Abstract
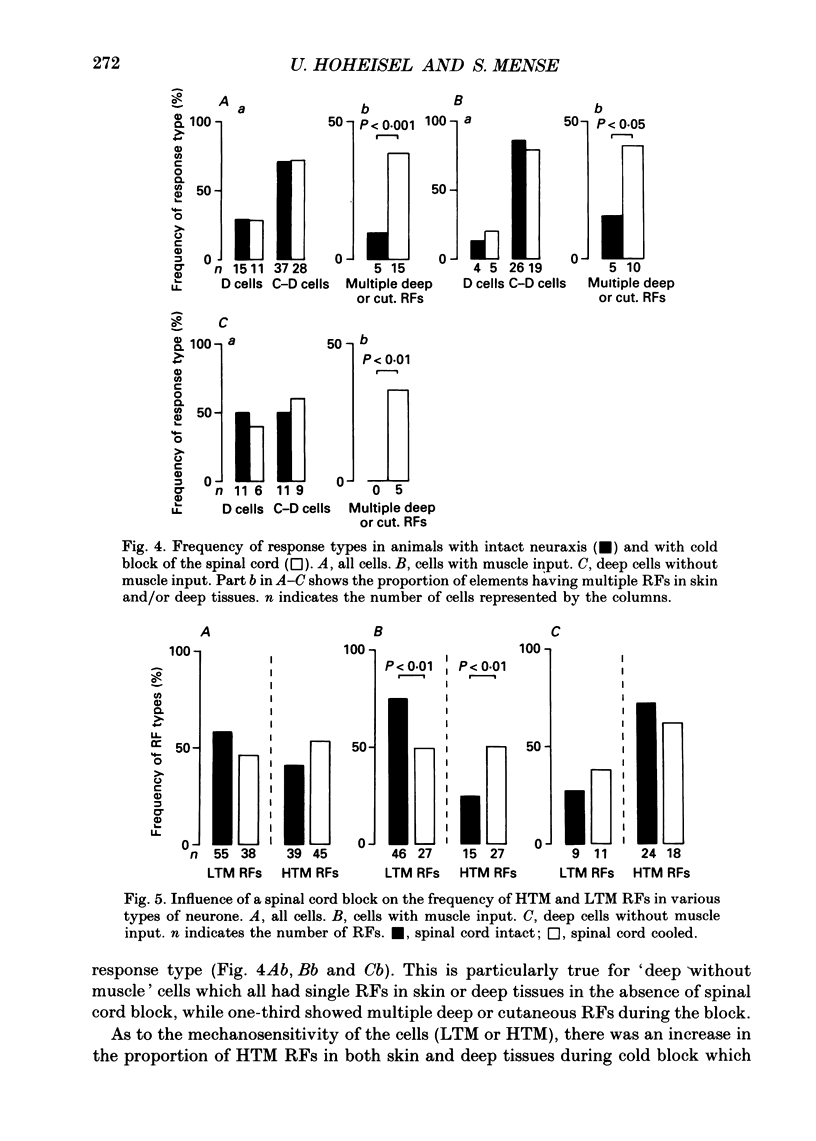
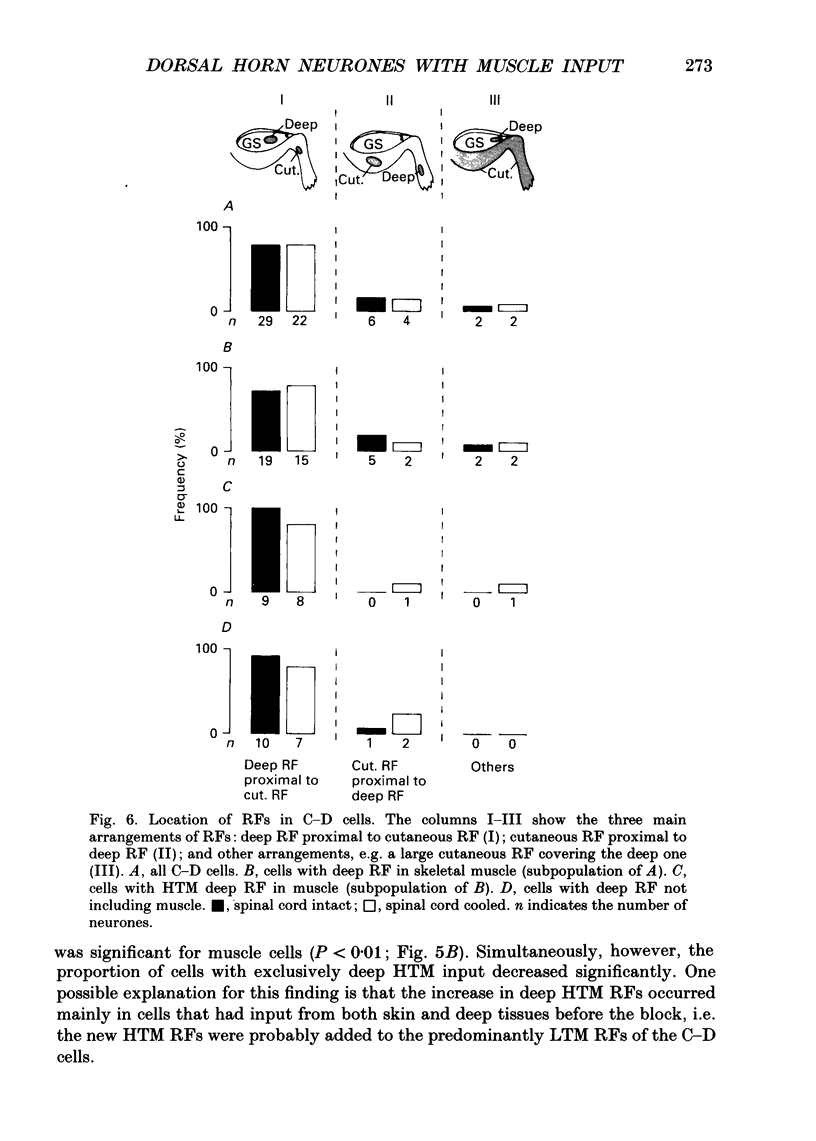
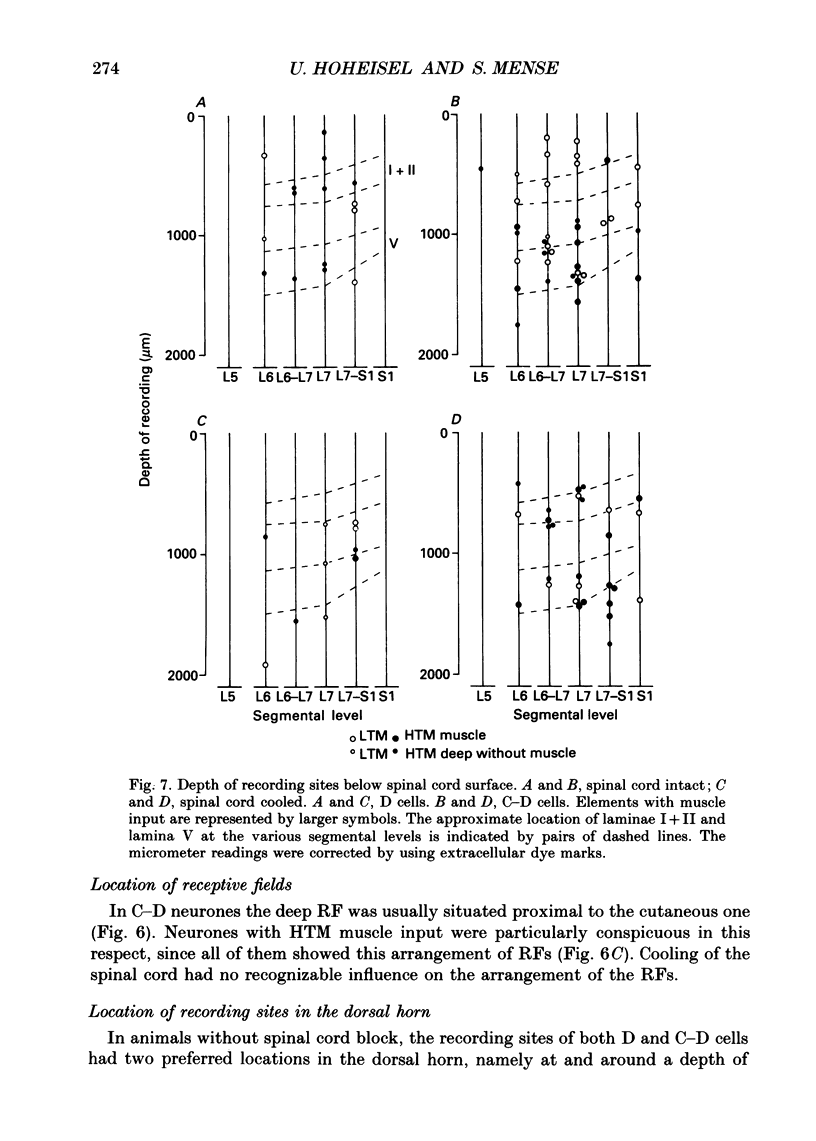
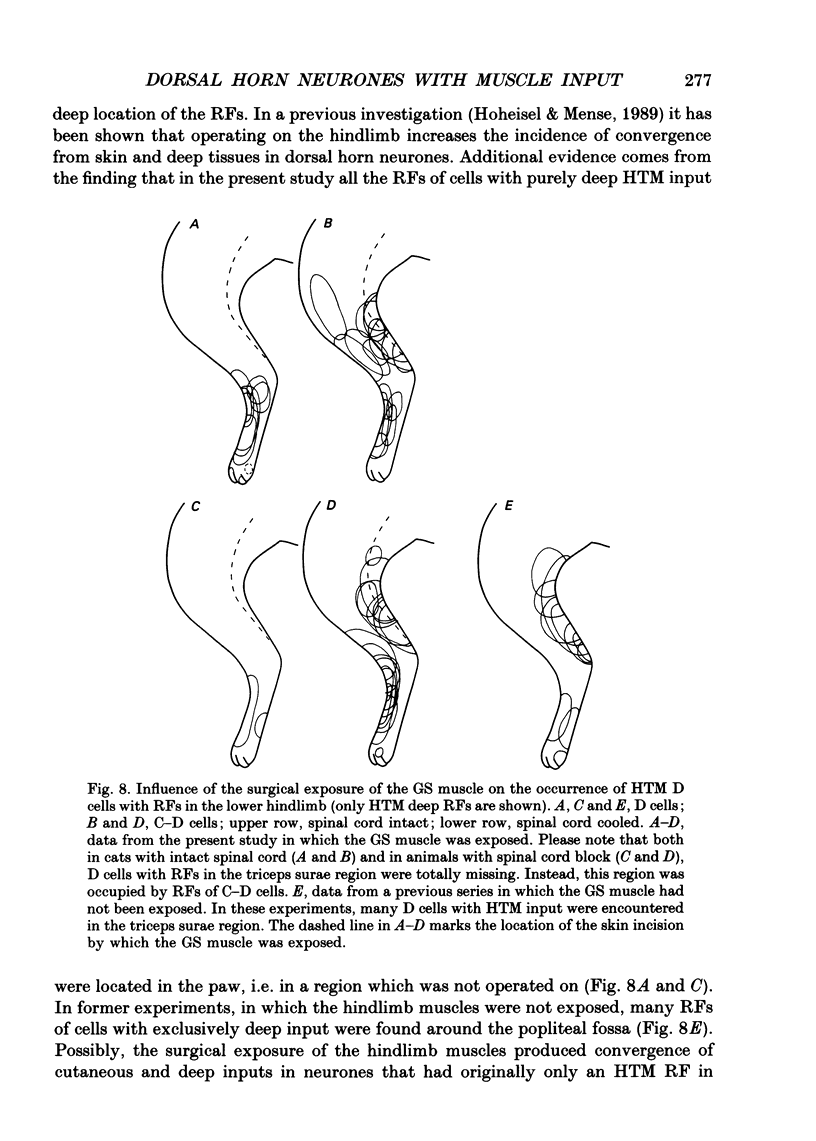
1. In chloralose-anaesthetized cats, lumbosacral dorsal horn neurones driven by receptors in skeletal muscle and other deep tissues (tendon, joint, bone) were studied. 2. Upon mechanical stimulation two main types of neurones were found: units having exclusive input from deep tissues (D cells, 28.8%) and units with input from both cutaneous (C) and deep (D) receptors (C-D cells, 71.2%). In both categories, low-threshold mechanosensitive (LTM) and high-threshold mechanosensitive (HTM) elements were present. 3. Neurones responding exclusively to noxious stimulation of skeletal muscle were not found; the input from muscle nociceptors converged on the dorsal horn cells together with other deep or cutaneous input. D cells with exclusively HTM input were numerous; these could from the anatomical basis for a specific spinal pathway for deep pain. 4. For C-D neurones with input from deep nociceptors the cutaneous receptive field (RF) was usually located distal to the deep one. This arrangement might be of relevance for the occurrence of hyperaesthetic skin distal to painful deep lesions. 5. Cold block of the spinal cord resulted in a marked increase in the neurones' mechanical responsiveness and in the number of RFs per neurone. Simultaneously, the proportion of HTM RFs increased, particularly in cells with input from skeletal muscle. 6. The recording sites in the dorsal horn were located in the superficial dorsal horn and in and around laminae V/VI. Evidence is presented that in dorsal horn cells with deep input not only the mechanical excitability but also the degree of convergence is controlled by descending spinal pathways.
