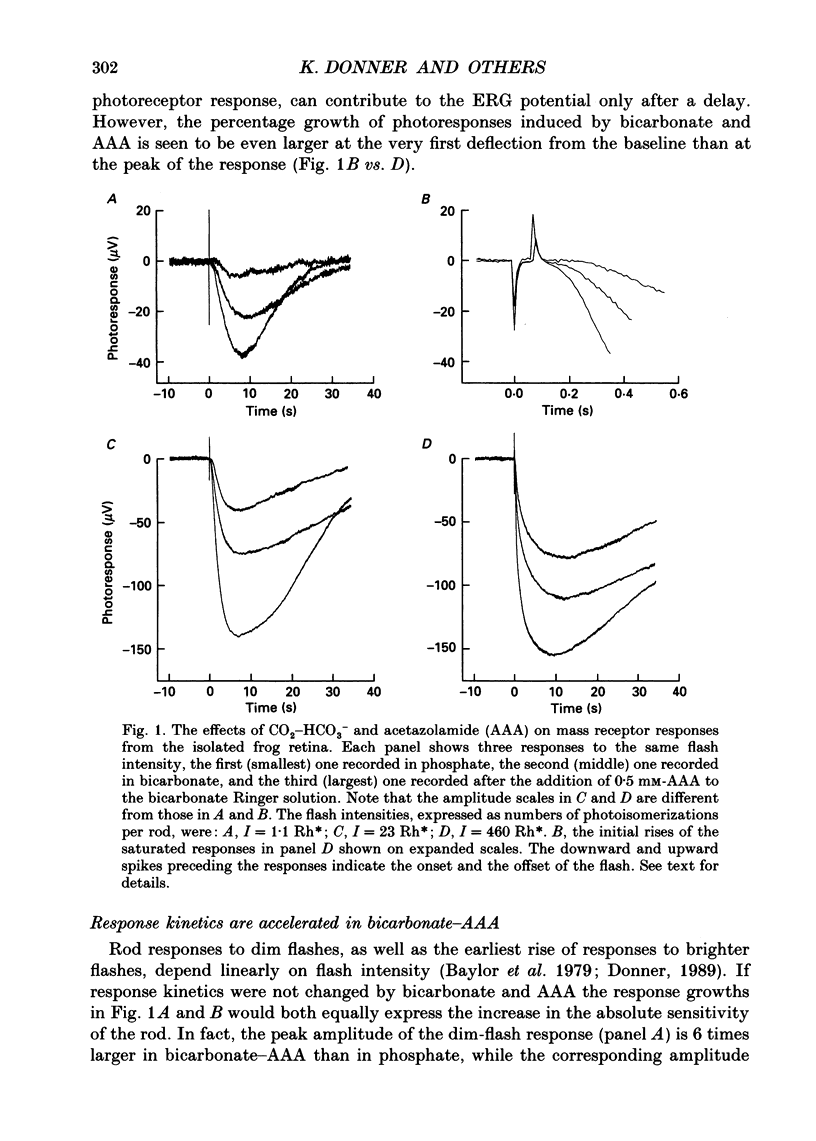
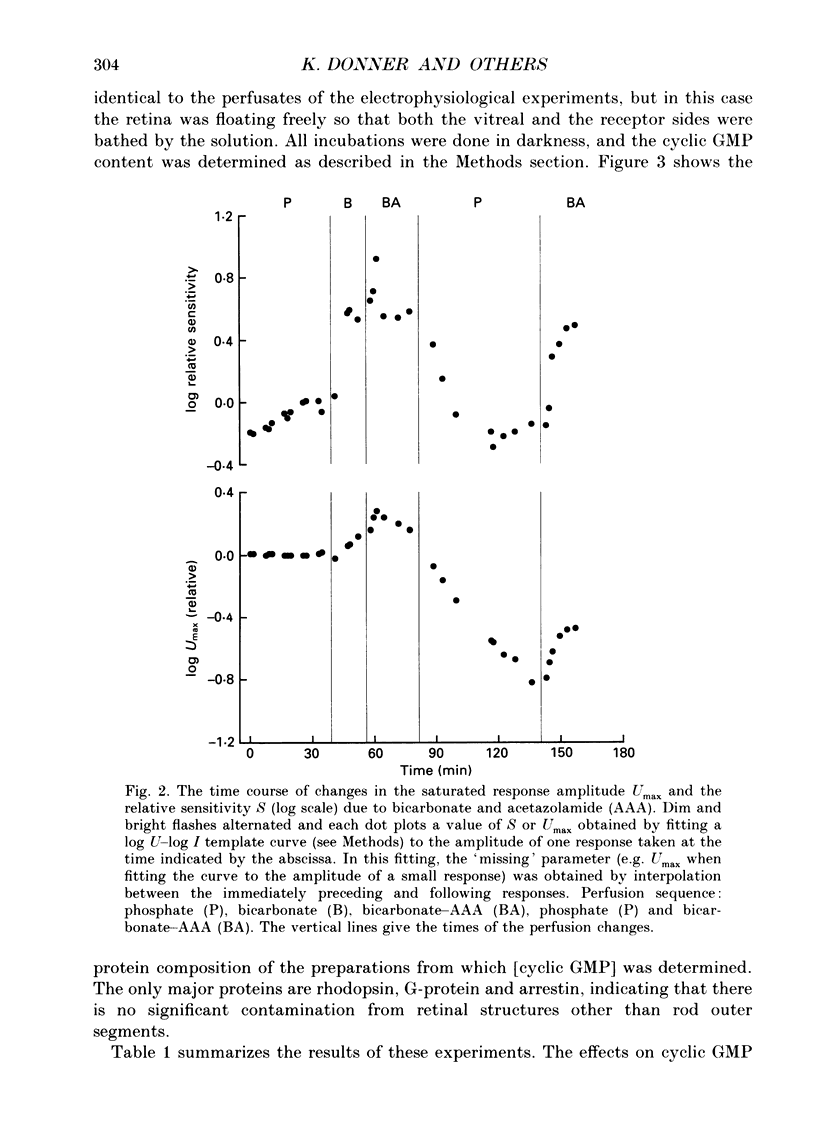
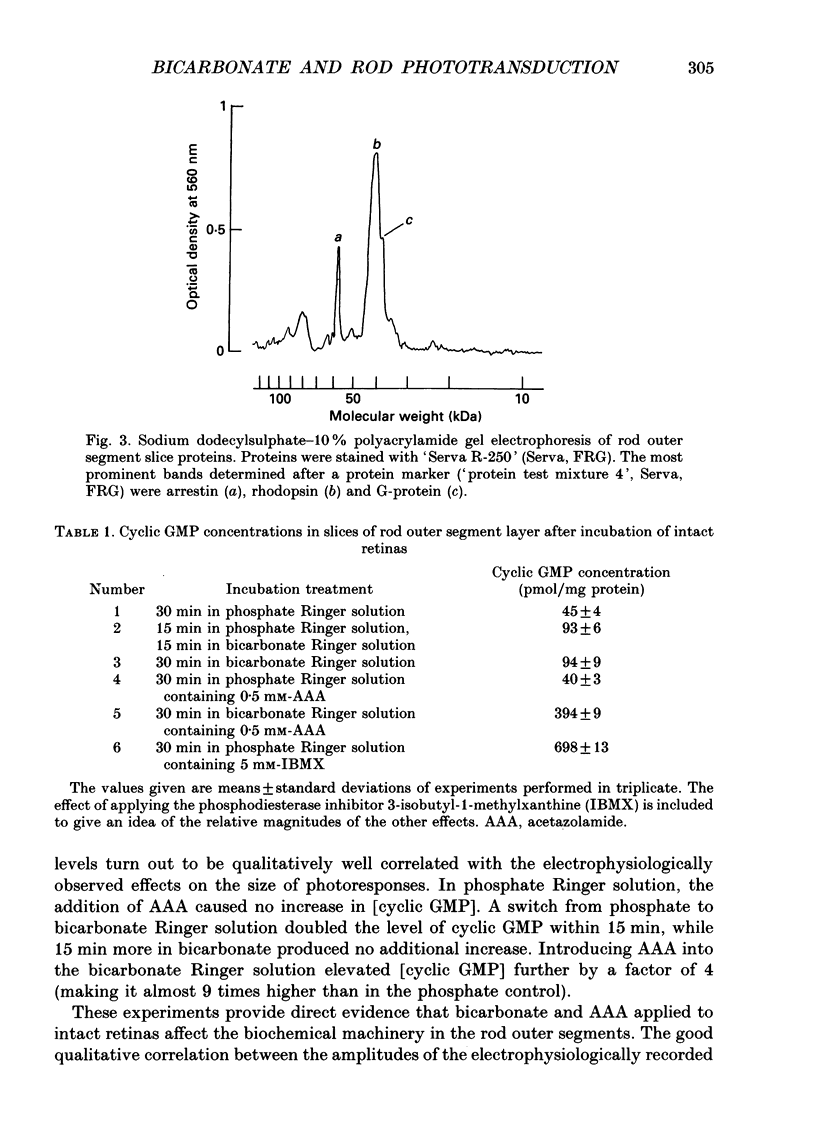
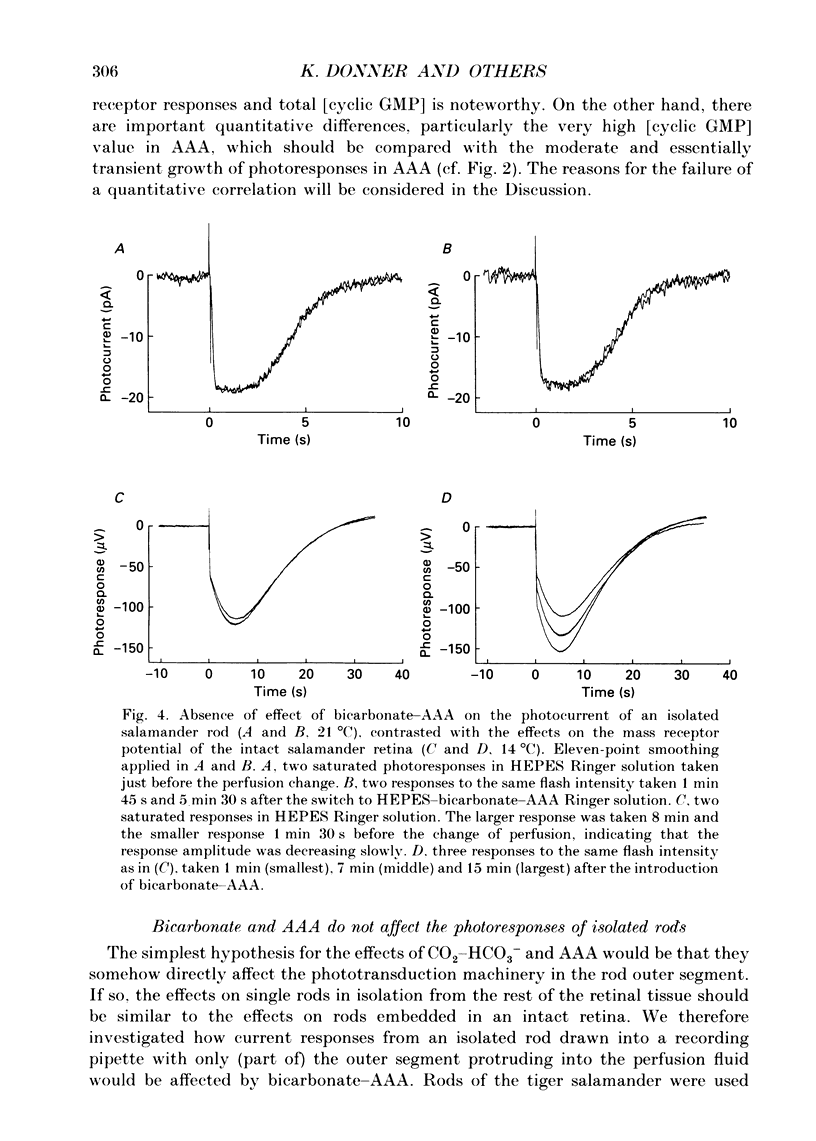
Abstract
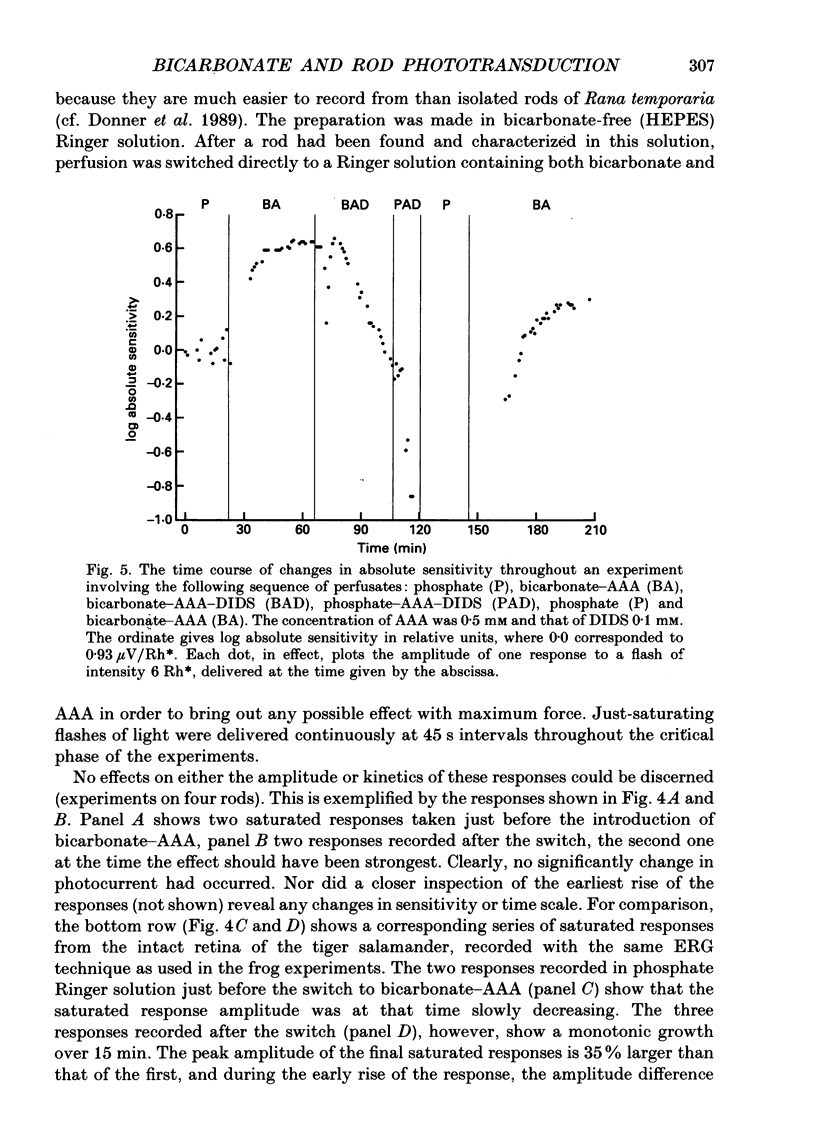
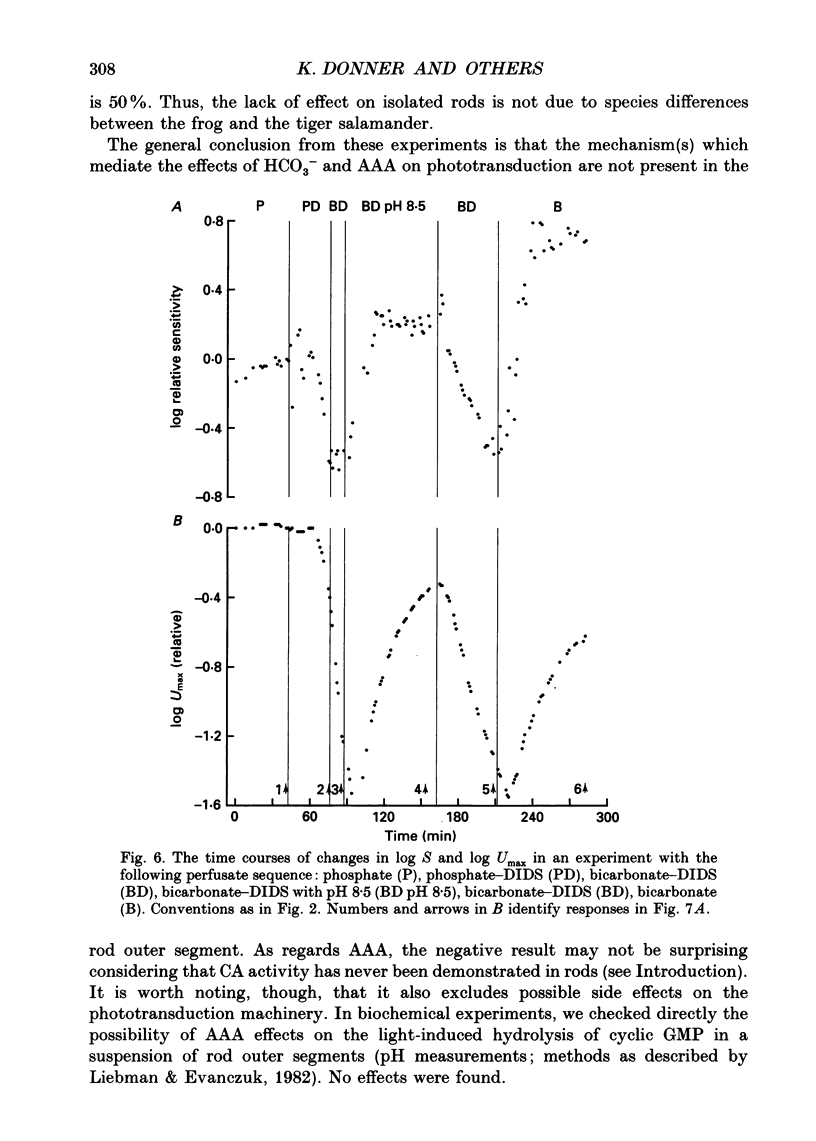
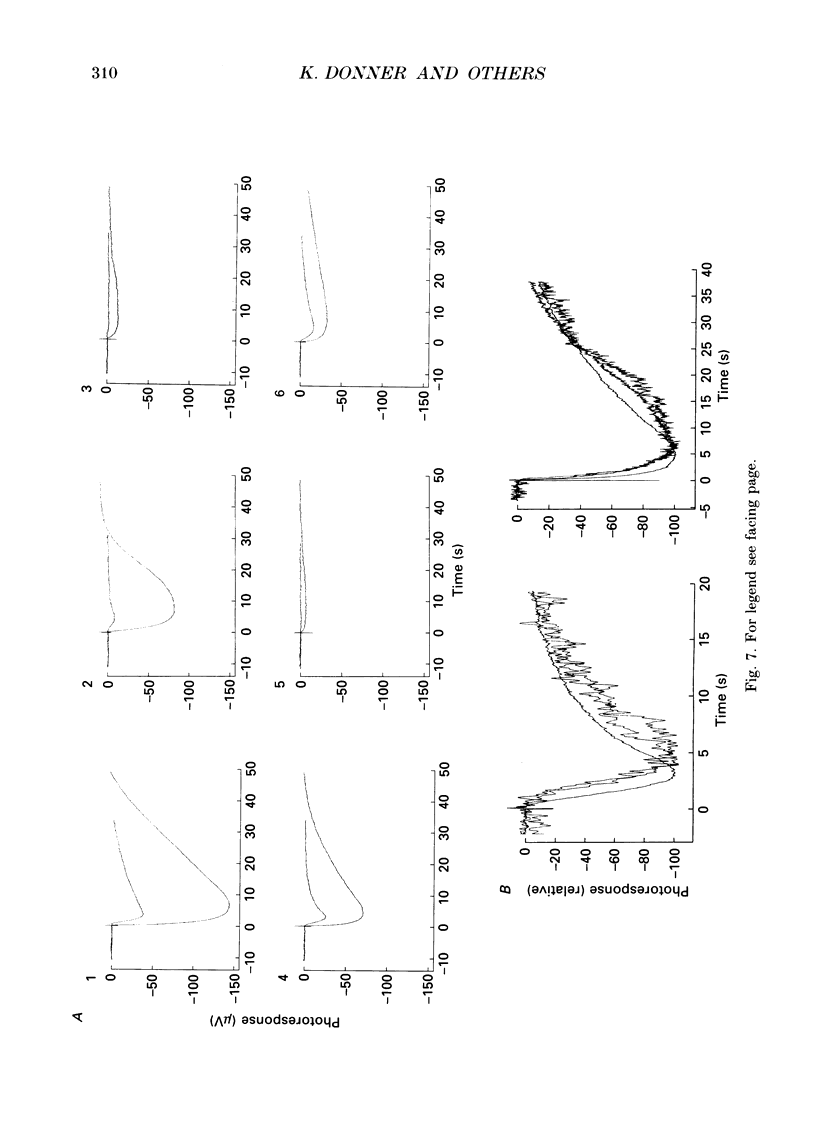
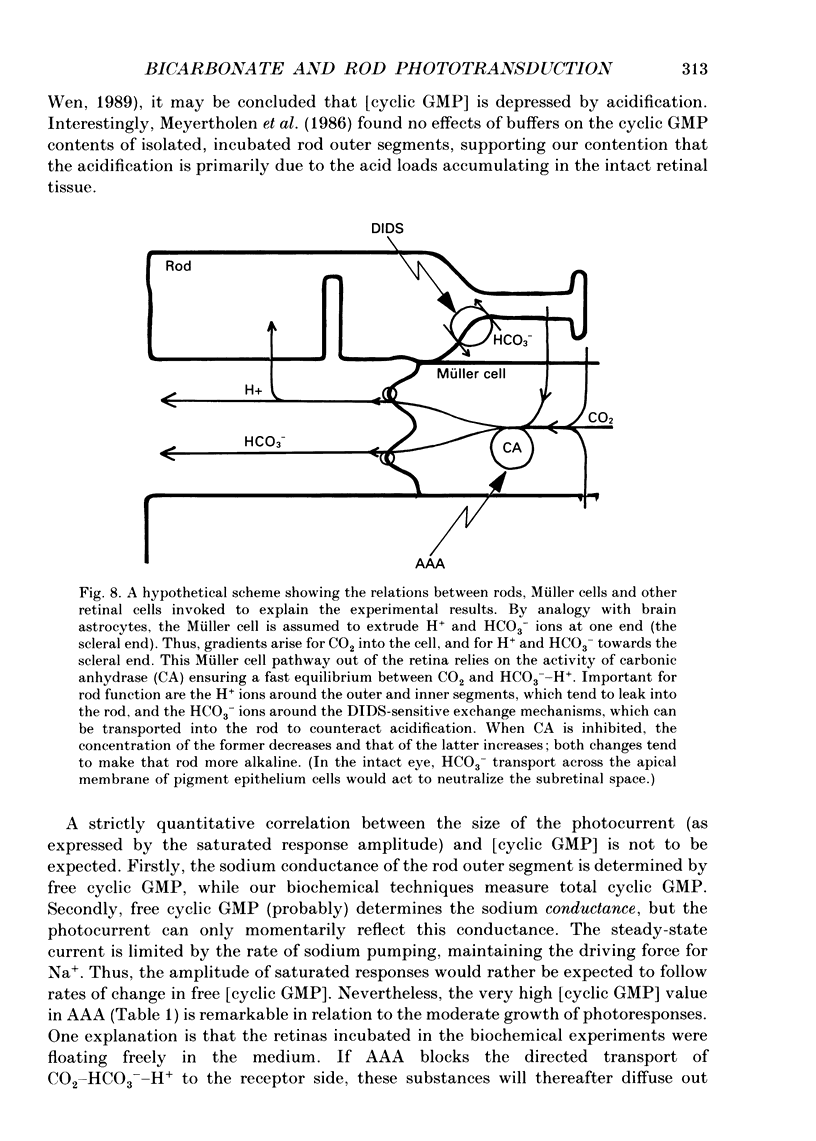
1. Effects on rod phototransduction following manipulation of retinal CO2-HCO3- and H+ fluxes were studied in dark-adapted retinas of the frog and the tiger salamander. 2. Rod photoresponses to brief flashes of light were recorded from the isolated sensory retina as electroretinogram mass receptor potentials and from isolated rods by the suction-pipette technique. The experimental treatments were: (1) varying [CO2] + [HCO3-] in the perfusion fluid: (2) applying acetazolamide (AAA), which inhibits the enzyme carbonic anhydrase (CA); and (3) applying 4,4'-diisothiocyanatostilbene-2,2'-disulphonic acid (DIDS) which blocks exchange mechanisms transporting HCO3- across cell membranes. 3. The concentration of the internal transmitter of the rods, cyclic GMP, was biochemically determined from the rod outer segment layer of retinas that had been incubated in the same solutions as were used for perfusion in the electrophysiological experiments. 