Abstract
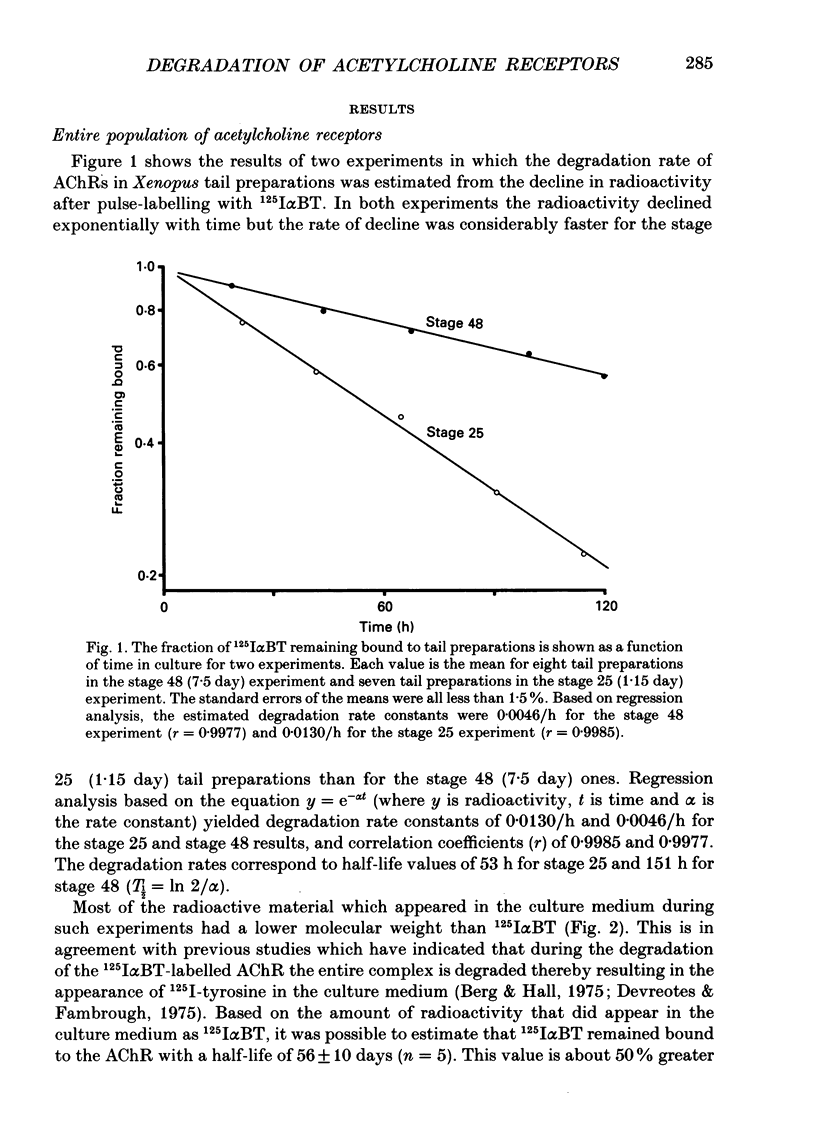
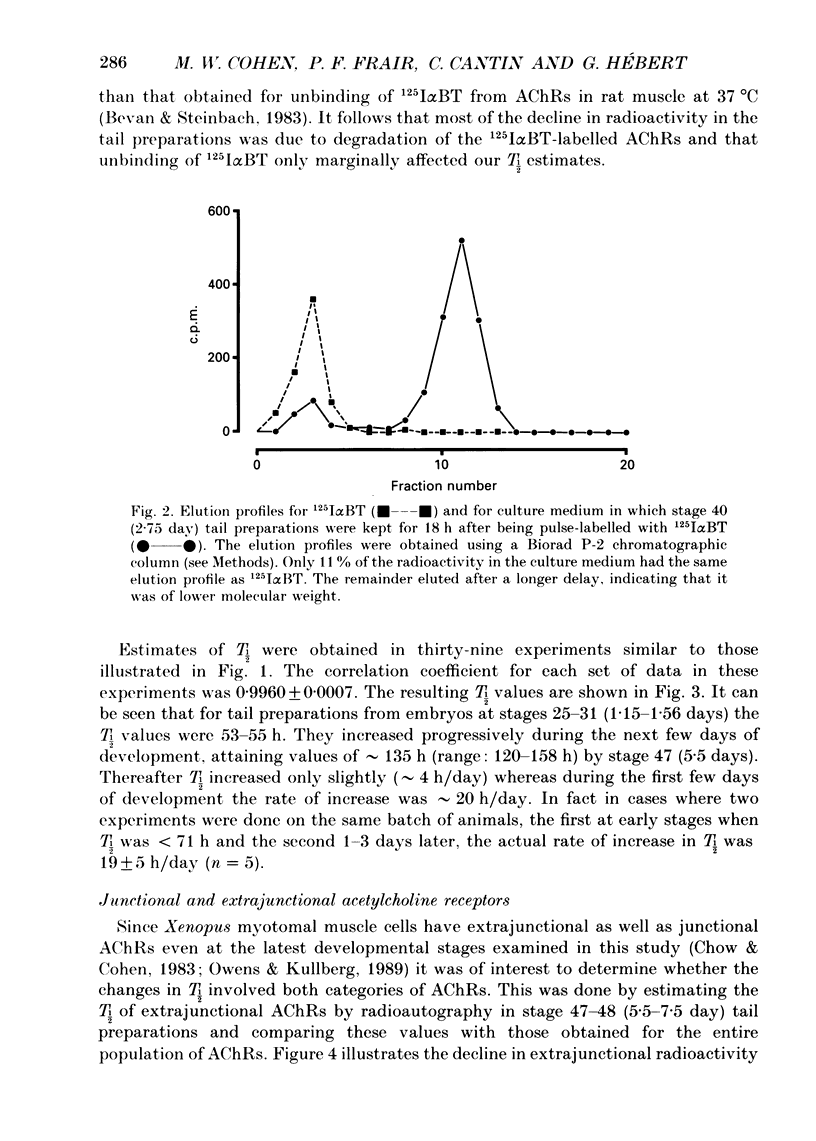
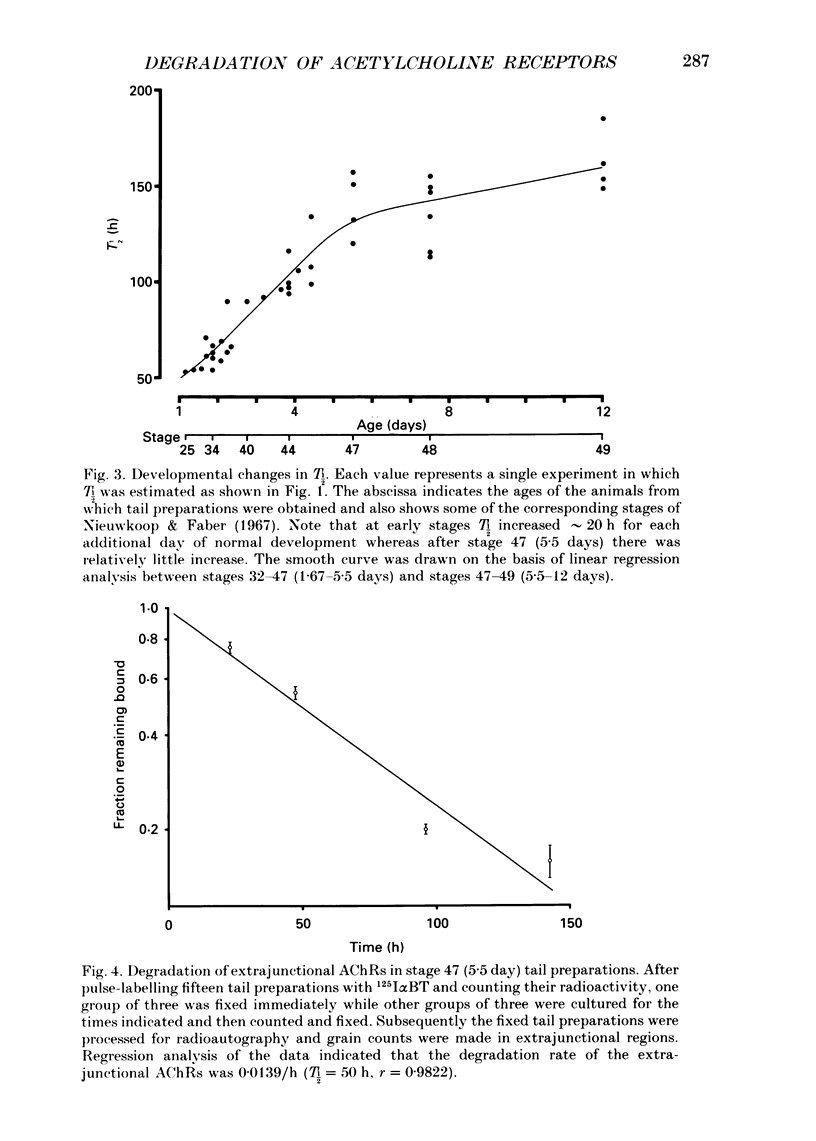
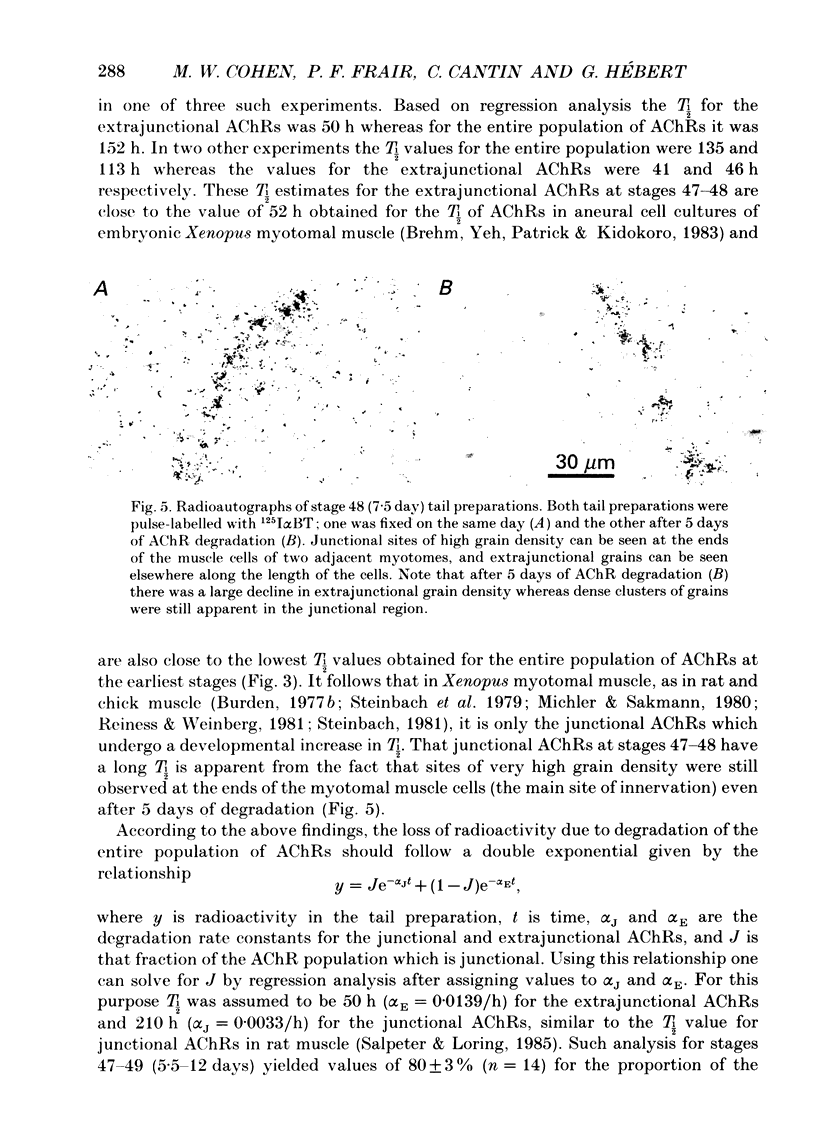
1. Tail preparations, containing myotomal muscle and associated spinal cord, were isolated from embryos and tadpoles of Xenopus laevis between stages 25 and 49 (1.15-12 days) and were pulse-labelled with 125I-alpha-bungarotoxin (125I alpha BT) so that the half-life (T1/2) of their acetylcholine receptors (AChRs) could be estimated in organ culture. 2. For the entire population of AChRs, estimates of T1/2 based on a single exponential decline in radioactivity (but see item 4 below) increased from 53-55 h at stages 25-31 (1.15-1.56 days) to approximately 135 h at stage 47 (5.5 days). Beyond stage 47 T1/2 increased only slightly. 3. Radioautographic estimates of the T1/2 of extrajunctional AChRs at stages 47-48 (5.5-7.5 days) were 41-50 h. It follows that the developmental change in the T1/2 of the entire population of AChRs was due to the junctional AChRs. 4. At stages 47-49 (5.5-12 days) the decline in radioactivity for the entire population of AChRs was fitted well by a double exponential. Assuming a T1/2 of 50 h for the extrajunctional AChRs and 210 h for the junctional AChRs, the correlation coefficient (r) was 0.9947 +/- 0.0014 (mean +/- S.E.M.; n = 14) and junctional AChRs were estimated to comprise 80 +/- 3% of the entire population. Similar analysis, as well as experiments in which the degradation of junctional AChRs was assessed by pulse-labelling with fluorescent alpha-bungarotoxin, suggested that at earlier stages of development the junctional AChRs have a shorter T1/2 and comprise a smaller fraction of the entire population. 