Full text
PDF


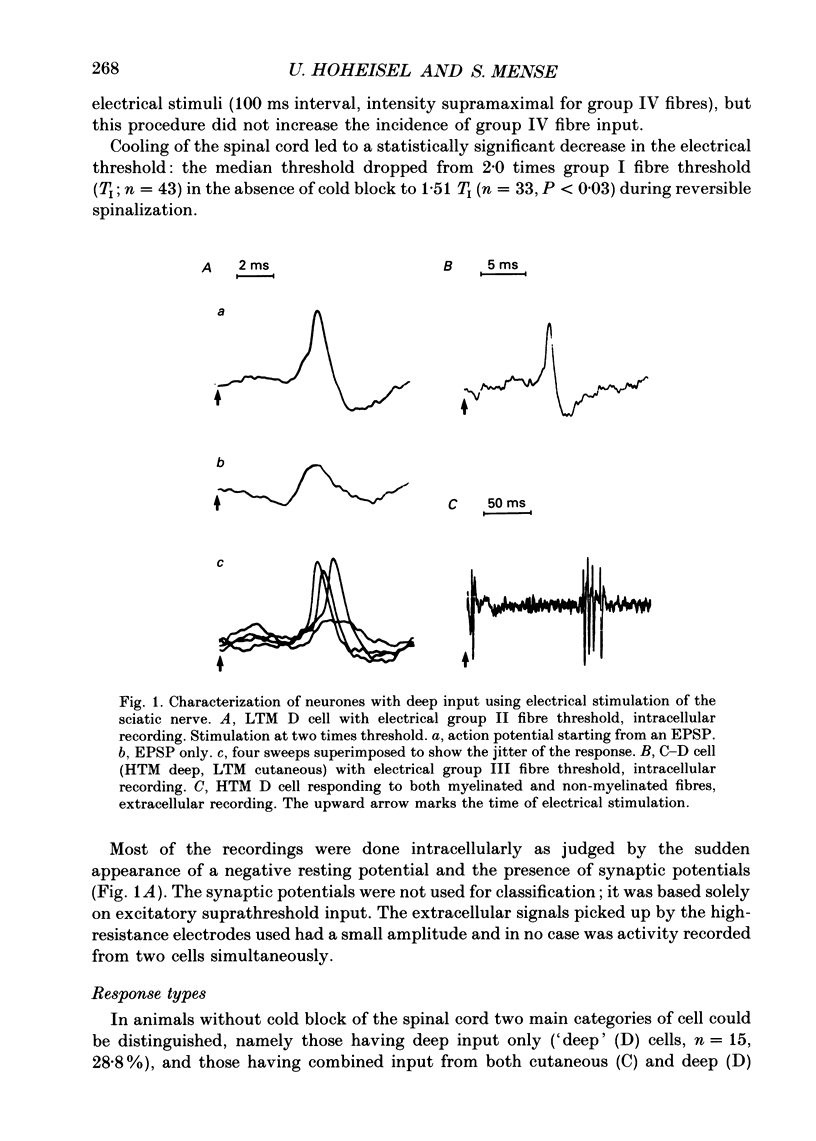
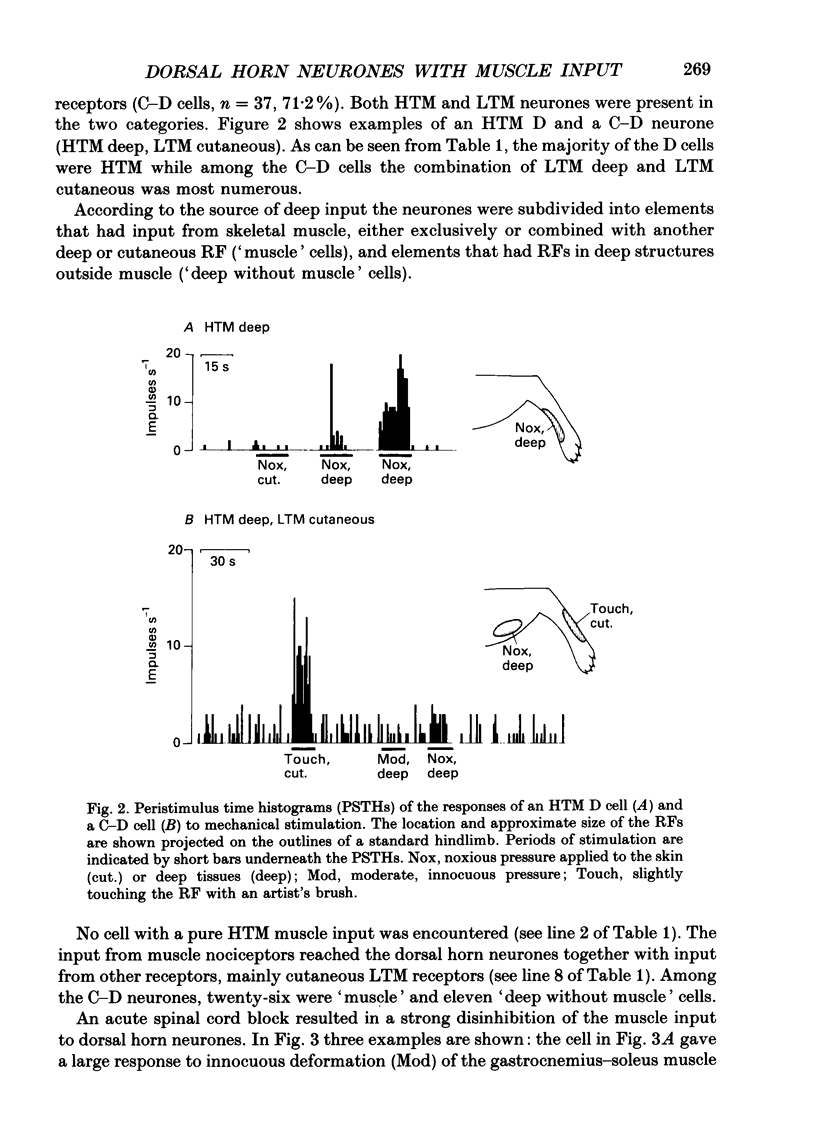
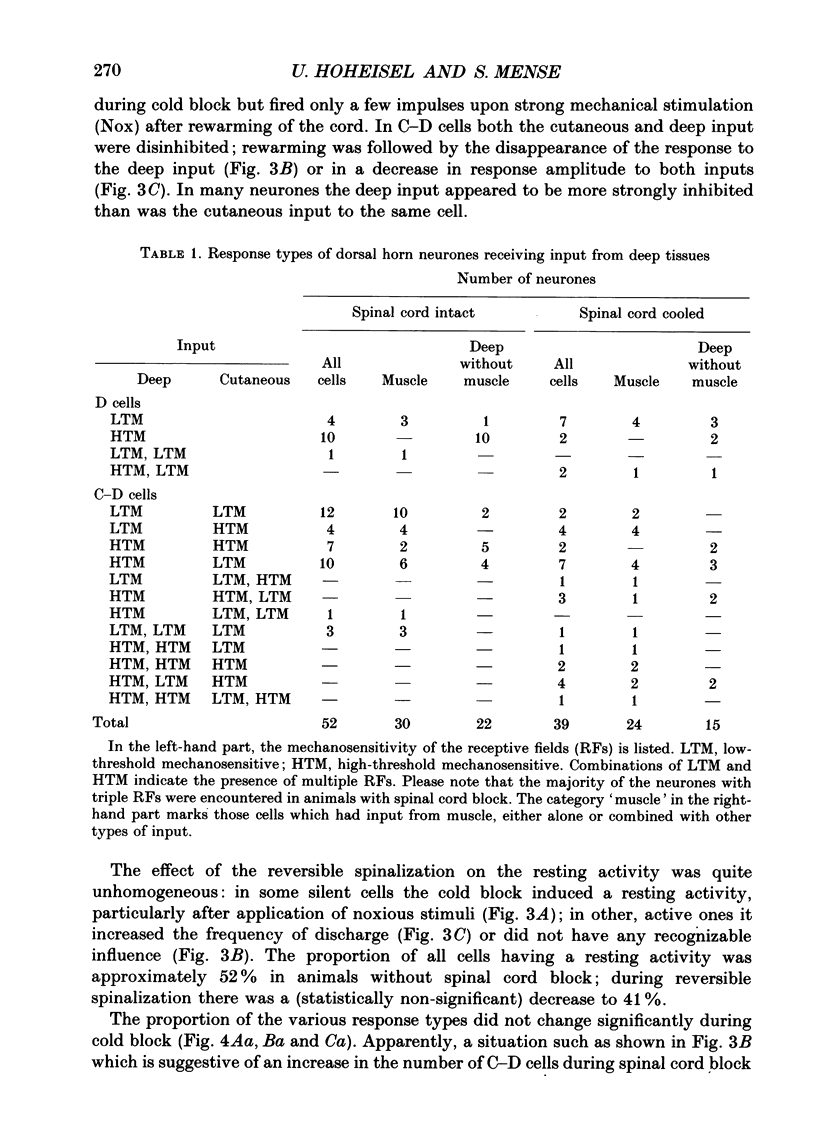
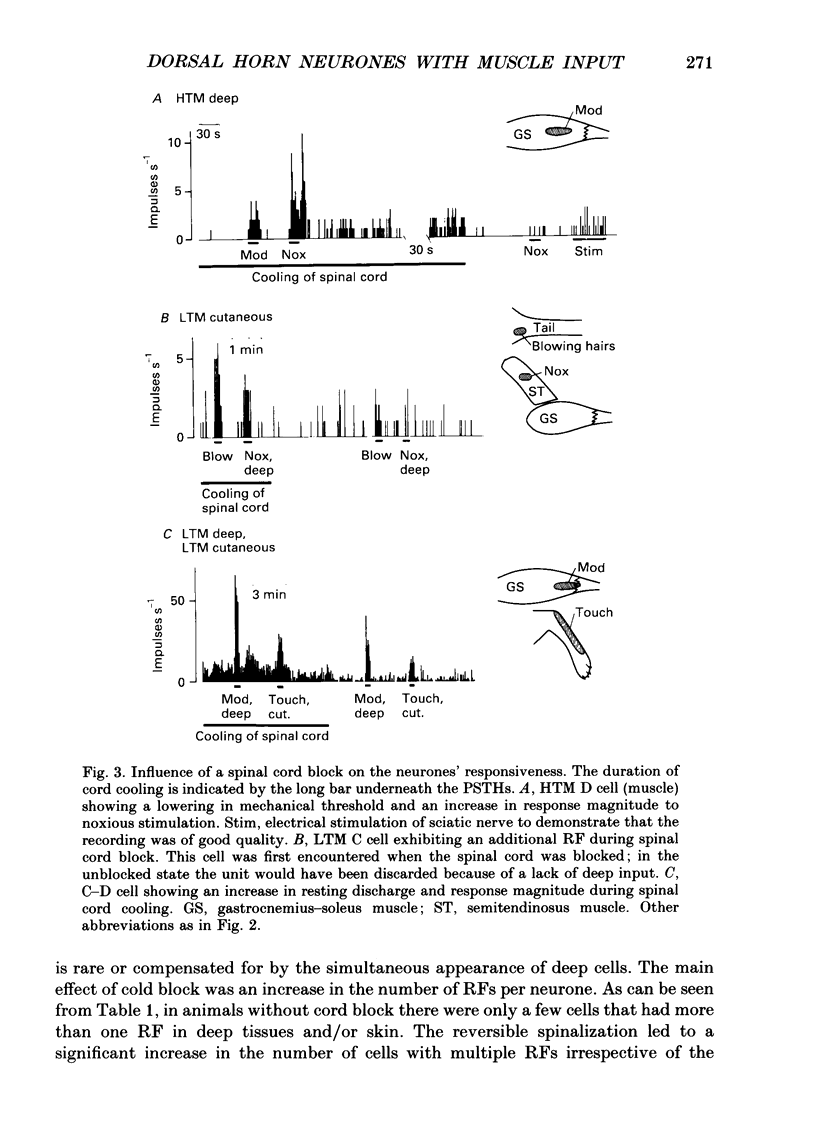





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amano N., Hu J. W., Sessle B. J. Responses of neurons in feline trigeminal subnucleus caudalis (medullary dorsal horn) to cutaneous, intraoral, and muscle afferent stimuli. J Neurophysiol. 1986 Feb;55(2):227–243. doi: 10.1152/jn.1986.55.2.227. [DOI] [PubMed] [Google Scholar]
- Aoyama M., Hongo T., Kudo N. Sensory input to cells of origin of uncrossed spinocerebellar tract located below Clarke's column in the cat. J Physiol. 1988 Apr;398:233–257. doi: 10.1113/jphysiol.1988.sp017040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Applebaum A. E., Leonard R. B., Kenshalo D. R., Jr, Martin R. F., Willis W. D. Nuclei in which functionally identified spinothalamic tract neurons terminate. J Comp Neurol. 1979 Dec 15;188(4):575–585. doi: 10.1002/cne.901880405. [DOI] [PubMed] [Google Scholar]
- Bennett G. J., Abdelmoumene M., Hayashi H., Dubner R. Physiology and morphology of substantia gelatinosa neurons intracellularly stained with horseradish peroxidase. J Comp Neurol. 1980 Dec 15;194(4):809–827. doi: 10.1002/cne.901940407. [DOI] [PubMed] [Google Scholar]