4. The introduction of 6 mM-sodium bicarbonate to replace half the buffer of a nominally CO2-HCO3(-)-free (12 mM-phosphate or HEPES, [Na+] constant) Ringer solution doubled the cyclic GMP concentration in the rod outer segment layer and increased the saturating response amplitude and the relative sensitivity of rods in the intact retina. 5. The introduction of 0.5 mM-AAA into bicarbonate-containing Ringer solution accelerated the growth of saturated responses and sensitivity. Incubation of the retina in AAA-bicarbonate Ringer solution elevated the concentration of cyclic GMP ninefold compared with the phosphate control. 6. No effects of switching to bicarbonate-AAA Ringer solution were observed in the photocurrent of isolated rods drawn into suction pipettes with only the outer segment protruding into the perfusion fluid. The target of AAA is probably the CA-containing Müller cell. 7. The introduction of DIDS into the perfusate (at normal pH 7.5) set off a continuous decay of photoresponses which finally abolished light sensitivity completely. The decay proceeded regardless of whether bicarbonate and AAA were present or not. 8. Rods that had lost their photosensitivity in DIDS recovered almost fully when the pH of the DIDS perfusate was raised to 8.5. They also recovered when DIDS was washed out with bicarbonate Ringer solution at constant pH (7.5). 9. It is proposed that all our treatments ultimately modulate the intracellular pH of the rods which is determined by the relative rates of H+ leakage and HCO3- transport into the cells.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baylor D. A., Lamb T. D., Yau K. W. The membrane current of single rod outer segments. J Physiol. 1979 Mar;288:589–611. [PMC free article] [PubMed] [Google Scholar]
- Biagi B. A. Effects of the anion transport inhibitor, SITS, on the proximal straight tubule of the rabbit perfused in vitro. J Membr Biol. 1985;88(1):25–31. doi: 10.1007/BF01871210. [DOI] [PubMed] [Google Scholar]
- Borgula G. A., Karwoski C. J., Steinberg R. H. Light-evoked changes in extracellular pH in frog retina. Vision Res. 1989;29(9):1069–1077. doi: 10.1016/0042-6989(89)90054-0. [DOI] [PubMed] [Google Scholar]
- Chesler M. Regulation of intracellular pH in reticulospinal neurones of the lamprey, Petromyzon marinus. J Physiol. 