5. The developmental increase in T1/2 occurred even when animals were raised in the anaesthetic tricaine or in tetrodotoxin, conditions which abolished all motor activity. 6. Developmental increases in T1/2 also occurred in culture but were smaller than those in vivo. The increases in culture did not occur amongst those AChRs which were pre-labelled with 125I alpha BT. 7. It is concluded that in Xenopus myotomal muscle the T1/2 of junctional AChRs begins to increase within a day after the onset of innervation and that the increase does not require nerve or muscle impulse activity. We suggest, among other possibilities, that it may depend upon incorporation of a different molecular species of AChR into the postsynaptic membrane.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avila O. L., Drachman D. B., Pestronk A. Neurotransmission regulates stability of acetylcholine receptors at the neuromuscular junction. J Neurosci. 1989 Aug;9(8):2902–2906. doi: 10.1523/JNEUROSCI.09-08-02902.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin T. J., Yoshihara C. M., Blackmer K., Kintner C. R., Burden S. J. Regulation of acetylcholine receptor transcript expression during development in Xenopus laevis. J Cell Biol. 1988 Feb;106(2):469–478. doi: 10.1083/jcb.106.2.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg D. K., Hall Z. W. Loss of alpha-bungarotoxin from junctional and extrajunctional acetylcholine receptors in rat diaphragm muscle in vivo and in organ culture. J Physiol. 1975 Nov;252(3):771–789. doi: 10.1113/jphysiol.1975.sp011169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betz H., Bourgeois J. P., Changeux J. P. Evolution of cholinergic proteins in developing slow and fast skeletal muscles in chick embryo. J Physiol. 