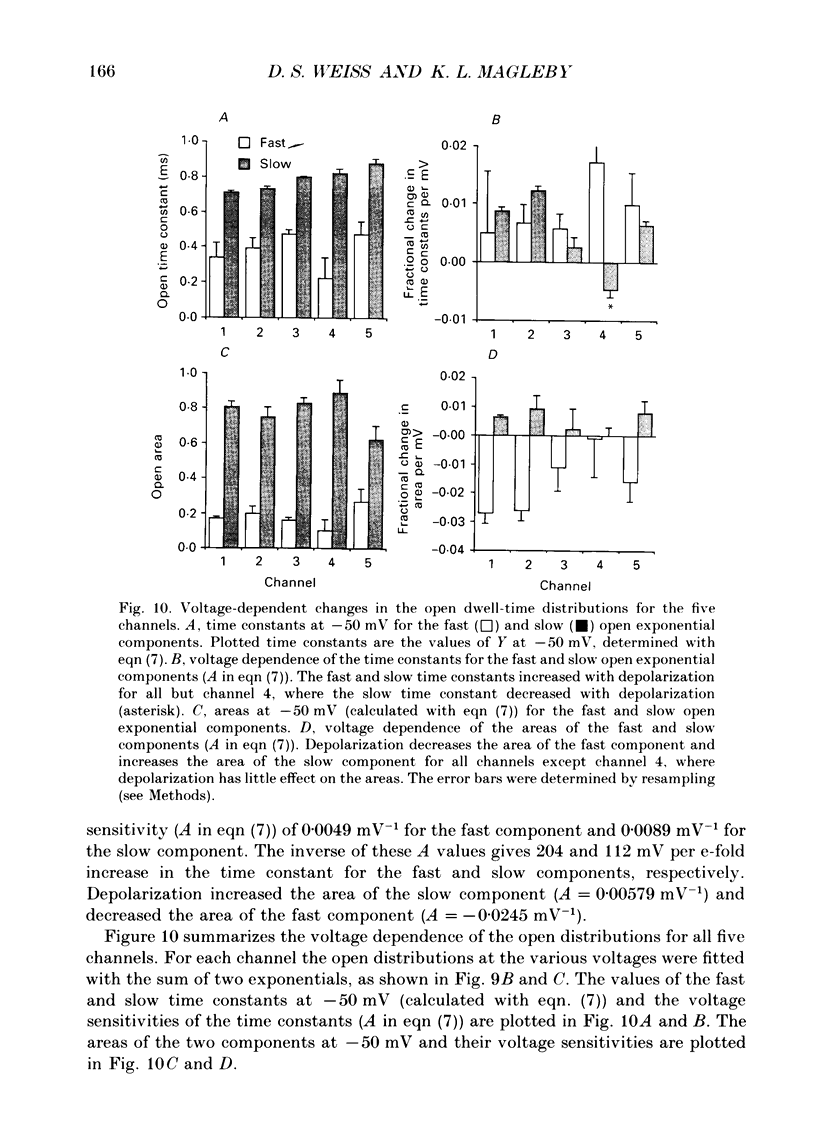
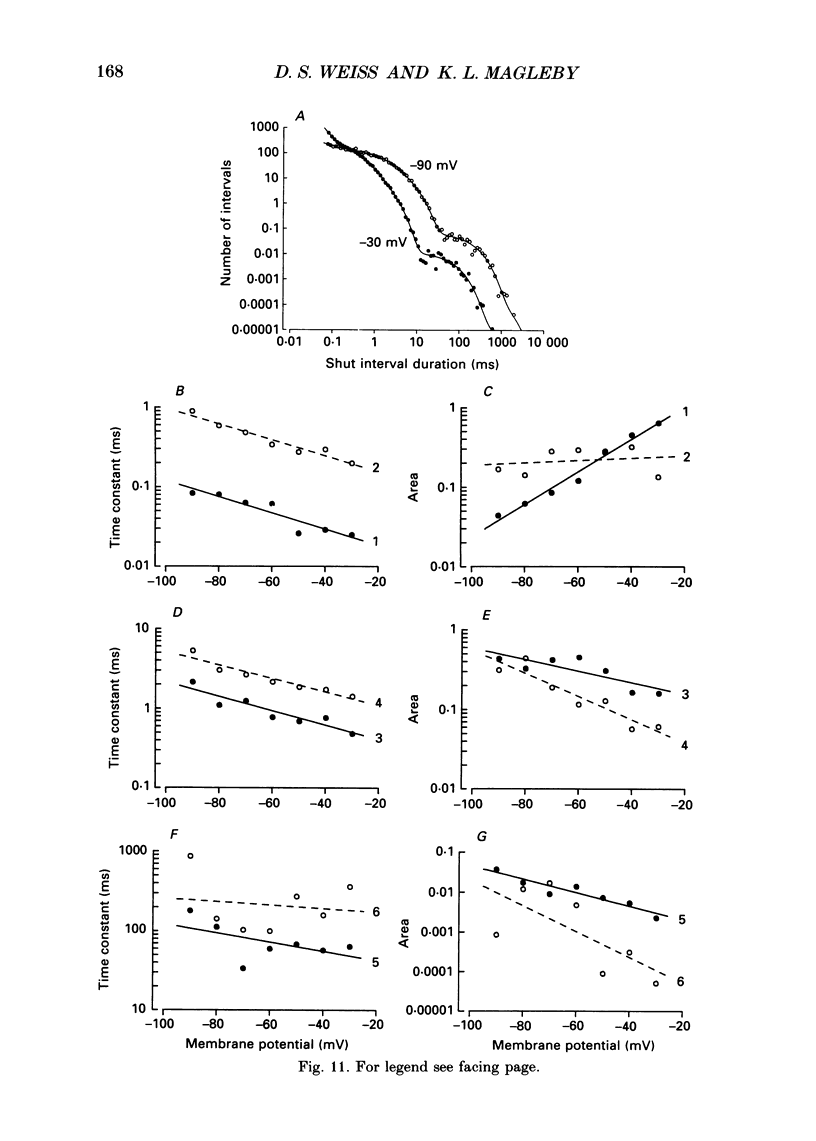
These references are in PubMed. This may not be the complete list of references from this article.
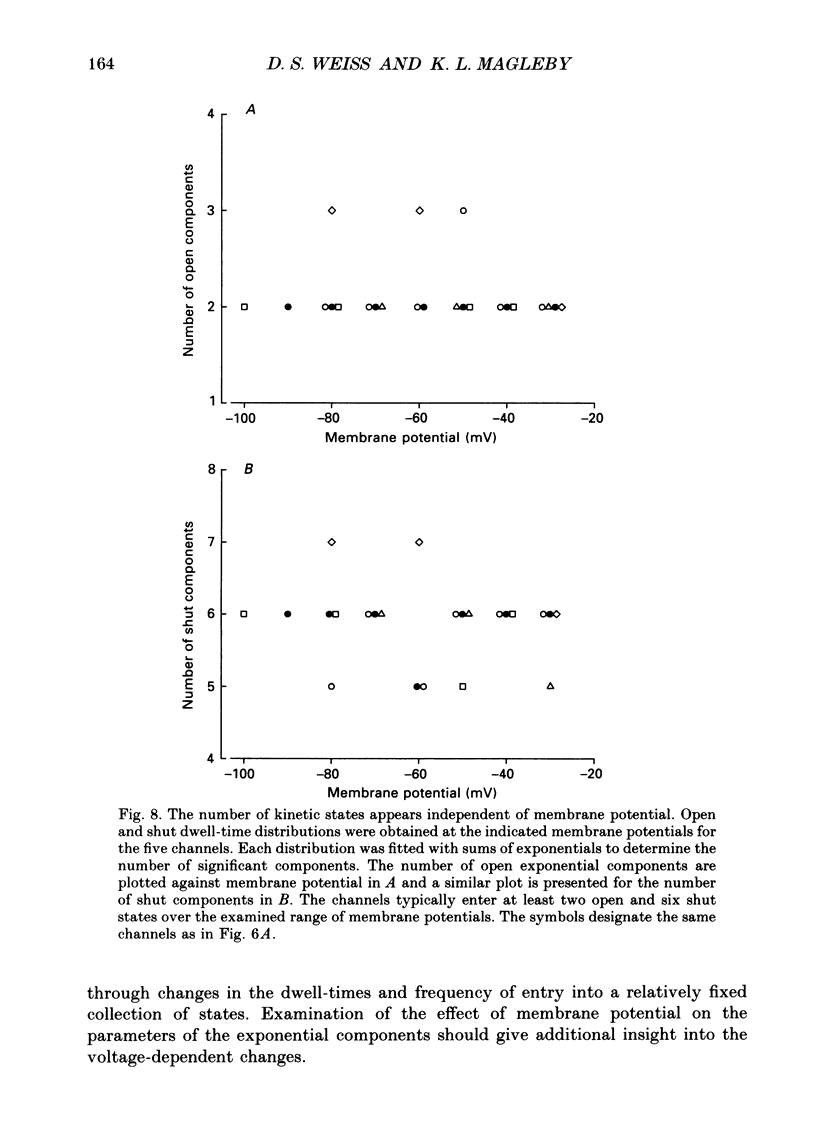
- Aldrich R. W., Corey D. P., Stevens C. F. A reinterpretation of mammalian sodium channel gating based on single channel recording. Nature. 1983 Dec 1;306(5942):436–441. doi: 10.1038/306436a0. [DOI] [PubMed] [Google Scholar]
- Armstrong C. M., Bezanilla F. Inactivation of the sodium channel. II. Gating current experiments. J Gen Physiol. 1977 Nov;70(5):567–590. doi: 10.1085/jgp.70.5.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett J. N., Barrett E. F., Dribin L. B. Calcium-dependent slow potassium conductance in rat skeletal myotubes. Dev Biol. 1981 Mar;82(2):258–266. doi: 10.1016/0012-1606(81)90450-4. [DOI] [PubMed] [Google Scholar]
- Barrett J. N., Magleby K. L., Pallotta B. S. Properties of single calcium-activated potassium channels in cultured rat muscle. J Physiol. 1982 Oct;331:211–230. doi: 10.1113/jphysiol.1982.sp014370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens M. I., Oberhauser A., Bezanilla F., Latorre R. Batrachotoxin-modified sodium channels from squid optic nerve in planar bilayers. Ion conduction and gating properties. J Gen Physiol. 1989 Jan;93(1):23–41. doi: 10.1085/jgp.93.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatz A. L., Magleby K. L. Adjacent interval analysis distinguishes among gating mechanisms for the fast chloride channel from rat skeletal muscle. J Physiol. 1989 Mar;410:561–585. doi: 10.1113/jphysiol.1989.sp017549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatz A. L., Magleby K. L. Correcting single channel data for missed events. Biophys J. 1986 May;49(5):967–980. doi: 10.1016/S0006-3495(86)83725-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatz A. L., Magleby K. L. Quantitative description of three modes of activity of fast chloride channels from rat skeletal muscle. J Physiol. 1986 Sep;378:141–174. doi: 10.1113/jphysiol.1986.sp016212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatz A. L., Magleby K. L. Single chloride-selective channels active at resting membrane potentials in cultured rat skeletal muscle. Biophys J. 1985 Jan;47(1):119–123. doi: 10.1016/S0006-3495(85)83884-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler A., Wei A. G., Baker K., Salkoff L. A family of putative potassium channel genes in Drosophila. Science. 