1986 Dec;381:241–261. doi: 10.1113/jphysiol.1986.sp016325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curci S., Debellis L., Frömter E. Evidence for rheogenic sodium bicarbonate cotransport in the basolateral membrane of oxyntic cells of frog gastric fundus. Pflugers Arch. 1987 May;408(5):497–504. doi: 10.1007/BF00585075. [DOI] [PubMed] [Google Scholar]
- Donner K., Hemilä S., Koskelainen A. Temperature-dependence of rod photoresponses from the aspartate-treated retina of the frog (Rana temporaria). Acta Physiol Scand. 1988 Dec;134(4):535–541. doi: 10.1111/j.1748-1716.1998.tb08528.x. [DOI] [PubMed] [Google Scholar]
- Donner K., Hemilä S. Rhodopsin phosphorylation inhibited by adenosine in frog rods: lack of effects on excitation. Comp Biochem Physiol A Comp Physiol. 1985;81(2):431–439. doi: 10.1016/0300-9629(85)90160-4. [DOI] [PubMed] [Google Scholar]
- Donner K. Visual latency and brightness: an interpretation based on the responses of rods and ganglion cells in the frog retina. Vis Neurosci. 1989 Jul;3(1):39–51. doi: 10.1017/s0952523800012499. [DOI] [PubMed] [Google Scholar]
- FUTTERMAN S., KINOSHITA J. H. Metabolism of the retina. I. Respiration of cattle retina. J Biol Chem. 1959 Apr;234(4):723–726. [PubMed] [Google Scholar]