1980 May;302:197–218. doi: 10.1113/jphysiol.1980.sp013238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan S., Steinbach J. H. Denervation increases the degradation rate of acetylcholine receptors at end-plates in vivo and in vitro. J Physiol. 1983 Mar;336:159–177. doi: 10.1113/jphysiol.1983.sp014574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan S., Steinbach J. H. The distribution of alpha-bungarotoxin binding sites of mammalian skeletal muscle developing in vivo. J Physiol. 1977 May;267(1):195–213. doi: 10.1113/jphysiol.1977.sp011808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackshaw S., Warner A. Onset of acetylcholine sensitivity and endplate activity in developing myotome muscles of Xenopus. Nature. 1976 Jul 15;262(5565):217–218. doi: 10.1038/262217a0. [DOI] [PubMed] [Google Scholar]
- Brehm P., Yeh E., Patrick J., Kidokoro Y. Metabolism of acetylcholine receptors on embryonic amphibian muscle. J Neurosci. 1983 Jan;3(1):101–107. doi: 10.1523/JNEUROSCI.03-01-00101.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burden S. Acetylcholine receptors at the neuromuscular junction: developmental change in receptor turnover. Dev Biol. 1977 Nov;61(1):79–85. doi: 10.1016/0012-1606(77)90343-8. [DOI] [PubMed] [Google Scholar]
- Burden S. Development of the neuromuscular junction in the chick embryo: the number, distribution, and stability of acetylcholine receptors. Dev Biol. 1977 Jun;57(2):317–329. doi: 10.1016/0012-1606(77)90218-4. [DOI] [PubMed] [Google Scholar]
- Chow I., Cohen M. W. Developmental changes in the distribution of acetylcholine receptors in the myotomes of Xenopus laevis. J Physiol. 1983 Jun;339:553–571. doi: 10.1113/jphysiol.1983.sp014733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen M. W., Greschner M., Tucci M. In vivo development of cholinesterase at a neuromuscular junction in the absence of motor activity in Xenopus laevis. J Physiol. 