1989 Feb 17;243(4893):943–947. doi: 10.1126/science.2493160. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Hawkes A. G. On the stochastic properties of single ion channels. Proc R Soc Lond B Biol Sci. 1981 Mar 6;211(1183):205–235. doi: 10.1098/rspb.1981.0003. [DOI] [PubMed] [Google Scholar]
- Colquhoun D. Single channel analysis costs time. Trends Pharmacol Sci. 1988 May;9(5):157–158. doi: 10.1016/0165-6147(88)90027-2. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hanke W., Miller C. Single chloride channels from Torpedo electroplax. Activation by protons. J Gen Physiol. 1983 Jul;82(1):25–45. doi: 10.1085/jgp.82.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartshorne R. P., Keller B. U., Talvenheimo J. A., Catterall W. A., Montal M. Functional reconstitution of the purified brain sodium channel in planar lipid bilayers. Proc Natl Acad Sci U S A. 1985 Jan;82(1):240–244. doi: 10.1073/pnas.82.1.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess P., Lansman J. B., Tsien R. W. Different modes of Ca channel gating behaviour favoured by dihydropyridine Ca agonists and antagonists. Nature. 1984 Oct 11;311(5986):538–544. doi: 10.1038/311538a0. [DOI] [PubMed] [Google Scholar]
- Horn R., Lange K. Estimating kinetic constants from single channel data. Biophys J. 1983 Aug;43(2):207–223. doi: 10.1016/S0006-3495(83)84341-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn R. Statistical methods for model discrimination. Applications to gating kinetics and permeation of the acetylcholine receptor channel. Biophys J. 1987 Feb;51(2):255–263. doi: 10.1016/S0006-3495(87)83331-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn R., Vandenberg C. A. Statistical properties of single sodium channels. J Gen Physiol. 1984 Oct;84(4):505–534. doi: 10.1085/jgp.84.4.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James W. M., Agnew W. S. Multiple oligosaccharide chains in the voltage-sensitive Na channel from electrophorus electricus: evidence for alpha-2,8-linked polysialic acid. Biochem Biophys Res Commun. 1987 Oct 29;148(2):817–826. doi: 10.1016/0006-291x(87)90949-1. [DOI] [PubMed] [Google Scholar]