- Gedney C., Ostroy S. E. Hydrogen ion effects of the vertebrate photoreceptor. The pK's of ionizable groups affecting cell permeability. Arch Biochem Biophys. 1978 May;188(1):105–113. doi: 10.1016/0003-9861(78)90362-4. [DOI] [PubMed] [Google Scholar]
- Kartner N., Ling V. Multidrug resistance in cancer. Sci Am. 1989 Mar;260(3):44–51. doi: 10.1038/scientificamerican0389-44. [DOI] [PubMed] [Google Scholar]
- Kimelberg H. K., Biddlecome S., Bourke R. S. SITS-inhibitable Cl- transport and Na+-dependent H+ production in primary astroglial cultures. Brain Res. 1979 Sep 7;173(1):111–124. [PubMed] [Google Scholar]
- Liebman P. A., Evanczuk A. T. Real time assay of rod disk membrane cGMP phosphodiesterase and its controller enzymes. Methods Enzymol. 1982;81:532–542. doi: 10.1016/s0076-6879(82)81074-4. [DOI] [PubMed] [Google Scholar]
- Liebman P. A., Mueller P., Pugh E. N., Jr Protons suppress the dark current of frog retinal rods. J Physiol. 1984 Feb;347:85–110. doi: 10.1113/jphysiol.1984.sp015055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linser P., Moscona A. A. Variable CA II compartmentalization in vertebrate retina. Ann N Y Acad Sci. 1984;429:430–446. doi: 10.1111/j.1749-6632.1984.tb12369.x. [DOI] [PubMed] [Google Scholar]