1984 Mar;348:57–66. doi: 10.1113/jphysiol.1984.sp015099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen M. W., Rodriguez-Marin E., Wilson E. M. Distribution of synaptic specializations along isolated motor units formed in Xenopus nerve-muscle cultures. J Neurosci. 1987 Sep;7(9):2849–2861. doi: 10.1523/JNEUROSCI.07-09-02849.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devreotes P. N., Fambrough D. M. Acetylcholine receptor turnover in membranes of developing muscle fibers. J Cell Biol. 1975 May;65(2):335–358. doi: 10.1083/jcb.65.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fambrough D. M. Control of acetylcholine receptors in skeletal muscle. Physiol Rev. 1979 Jan;59(1):165–227. doi: 10.1152/physrev.1979.59.1.165. [DOI] [PubMed] [Google Scholar]
- Fischbach G. D., Schuetze S. M. A post-natal decrease in acetylcholine channel open time at rat end-plates. J Physiol. 1980 Jun;303:125–137. doi: 10.1113/jphysiol.1980.sp013275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfarb J., Cantin C., Cohen M. W. Intracellular and surface acetylcholine receptors during the normal development of a frog skeletal muscle. J Neurosci. 1990 Feb;10(2):500–507. doi: 10.1523/JNEUROSCI.10-02-00500.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullberg R. W., Brehm P., Steinbach J. H. Nonjunctional acetylcholine receptor channel open time decreases during development of Xenopus muscle. Nature. 1981 Jan 29;289(5796):411–413. doi: 10.1038/289411a0. [DOI] [PubMed] [Google Scholar]
- Kullberg R. W., Lentz T. L., Cohen M. W. Development of the myotomal neuromuscular junction in Xenopus laevis: an electrophysiological and fine-structural study. Dev Biol. 1977 Oct 1;60(1):101–129. doi: 10.1016/0012-1606(77)90113-0. [DOI] [PubMed] [Google Scholar]