- Keller B. U., Hartshorne R. P., Talvenheimo J. A., Catterall W. A., Montal M. Sodium channels in planar lipid bilayers. Channel gating kinetics of purified sodium channels modified by batrachotoxin. J Gen Physiol. 1986 Jul;88(1):1–23. doi: 10.1085/jgp.88.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacerda A. E., Brown A. M. Nonmodal gating of cardiac calcium channels as revealed by dihydropyridines. J Gen Physiol. 1989 Jun;93(6):1243–1273. doi: 10.1085/jgp.93.6.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemos J. R., Novak-Hofer I., Levitan I. B. Regulation of ion channel activity by protein phosphorylation. Prog Brain Res. 1986;69:107–118. doi: 10.1016/s0079-6123(08)61052-3. [DOI] [PubMed] [Google Scholar]
- Magleby K. L., Stevens C. F. The effect of voltage on the time course of end-plate currents. J Physiol. 1972 May;223(1):151–171. doi: 10.1113/jphysiol.1972.sp009839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McManus O. B., Blatz A. L., Magleby K. L. Sampling, log binning, fitting, and plotting durations of open and shut intervals from single channels and the effects of noise. Pflugers Arch. 1987 Nov;410(4-5):530–553. doi: 10.1007/BF00586537. [DOI] [PubMed] [Google Scholar]
- McManus O. B., Magleby K. L. Kinetic states and modes of single large-conductance calcium-activated potassium channels in cultured rat skeletal muscle. J Physiol. 1988 Aug;402:79–120. doi: 10.1113/jphysiol.1988.sp017195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McManus O. B., Magleby K. L. Kinetic time constants independent of previous single-channel activity suggest Markov gating for a large conductance Ca-activated K channel. J Gen Physiol. 1989 Dec;94(6):1037–1070. doi: 10.1085/jgp.94.6.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millhauser G. L., Salpeter E. E., Oswald R. E. Rate-amplitude correlation from single-channel records. A hidden structure in ion channel gating kinetics? Biophys J. 1988 Dec;54(6):1165–1168. doi: 10.1016/S0006-3495(88)83051-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moczydlowski E., Latorre R. Gating kinetics of Ca2+-activated K+ channels from rat muscle incorporated into planar lipid bilayers. Evidence for two voltage-dependent Ca2+ binding reactions. J Gen Physiol. 