- Maren T. H. Carbonic anhydrase: chemistry, physiology, and inhibition. Physiol Rev. 1967 Oct;47(4):595–781. doi: 10.1152/physrev.1967.47.4.595. [DOI] [PubMed] [Google Scholar]
- Maren T. H. Use of inhibitors in physiological studies of carbonic anhydrase. Am J Physiol. 1977 Apr;232(4):F291–F297. doi: 10.1152/ajprenal.1977.232.4.F291. [DOI] [PubMed] [Google Scholar]
- Meyertholen E. P., Wilson M. J., Ostroy S. E. Removing bicarbonate/CO2 reduces the cGMP concentration of the vertebrate photoreceptor to the levels normally observed on illumination. Biochem Biophys Res Commun. 1980 Sep 30;96(2):785–792. doi: 10.1016/0006-291x(80)91423-0. [DOI] [PubMed] [Google Scholar]
- Meyertholen E. P., Wilson M. J., Ostroy S. E. The effects of hepes, bicarbonate and calcium on the cGMP content of vertebrate rod photoreceptors and the isolated electrophysiological effects of cGMP and calcium. Vision Res. 1986;26(4):521–533. doi: 10.1016/0042-6989(86)90001-5. [DOI] [PubMed] [Google Scholar]
- Miki N., Baraban J. M., Keirns J. J., Boyce J. J., Bitensky M. W. Purification and properties of the light-activated cyclic nucleotide phosphodiesterase of rod outer segments. J Biol Chem. 1975 Aug 25;250(16):6320–6327. [PubMed] [Google Scholar]