- Cervero F., Iggo A., Ogawa H. Nociceptor-driven dorsal horn neurones in the lumbar spinal cord of the cat. Pain. 1976 Mar;2(1):5–24. doi: 10.1016/0304-3959(76)90042-7. [DOI] [PubMed] [Google Scholar]
- Cervero F. Somatic and visceral inputs to the thoracic spinal cord of the cat: effects of noxious stimulation of the biliary system. J Physiol. 1983 Apr;337:51–67. doi: 10.1113/jphysiol.1983.sp014611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervero F. Supraspinal connections of neurones in the thoracic spinal cord of the cat: ascending projections and effects of descending impulses. Brain Res. 1983 Sep 26;275(2):251–261. doi: 10.1016/0006-8993(83)90986-1. [DOI] [PubMed] [Google Scholar]
- Christensen B. N., Perl E. R. Spinal neurons specifically excited by noxious or thermal stimuli: marginal zone of the dorsal horn. J Neurophysiol. 1970 Mar;33(2):293–307. doi: 10.1152/jn.1970.33.2.293. [DOI] [PubMed] [Google Scholar]
- Craig A. D., Kniffki K. D. Spinothalamic lumbosacral lamina I cells responsive to skin and muscle stimulation in the cat. J Physiol. 1985 Aug;365:197–221. doi: 10.1113/jphysiol.1985.sp015767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duggan A. W., Griersmith B. T., North R. A. Morphine and supraspinal inhibition of spinal neurones: evidence that morphine decreases tonic descending inhibition in the anaesthetized cat. Br J Pharmacol. 