1983 Oct;82(4):511–542. doi: 10.1085/jgp.82.4.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E., Sakmann B. Voltage-dependence of drug-induced conductance in frog neuromuscular junction. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2140–2144. doi: 10.1073/pnas.72.6.2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilius B. Modal gating behavior of cardiac sodium channels in cell-free membrane patches. Biophys J. 1988 Jun;53(6):857–862. doi: 10.1016/S0006-3495(88)83166-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patlak J. B., Gration K. A., Usherwood P. N. Single glutamate-activated channels in locust muscle. Nature. 1979 Apr 12;278(5705):643–645. doi: 10.1038/278643a0. [DOI] [PubMed] [Google Scholar]
- Patlak J. B., Ortiz M., Horn R. Opentime heterogeneity during bursting of sodium channels in frog skeletal muscle. Biophys J. 1986 Mar;49(3):773–777. doi: 10.1016/S0006-3495(86)83704-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patlak J. B., Ortiz M. Two modes of gating during late Na+ channel currents in frog sartorius muscle. J Gen Physiol. 1986 Feb;87(2):305–326. doi: 10.1085/jgp.87.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux B., Sauvé R. A general solution to the time interval omission problem applied to single channel analysis. Biophys J. 1985 Jul;48(1):149–158. doi: 10.1016/S0006-3495(85)83768-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz T. L., Tempel B. L., Papazian D. M., Jan Y. N., Jan L. Y. Multiple potassium-channel components are produced by alternative splicing at the Shaker locus in Drosophila. Nature. 1988 Jan 14;331(6152):137–142. doi: 10.1038/331137a0. [DOI] [PubMed] [Google Scholar]
- Weiss D. S. Membrane potential modulates the activation of GABA-gated channels. J Neurophysiol. 1988 Feb;59(2):514–527. doi: 10.1152/jn.1988.59.2.514. [DOI] [PubMed] [Google Scholar]
- White M. M., Bezanilla F. Activation of squid axon K+ channels. Ionic and gating current studies. J Gen Physiol. 1985 Apr;85(4):539–554. doi: 10.1085/jgp.85.4.539. [DOI] [PMC free article] [PubMed] [Google Scholar]