- Musser G. L., Rosen S. Localization of carbonic anhydrase activity in the vertebrate retina. Exp Eye Res. 1973 Jan 1;15(1):105–119. doi: 10.1016/0014-4835(73)90195-4. [DOI] [PubMed] [Google Scholar]
- Oakley B., 2nd, Wen R. Extracellular pH in the isolated retina of the toad in darkness and during illumination. J Physiol. 1989 Dec;419:353–378. doi: 10.1113/jphysiol.1989.sp017876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passow H. Molecular aspects of band 3 protein-mediated anion transport across the red blood cell membrane. Rev Physiol Biochem Pharmacol. 1986;103:61–203. doi: 10.1007/3540153330_2. [DOI] [PubMed] [Google Scholar]
- Russell J. M., Boron W. F. Role of choloride transport in regulation of intracellular pH. Nature. 1976 Nov 4;264(5581):73–74. doi: 10.1038/264073a0. [DOI] [PubMed] [Google Scholar]
- Sillman A. J., Ito H., Tomita T. Studies on the mass receptor potential of the isolated frog retina. I. General properties of the response. Vision Res. 1969 Dec;9(12):1435–1442. doi: 10.1016/0042-6989(69)90059-5. [DOI] [PubMed] [Google Scholar]
- Sillman A. J., Owen W. G., Fernandez H. R. The generation of the late receptor potential: an excitation-inhibition phenomenon. Vision Res. 1972 Sep;12(9):1519–1531. doi: 10.1016/0042-6989(72)90177-0. [DOI] [PubMed] [Google Scholar]