1980 Jul;69(3):461–466. doi: 10.1111/j.1476-5381.1980.tb07035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., ECCLES R. M., LUNDBERG A. Types of neurone in and around the intermediate nucleus of the lumbosacral cord. J Physiol. 1960 Nov;154:89–114. doi: 10.1113/jphysiol.1960.sp006566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., OSCARSSON O., WILLIS W. D. Synaptic action of group I and II afferent fibres of muscle on the cells of the dorsal spinocerebellar tract. J Physiol. 1961 Oct;158:517–543. doi: 10.1113/jphysiol.1961.sp006783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgley S. A., Jankowska E. An interneuronal relay for group I and II muscle afferents in the midlumbar segments of the cat spinal cord. J Physiol. 1987 Aug;389:647–674. doi: 10.1113/jphysiol.1987.sp016676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrington D. G., Sorkin L. S., Willis W. D., Jr Responses of spinothalamic tract cells in the cat cervical spinal cord to innocuous and graded noxious stimuli. Somatosens Res. 1986;3(4):339–358. doi: 10.3109/07367228609144592. [DOI] [PubMed] [Google Scholar]
- Foreman R. D., Blair R. W., Weber R. N. Viscerosomatic convergence onto T2-T4 spinoreticular, spinoreticular-spinothalamic, and spinothalamic tract neurons in the cat. Exp Neurol. 1984 Sep;85(3):597–619. doi: 10.1016/0014-4886(84)90034-7. [DOI] [PubMed] [Google Scholar]