- Kullberg R., Kasprzak H. Gating kinetics of nonjunctional acetylcholine receptor channels in developing Xenopus muscle. J Neurosci. 1985 Apr;5(4):970–976. doi: 10.1523/JNEUROSCI.05-04-00970.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullberg R., Owens J. L., Vickers J. Development of synaptic currents in immobilized muscle of Xenopus laevis. J Physiol. 1985 Jul;364:57–68. doi: 10.1113/jphysiol.1985.sp015729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loring R. H., Salpeter M. M. Denervation increases turnover rate of junctional acetylcholine receptors. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2293–2297. doi: 10.1073/pnas.77.4.2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lømo T., Slater C. R. Control of junctional acetylcholinesterase by neural and muscular influences in the rat. J Physiol. 1980 Jun;303:191–202. doi: 10.1113/jphysiol.1980.sp013280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthew W. D., Tsavaler L., Reichardt L. F. Identification of a synaptic vesicle-specific membrane protein with a wide distribution in neuronal and neurosecretory tissue. J Cell Biol. 1981 Oct;91(1):257–269. doi: 10.1083/jcb.91.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michler A., Sakmann B. Receptor stability and channel conversion in the subsynaptic membrane of the developing mammalian neuromuscular junction. Dev Biol. 1980 Nov;80(1):1–17. doi: 10.1016/0012-1606(80)90494-7. [DOI] [PubMed] [Google Scholar]
- Owens J. L., Kullberg R. In vivo development of nicotinic acetylcholine receptor channels in Xenopus myotomal muscle. J Neurosci. 1989 Mar;9(3):1018–1028. doi: 10.1523/JNEUROSCI.09-03-01018.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakmann B., Brenner H. R. Change in synaptic channel gating during neuromuscular development. Nature. 1978 Nov 23;276(5686):401–402. doi: 10.1038/276401a0. [DOI] [PubMed] [Google Scholar]