- Steiner A. L., Parker C. W., Kipnis D. M. Radioimmunoassay for cyclic nucleotides. I. Preparation of antibodies and iodinated cyclic nucleotides. J Biol Chem. 1972 Feb 25;247(4):1106–1113. [PubMed] [Google Scholar]
- Thomas R. C. Experimental displacement of intracellular pH and the mechanism of its subsequent recovery. J Physiol. 1984 Sep;354:3P–22P. doi: 10.1113/jphysiol.1984.sp015397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas R. C. The role of bicarbonate, chloride and sodium ions in the regulation of intracellular pH in snail neurones. J Physiol. 1977 Dec;273(1):317–338. doi: 10.1113/jphysiol.1977.sp012096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler B. S. Buffer dependence of retinal glycolysis and ERG potentials. Exp Eye Res. 1986 Jun;42(6):585–593. doi: 10.1016/0014-4835(86)90048-5. [DOI] [PubMed] [Google Scholar]
- Winkler B. S. Glycolytic and oxidative metabolism in relation to retinal function. J Gen Physiol. 1981 Jun;77(6):667–692. doi: 10.1085/jgp.77.6.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zak P. P., Lelekova T. V., Ostrovskii M. A. Raspredelenie atsetikholina v sloiakh setchatki glaza liagushki. Fiziol Zh SSSR Im I M Sechenova. 1974 Sep;60(9):1397–1402. [PubMed] [Google Scholar]