- Hockaday J. M., Whitty C. W. Patterns of referred pain in the normal subject. Brain. 1967 Sep;90(3):481–496. doi: 10.1093/brain/90.3.481. [DOI] [PubMed] [Google Scholar]
- Hoheisel U., Mense S. Long-term changes in discharge behaviour of cat dorsal horn neurones following noxious stimulation of deep tissues. Pain. 1989 Feb;36(2):239–247. doi: 10.1016/0304-3959(89)90029-8. [DOI] [PubMed] [Google Scholar]
- Hong S. K., Kniffke K. D., Mense S., Schmidt R. F., Wendisch M. Descending influences on the responses of spinocervical tract neurones to chemical stimulation of fine muscle afferents. J Physiol. 1979 May;290(2):129–140. doi: 10.1113/jphysiol.1979.sp012764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kniffki K. D., Schomburg E. D., Steffens H. Convergence in segmental reflex pathways from fine muscle afferents and cutaneous or group II muscle afferents to alpha-motoneurones. Brain Res. 1981 Aug 10;218(1-2):342–346. doi: 10.1016/0006-8993(81)91312-3. [DOI] [PubMed] [Google Scholar]
- Mendell L. M. Physiological properties of unmyelinated fiber projection to the spinal cord. Exp Neurol. 1966 Nov;16(3):316–332. doi: 10.1016/0014-4886(66)90068-9. [DOI] [PubMed] [Google Scholar]