- Salpeter M. M., Cooper D. L., Levitt-Gilmour T. Degradation rates of acetylcholine receptors can be modified in the postjunctional plasma membrane of the vertebrate neuromuscular junction. J Cell Biol. 1986 Oct;103(4):1399–1403. doi: 10.1083/jcb.103.4.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salpeter M. M., Loring R. H. Nicotinic acetylcholine receptors in vertebrate muscle: properties, distribution and neural control. Prog Neurobiol. 1985;25(4):297–325. doi: 10.1016/0301-0082(85)90018-8. [DOI] [PubMed] [Google Scholar]
- Schuetze S. M., Role L. W. Developmental regulation of nicotinic acetylcholine receptors. Annu Rev Neurosci. 1987;10:403–457. doi: 10.1146/annurev.ne.10.030187.002155. [DOI] [PubMed] [Google Scholar]
- Schuetze S. M. The acetylcholine channel open time in chick muscle is not decreased following innervation. J Physiol. 1980 Jun;303:111–124. doi: 10.1113/jphysiol.1980.sp013274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyng S. L., Salpeter M. M. Degradation rate of acetylcholine receptors inserted into denervated vertebrate neuromuscular junctions. J Cell Biol. 1989 Feb;108(2):647–651. doi: 10.1083/jcb.108.2.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbach J. H., Merlie J., Heinemann S., Bloch R. Degradation of junctional and extrajunctional acetylcholine receptors by developing rat skeletal muscle. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3547–3551. doi: 10.1073/pnas.76.7.3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg C. B., Hall Z. W. Junctional form of acetylcholinesterase restored at nerve-free endplates. Dev Biol. 1979 Feb;68(2):631–635. doi: 10.1016/0012-1606(79)90233-1. [DOI] [PubMed] [Google Scholar]