- Mense S., Meyer H. Different types of slowly conducting afferent units in cat skeletal muscle and tendon. J Physiol. 1985 Jun;363:403–417. doi: 10.1113/jphysiol.1985.sp015718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menétrey D., Giesler G. J., Jr, Besson J. M. An analysis of response properties of spinal cord dorsal horn neurones to nonnoxious and noxious stimuli in the spinal rat. Exp Brain Res. 1977 Jan 18;27(1):15–33. doi: 10.1007/BF00234822. [DOI] [PubMed] [Google Scholar]
- Price D. D., Dubner R. Neurons that subserve the sensory-discriminative aspects of pain. Pain. 1977 Aug;3(4):307–338. doi: 10.1016/0304-3959(77)90063-X. [DOI] [PubMed] [Google Scholar]
- Schaible H. G., Schmidt R. F., Willis W. D. Convergent inputs from articular, cutaneous and muscle receptors onto ascending tract cells in the cat spinal cord. Exp Brain Res. 1987;66(3):479–488. doi: 10.1007/BF00270680. [DOI] [PubMed] [Google Scholar]
- Schaible H. G., Schmidt R. F., Willis W. D. Responses of spinal cord neurones to stimulation of articular afferent fibres in the cat. J Physiol. 1986 Mar;372:575–593. doi: 10.1113/jphysiol.1986.sp016026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOSS R. E. Studies of the spinal cord. Part 3. Pathways for deep pain within the spinal cord and brain. Neurology. 1953 Mar;3(3):163–175. doi: 10.1212/wnl.3.3.163. [DOI] [PubMed] [Google Scholar]


